Characterization and Electrical Properties of PVA Films with Self-Assembled Chitosan-AuNPs/SWCNT-COOH Nanostructures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of Gold Nanoparticles and Chitosan-AuNPS/SWCNT-COOH Nanostructures
2.3. Nanostructured Film Formation of PVA/Chitosan-AuNPs/(SWCNT-COOH)
2.4. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vert, M. Aliphatic polyesters: Great degradable polymers that cannot do everything. Biomacromolecules 2005, 6, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Basavaraja, C.; Thinh, P.X.; Huh, D.S. Structural characterization and DC conductivity of honeycomb-patterned poly(ε-caprolactone)/gold nanoparticle-reduced graphite oxide composite films. Mater. Lett. 2013, 90, 14–18. [Google Scholar] [CrossRef]
- Idris, N.H.; Majid, S.R.; Khiar, A.S.A.; Hassan, M.F.; Arof, A.K. Conductivity studies on chitosan/PEO blends with LiTFSI salt. Ionics (Kiel) 2005, 11, 375–377. [Google Scholar] [CrossRef]
- Bhumkar, D.R.; Joshi, H.M.; Sastry, M.; Pokharkar, V.B. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm. Res. 2007, 24, 1415–1426. [Google Scholar] [CrossRef] [Green Version]
- Boca, S.C.; Potara, M.; Toderas, F.; Stephan, O.; Baldeck, P.L.; Astilean, S. Uptake and biological effects of chitosan-capped gold nanoparticles on Chinese Hamster Ovary cells. Mater. Sci. Eng. C 2011, 31, 184–189. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, P.; Ma, X. Preparation and catalytic properties of magnetic rectorite-chitosan-Au composites. J. Alloys Compd. 2017, 690, 381–389. [Google Scholar] [CrossRef]
