Ultraviolet Lithography-Based Ceramic Manufacturing (UV-LCM) of the Aluminum Nitride (AlN)-Based Photocurable Dispersions
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Powder Surface Treatment with Dispersant and Reactive Dispersion Preparation
2.3. Three-Dimensional Shaping via LCM
2.4. Reference Samples Preparation
2.5. Debinding and Sintering
2.6. Characterization Techniques
3. Results and Discussion
3.1. Dispersion Preparation and Characterization
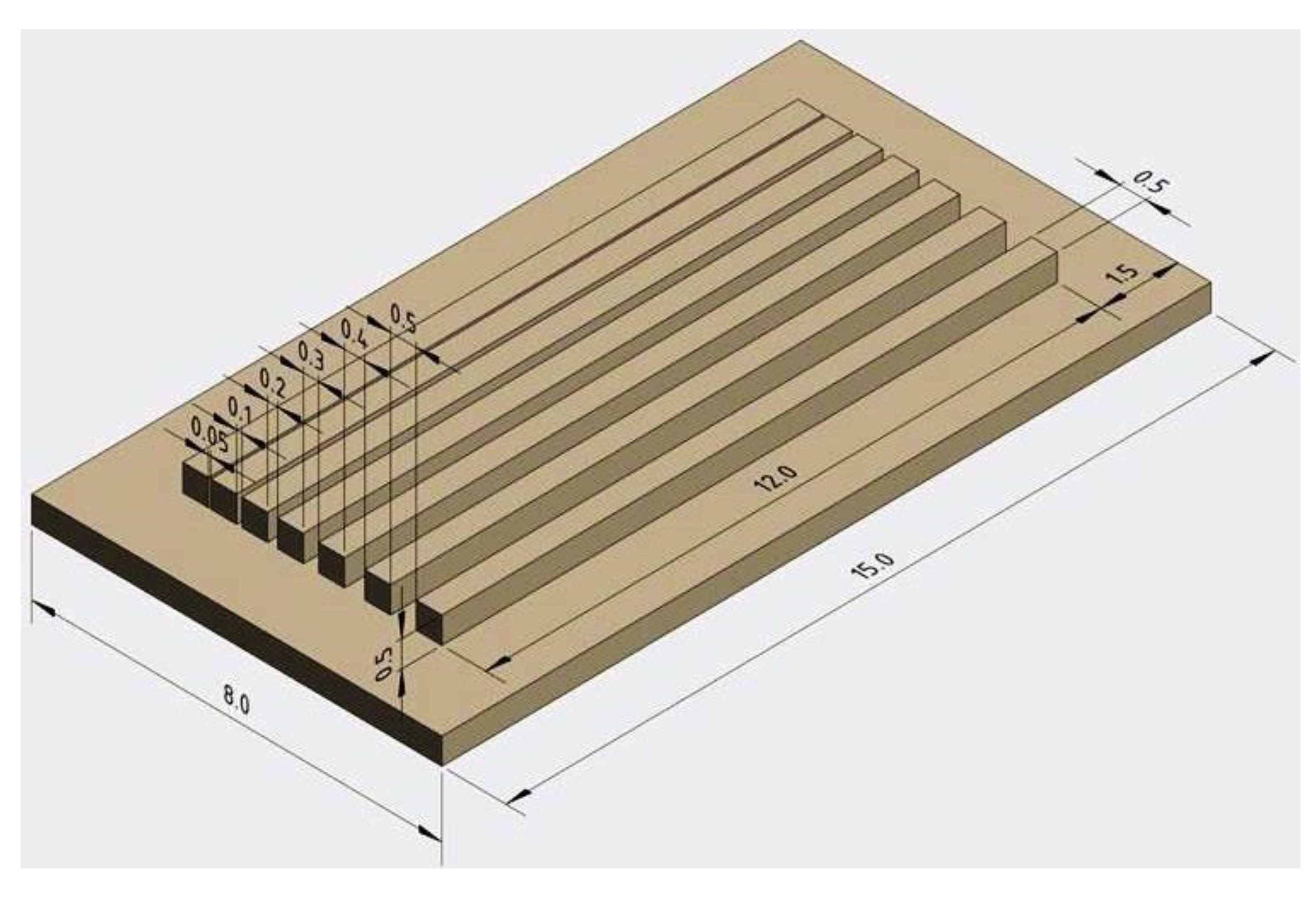
3.2. Shaping of Three-Dimensional Structures
3.3. Debinding and Sintering of AlN Pieces
4. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sheppard, L.M. Aluminum nitride: A versatile but challenging material. Am. Ceram. Soc. Bull. 1990, 69, 1801–1812. [Google Scholar]
- Descamps, M.; Moreau, G.; Mascart, M.; Thierry, B. Processing of aluminum nitride powder by the tape-casting process. J. Eur. Ceram. Soc. 1994, 13, 221–228. [Google Scholar] [CrossRef]
- Chartier, T.; Streicher, E.; Boch, P. Preparation and characterization of tape cast aluminum nitride substrates. J. Eur. Ceram. Soc. 1992, 9, 231–242. [Google Scholar] [CrossRef]
- Luo, X.J.; Zhang, B.L.; Li, W.L.; Zhuang, H.R. Preparation of aluminum nitride green sheets by aqueous tape casting. Ceram. Int. 2004, 30, 2099–2103. [Google Scholar] [CrossRef]
- Kata, D. Spiekany Azotek Glinu o Wysokim Przewodnictwie Cieplnym; Wydawnictwa AGH: Kraków, Poland, 2015. [Google Scholar]
- Baik, Y.; Drew, R. Aluminum Nitride: Processing and Applications. Key Eng. Mater. 1996, 122–124, 553–570. [Google Scholar] [CrossRef]
- Lee, H.M.; Bharathi, K.; Kim, D.K. Processing and Characterization of Aluminum Nitride Ceramics for High Thermal Conductivity. Adv. Eng. Mater. 2014, 16, 655–669. [Google Scholar] [CrossRef]
- Bellosi, A.; Esposito, L.; Scafe, E.; Fabbri, L. The Influence of Microstructure on the Thermal Conductivity of Aluminum Nitride. J. Mater. Sci. 1994, 29, 5014–5022. [Google Scholar] [CrossRef]
- Virkar, A.; Jackson, T.B.; Cutler, R.A. Thermodynamic and Kinetic Effects of Oxygen Rmoval on the Thermal Conductivity of Aluminum Nitride. J. Am. Ceram. Soc. 1989, 72, 2031–2042. [Google Scholar] [CrossRef]
- Watari, K.; Ishizaki, K.; Fujikawa, T. Thermal Conduction Mechanism of Aluminum Nitride Ceramics. J. Mater. Sci. 1992, 27, 2627–2630. [Google Scholar] [CrossRef]