- Shi, H.; Xu, N.; Zhao, D.; Xu, B.Q. Immobilized PVA-stabilized gold nanoparticles on silica show an unusual selectivity in the hydrogenation of cinnamaldehyde. Catal. Commun. 2008, 9, 1949–1954. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Wu, Q.; Chen, Z.; Lin, X. A nonenzymatic hydrogen peroxide sensor based on Au-Ag nanotubes and chitosan film. J. Electroanal. Chem. 2014, 735, 19–23. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Tu, F.; Yao, C. Ultrasensitive enzyme-free electrochemical immunoassay for free thyroxine based on three dimensionally ordered macroporous chitosan-Au nanoparticles hybrid film. Biosens. Bioelectron. 2014, 59, 377–383. [Google Scholar] [CrossRef]
- Roushani, M.; Jalilian, Z.; Nezhadali, A. A novel electrochemical sensor based on electrode modified with gold nanoparticles and molecularly imprinted polymer for rapid determination of trazosin. Colloids Surfaces B Biointerfaces 2018, 172, 594–600. [Google Scholar] [CrossRef]
- Wu, C.M.; Yu, S.A.; Lin, S.L. Graphene modified electrospun poly(vinyl alcohol) nanofibrous membranes for glucose oxidase immobilization. Express Polym. Lett. 2014, 8, 565–573. [Google Scholar] [CrossRef]
- Doretti, L.; Ferrara, D.; Gattolin, P.; Lora, S.; Schiavon, F.; Veronese, F.M. PEG-modified glucose oxidase immobilized on a PVA cryogel membrane for amperometric biosensor applications. Talanta 1998, 45, 891–898. [Google Scholar] [CrossRef]
- Ren, G.; Xu, X.; Liu, Q.; Cheng, J.; Yuan, X.; Wu, L.; Wan, Y. Electrospun poly(vinyl alcohol)/glucose oxidase biocomposite membranes for biosensor applications. React. Funct. Polym. 2006, 66, 1559–1564. [Google Scholar] [CrossRef]
- Wang, L.; Wen, W.; Xiong, H.; Zhang, X.; Gu, H.; Wang, S. A novel amperometric biosensor for superoxide anion based on superoxide dismutase immobilized on gold nanoparticle-chitosan-ionic liquid biocomposite film. Anal. Chim. Acta 2013, 758, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Patolsky, F.; Katz, E.; Hainfeld, J.F.; Willner, I. Plugging into enzymes: Nanowiring of redox enzymes by a gold nanoparticle. Science 2003, 299, 1877–1881. [Google Scholar] [CrossRef] [PubMed]
- Parlak, O.; İncel, A.; Uzun, L.; Turner, A.P.F.; Tiwari, A. Structuring Au nanoparticles on two-dimensional MoS2 nanosheets for electrochemical glucose biosensors. Biosens. Bioelectron. 2017, 89, 545–550. [Google Scholar] [CrossRef]
- Musto, P.; Russo, P.; Cimino, F.; Acierno, D.; Lupò, G.; Petrarca, C. Dielectric behavior of biopolymer based composites containing multi wall carbon nanotubes: Effect of filler content and aspect ratio. Eur. Polym. J. 2015, 64, 170–178. [Google Scholar] [CrossRef]
- Seo, J.W.T.; Yoder, N.L.; Shastry, T.A.; Humes, J.J.; Johns, J.E.; Green, A.A.; Hersam, M.C. Diameter refinement of semiconducting arc discharge single-walled carbon nanotubes via density gradient ultracentrifugation. J. Phys. Chem. Lett. 2013, 4, 2805–2810. [Google Scholar] [CrossRef]
- Amekpewu, M.; Abukari, S.S.; Adu, K.W.; Mensah, S.Y.; Mensah, N.G. Effect of hot electrons on the electrical conductivity of carbon nanotubes under the influence of applied dc field. Eur. Phys. J. B 2015, 88, 1–6. [Google Scholar] [CrossRef]
- Pan, M.; Yin, Z.; Liu, K.; Du, X.; Liu, H.; Wang, S. Carbon-based nanomaterials in sensors for food safety. Nanomaterials 2019, 9, 1330. [Google Scholar] [CrossRef] [Green Version]
- Salvo-Comino, C.; Rassas, I.; Minot, S.; Bessueille, F.; Arab, M.; Chevallier, V.; Rodriguez-Mendez, M.L.; Errachid, A.; Jaffrezic-Renault, N. Voltammetric sensor based on molecularly imprinted chitosan-carbon nanotubes decorated with gold nanoparticles nanocomposite deposited on boron-doped diamond electrodes for catechol detection. Materials 2020, 13, 688. [Google Scholar] [CrossRef] [Green Version]
- Sainsbury, T.; Stolarczyk, J.; Fitzmaurice, D. An experimental and theoretical study of the self-assembly of gold nanoparticles at the surface of functionalized multiwalled carbon nanotubes. J. Phys. Chem. B 2005, 109, 16310–16325. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Lee, H.Y. Size control of aggregations via self-assembly of amphiphilic gold nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 574–582. [Google Scholar] [CrossRef]
- Sainsbury, T.; Ikuno, T.; Okawa, D.; Pacilé, D.; Fréchet, J.M.J.; Zettl, A. Self-assembly of gold nanoparticles at the surface of amine- and thiol-functionalized boron nitride nanotubes. J. Phys. Chem. C 2007, 111, 12992–12999. [Google Scholar] [CrossRef] [Green Version]
- Castellanos, S.G.; Fernández, V.V.A.; Aguilar, J.; Moscoso-Sánchez, F.J.; Ceja, I.; Canché-Escamilla, G.; Barrera, A. Synthesis and characterization of poly(methyl methacrylate)-boehmite nanocomposites by direct microemulsion polymerization combined with the in-situ sol-gel method. Polymer (Guildf) 2019, 163, 134–143. [Google Scholar] [CrossRef]
- Sandeep, S.; Nagashree, K.; Maiyalagan, T.; Keerthiga, G. Photocatalytic degradation of 2,4-dichlorophenoxyacetic acid—A comparative study in hydrothermal TiO2 and commercial TiO2. Appl. Surf. Sci. 2018, 449, 371–379. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol-gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Wang, B.; Chen, K.; Jiang, S.; Reincke, F.; Tong, W.; Wang, D.; Gao, C. Chitosan-mediated synthesis of gold nanoparticles on patterned poly(dimethylsiloxane) surfaces. Biomacromolecules 2006, 7, 1203–1209. [Google Scholar] [CrossRef]
- Guibal, E. Heterogeneous catalysis on chitosan-based materials: A review. Prog. Polym. Sci. 2005, 30, 71–109. [Google Scholar] [CrossRef]
- Huang, H.; Yang, X. Synthesis of chitosan-stabilized gold nanoparticles in the absence/presence of tripolyphosphate. Biomacromolecules 2004, 5, 2340–2346. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Wang, L.; Deng, Y.; Zhou, S. Preparation, Structure and Properties of Acid aqueous solution plasticized thermoplastic chitosan. Polymers 2019, 11, 818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meen, T.H.; Tsai, J.K.; Chao, S.M.; Lin, Y.C.; Wu, T.C.; Chang, T.Y.; Ji, L.W.; Water, W.; Chen, W.R.; Tang, I.T.; et al. Surface plasma resonant effect of gold nanoparticles on the photoelectrodes of dye-sensitized solar cells. Nanoscale Res. Lett. 2013, 8, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maye, M.M.; Chun, S.C.; Han, L.; Rabinovich, D.; Zhong, C.J. Novel spherical assembly of gold nanoparticles mediated by a tetradentate thioether. J. Am. Chem. Soc. 2002, 124, 4958–4959. [Google Scholar] [CrossRef]
- Van Herrikhuyzen, J.; Willems, R.; George, S.J.; Flipse, C.; Gielen, J.C.; Christianen, P.C.M.; Schenning, A.P.H.J.; Meskers, S.C.J. Atomic force microscopy nanomanipulation of shape persistent, spherical, self-assembled aggregates of gold nanoparticles. ACS Nano 2010, 4, 6501–6508. [Google Scholar] [CrossRef] [Green Version]
- Franconetti, A.; Carnerero, J.M.; Prado-Gotor, R.; Cabrera-Escribano, F.; Jaime, C. Chitosan as a capping agent: Insights on the stabilization of gold nanoparticles. Carbohydr. Polym. 2019, 207, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Thandapani, G.; Supriya Prasad, P.; Sudha, P.N.; Sukumaran, A. Size optimization and in vitro biocompatibility studies of chitosan nanoparticles. Int. J. Biol. Macromol. 2017, 104, 1794–1806. [Google Scholar] [CrossRef]
- Gracheva, T.A.; Kuz’micheva, T.A.; Perevezentsev, V.N.; Smirnova, L.A.; Mochalova, A.E.; Salomatina, E.B. Kinetics and mechanisms of the UV-radiation-assisted formation of gold nanoparticles in HAuCl4-doped chitosan solutions. Tech. Phys. 2017, 62, 1228–1232. [Google Scholar] [CrossRef]
- Branca, C.; D’Angelo, G.; Crupi, C.; Khouzami, K.; Rifici, S.; Ruello, G.; Wanderlingh, U. Role of the OH and NH vibrational groups in polysaccharide-nanocomposite interactions: A FTIR-ATR study on chitosan and chitosan/clay films. Polymer (Guildf) 2016, 99, 614–622. [Google Scholar] [CrossRef]
- Sanyakamdhorn, S.; Agudelo, D.; Tajmir-Riahi, H.A. Encapsulation of antitumor drug doxorubicin and its analogue by chitosan nanoparticles. Biomacromolecules 2013, 14, 557–563. [Google Scholar] [CrossRef]
- Corazzari, I.; Nisticò, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Nogueira, A.F.; Lomba, B.S.; Soto-Oviedo, M.A.; Correia, C.R.D.; Corio, P.; Furtado, C.A.; Hümmelgen, I.A. Polymer solar cells using single-wall carbon nanotubes modified with thiophene pedant groups. J. Phys. Chem. C 2007, 111, 18431–18438. [Google Scholar] [CrossRef]
- Coimbra, P.; Ferreira, P.; de Sousa, H.C.; Batista, P.; Rodrigues, M.A.; Correia, I.J.; Gil, M.H. Preparation and chemical and biological characterization of a pectin/chitosan polyelectrolyte complex scaffold for possible bone tissue engineering applications. Int. J. Biol. Macromol. 2011, 48, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, A.; Susi, T.; Kauppinen, E.I.; Vijayaraghavan, A. Growth, dispersion, and electronic devices of nitrogen-doped single-wall carbon nanotubes. Phys. Status Solidi Basic Res. 2012, 249, 2416–2419. [Google Scholar] [CrossRef]
- Dass, D. Structural parameters, electronic properties, and band gaps of a single walled carbon nanotube: A pz orbital tight binding study. Superlattices Microstruct. 2018, 120, 108–126. [Google Scholar] [CrossRef]
- Usman, F.; Dennis, J.O.; Seong, K.C.; Yousif Ahmed, A.; Meriaudeau, F.; Ayodele, O.B.; Tobi, A.R.; Rabih, A.A.S.; Yar, A. Synthesis and characterisation of a ternary composite of polyaniline, reduced graphene-oxide and chitosan with reduced optical band gap and stable aqueous dispersibility. Results Phys. 2019, 15. [Google Scholar] [CrossRef]
- Lioudakis, E.; Kanari, C.; Othonos, A.; Alexandrou, I. Direct observation of excitons in polymer/carbon nanotube composites at room temperature: The influence of nanotube concentration. Diam. Relat. Mater. 2008, 17, 1600–1603. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Dumitrescu, I.; Wilson, N.R.; Macpherson, J.V. Functionalizing single-walled carbon nanotube networks: Effect on electrical and electrochemical properties. J. Phys. Chem. C 2007, 111, 12944–12953. [Google Scholar] [CrossRef]
- Sun, H.Y.; Pu, J.H.; Tang, G.H. High-performance thermogalvanic cell based on organic nanofluids. Wuli Huaxue Xuebao Acta Phys. Chim. Sin. 2016, 32, 2555–2562. [Google Scholar] [CrossRef]
- Xia, J.; Song, L.X.; Liu, W.; Teng, Y.; Wang, Q.S.; Zhao, L.; Ruan, M.M. Highly monodisperse Cu3Mo2O9 micropompons with excellent performance in photocatalysis, photocurrent response and lithium storage. RSC Adv. 2015, 5, 12015–12024. [Google Scholar] [CrossRef]
- Skorikov, V.M.; Zakharov, I.S.; Volkov, V.V.; Umrikhin, V.V.; Kisteneva, M.G. Effects of doping, gamma irradiation, and annealing on the temperature dependence of the photocurrent through bismuth titanate crystals. Inorg. Mater. 2001, 37, 1155–1160. [Google Scholar] [CrossRef]



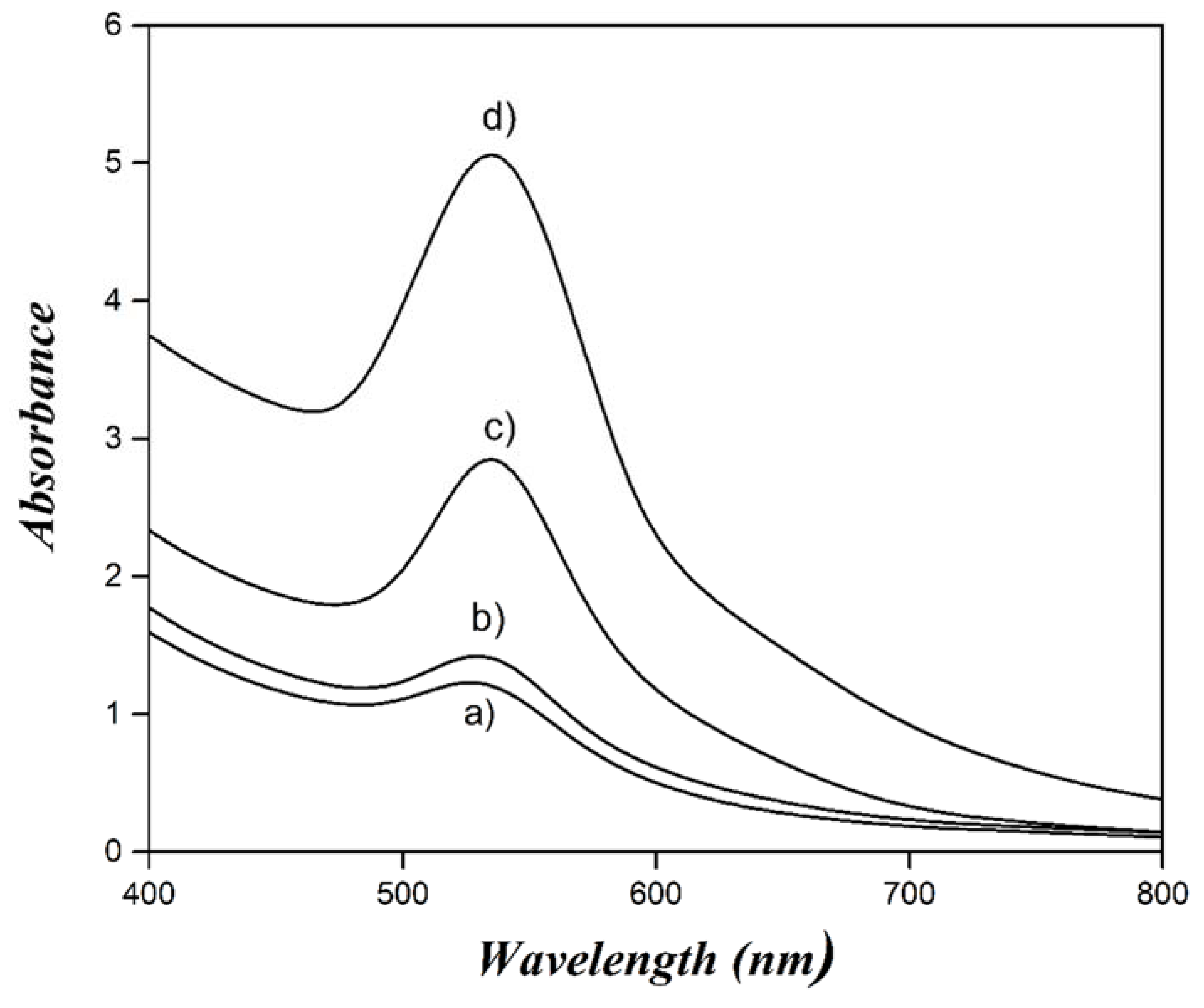

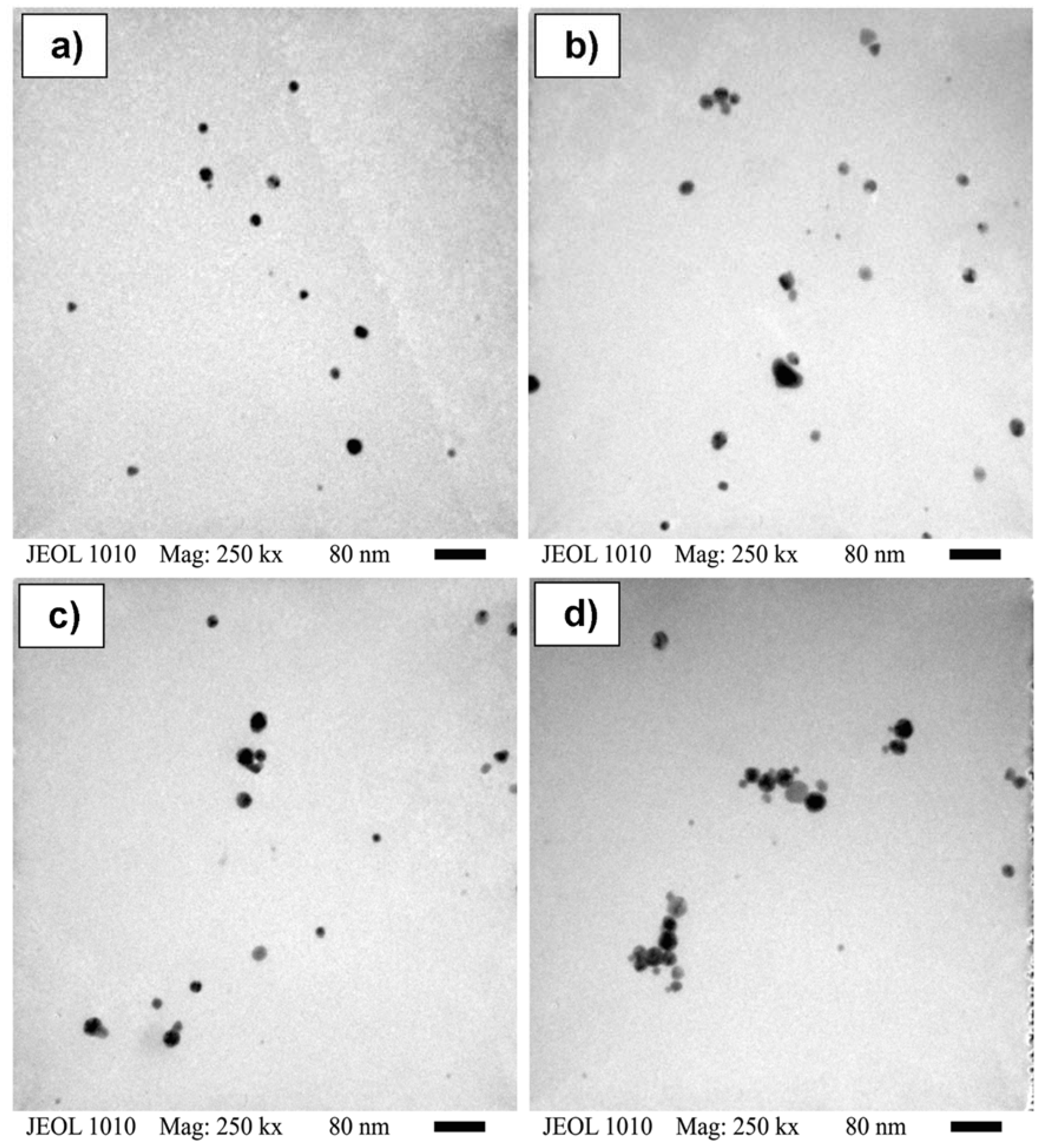
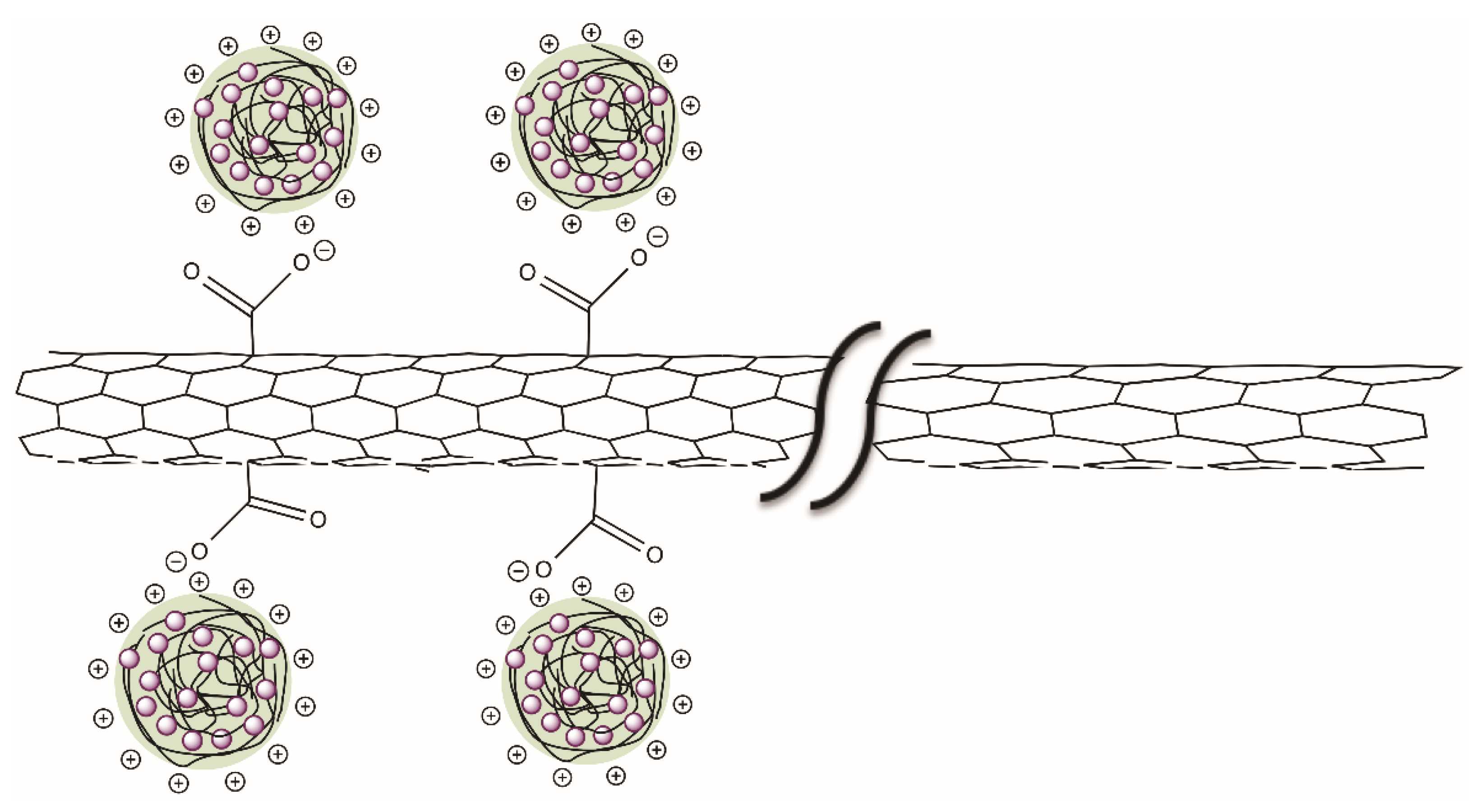



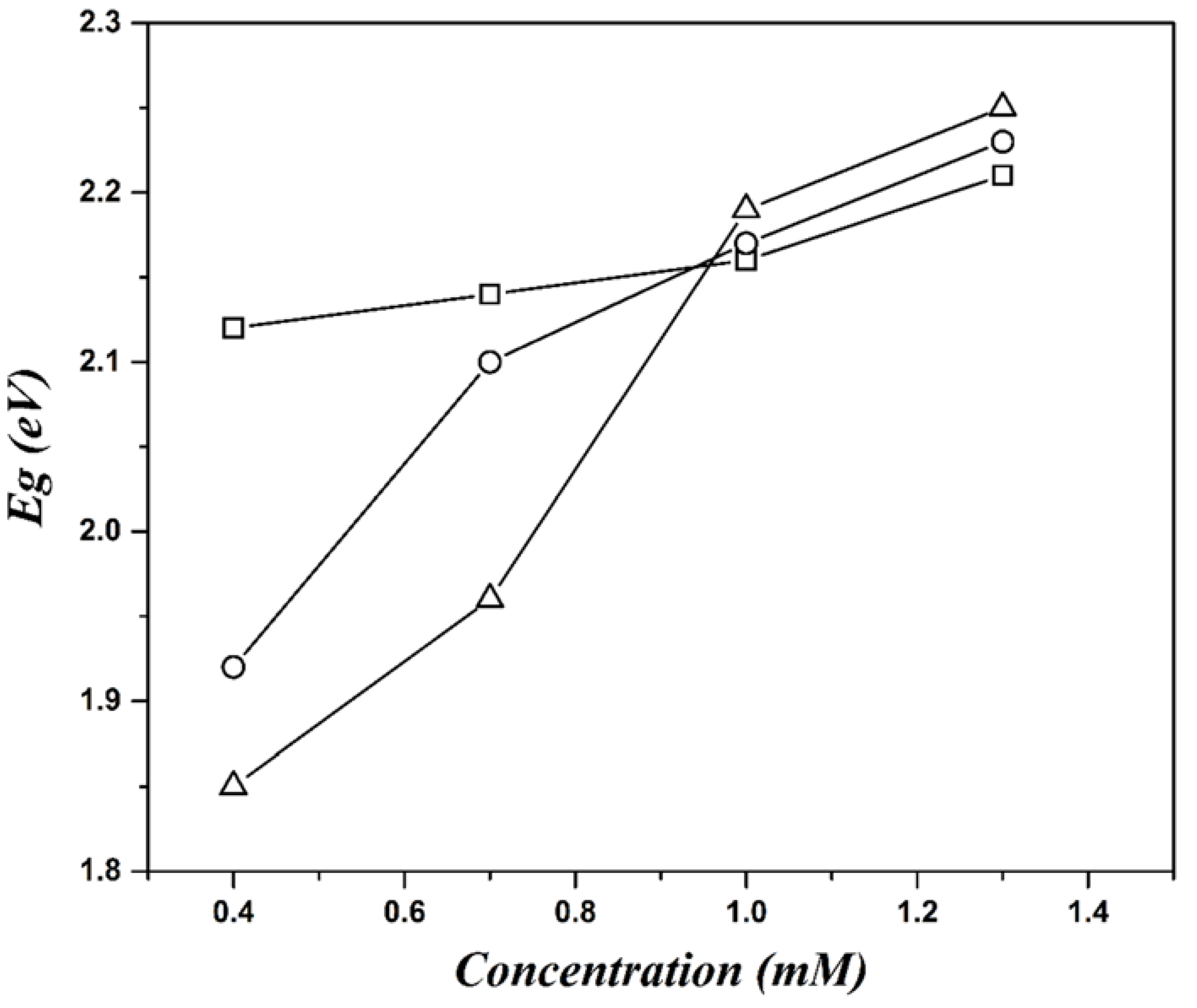

| Concentration (mM) | Dn (nm) | Dz (nm) | PDI |
|---|---|---|---|
| 0.4 | 209 | 320 | 1.4 |
| 0.7 | 160 | 246 | 1.4 |
| 1.0 | 114 | 203 | 1.5 |
| 1.3 | 90 | 187 | 1.6 |
| Concentration (mM) | L (nm) | D (nm) |
|---|---|---|
| 0.4 | 13.4 ± 1.5 | 15 ± 2.3 |
| 0.7 | 14.1 ± 1.7 | 17 ± 2.8 |
| 1.0 | 17.1 ± 2.1 | 19 ± 3.0 |
| 1.3 | 23.4 ± 2.6 | 24 ± 4.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceja, I.; González-Íñiguez, K.J.; Carreón-Álvarez, A.; Landazuri, G.; Barrera, A.; Casillas, J.E.; Fernández-Escamilla, V.V.A.; Aguilar, J. Characterization and Electrical Properties of PVA Films with Self-Assembled Chitosan-AuNPs/SWCNT-COOH Nanostructures. Materials 2020, 13, 4138. https://doi.org/10.3390/ma13184138
Ceja I, González-Íñiguez KJ, Carreón-Álvarez A, Landazuri G, Barrera A, Casillas JE, Fernández-Escamilla VVA, Aguilar J. Characterization and Electrical Properties of PVA Films with Self-Assembled Chitosan-AuNPs/SWCNT-COOH Nanostructures. Materials. 2020; 13(18):4138. https://doi.org/10.3390/ma13184138
Chicago/Turabian StyleCeja, Israel, Karla Josefina González-Íñiguez, Alejandra Carreón-Álvarez, Gabriel Landazuri, Arturo Barrera, José Eduardo Casillas, Víctor Vladimir A. Fernández-Escamilla, and Jacobo Aguilar. 2020. "Characterization and Electrical Properties of PVA Films with Self-Assembled Chitosan-AuNPs/SWCNT-COOH Nanostructures" Materials 13, no. 18: 4138. https://doi.org/10.3390/ma13184138