- Kobayashi, R.; Moriya, Y.; Imamura, M.; Oosawa, K.; Oh-Ishi, K. Relation Between Oxygen Concentration in AlN Lattice and Thermal Conductivity of AlN Ceramics Sintered with Various Sintering Additives. J. Ceram. Soc. Japan. 2011, 119, 291–294. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Utsimu, K.; Takamizawa, H. Development and Microstructural Characterization of High-Thermal-Conductivity Aluminum Nitride Ceramics. J. Am. Ceram. Soc. 1988, 71, 588–594. [Google Scholar] [CrossRef]
- Jackson, T.B.; More, K.L.; Dinwiddie, B.R.; Cutler, R.A. High-Thermal-Conductivity Aluminum Nitride Ceramics: The Effect of Thermodynamic, Kinetic, and Microstructural Factors. J. Am. Ceram. Soc. 1997, 80, 1421–1435. [Google Scholar] [CrossRef]
- Slack, G.A.; Tanzilli, R.A.; Pohl, R.O.; Vandersande, J.W. The Intrinsic Thermal Conductivity of AlN. J. Phys. Chem. Solids. 1987, 48, 641–647. [Google Scholar] [CrossRef]
- Slack, G.A. Nonmetallic Crystals with High Thermal Conductivity. J. Phys. Chem. Solids. 1973, 34, 321–355. [Google Scholar] [CrossRef]
- Schwentenwein, M.; Schneider, P.; Homa, J. Lithography-based Ceramic Manufacturing: A Novel Technique for Additive Manufacturing of High-Performance Ceramics. Adv. Sci. Technol. 2014, 88, 60–64. [Google Scholar] [CrossRef]
- Brady, G.A.; Halloran, J.W. Stereolithography of ceramic suspensions. Rapid Prototyp. J. 1997, 3, 61–65. [Google Scholar] [CrossRef]
- Hinczewski, C.; Corbel, S.; Chartier, T. Stereolithography for the fabrication of ceramic three-dimensional parts. Rapid Prototyp. J. 1998, 4, 104–111. [Google Scholar] [CrossRef]
- Bertsch, A.; Jiguet, S.; Renaud, P. Microfabrication of Ceramic Components by Microstereolithography. J. Micromech. Microeng. 2004, 14, 197–203. [Google Scholar] [CrossRef]
- Homa, J.; Schwentenwein, M. A Novel Additive Manufacturing Technology for High-Performance Ceramics. Adv. Process. Manuf. Technol. Nanostruct. Multifunct. Mater. 2014, 35, 33–40. [Google Scholar]
- Schwentenwein, M.; Homa, J. Additive manufacturing of dense alumina ceramics. Int. J. Appl. Ceram. Technol. 2015, 12, 1–7. [Google Scholar] [CrossRef]
- Johansson, E.; Lidström, O.; Johansson, J.; Lyckfeldt, O.; Adolfsson, E. Influence of Resin Composition on the Defect Formation in Alumina Manufactured by Stereolithography. Materials 2017, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Varghese, G.; Moral, M.; Castro-garcía, M.; López-lópez, J.J.; Marín-rueda, J.R.; Yagüe-alcaraz, V.; Hernández-Afonso, L.; CarlosRuiz-Morales, J.; Canales-Vázque, J. Fabrication and characterization of ceramics via low-cost DLP 3D printing. Bol. Soc. Eep. Ceram. V. 2018, 57, 9–18. [Google Scholar] [CrossRef]
- Borlaf, M.; Serra-Capdevila, A.; Colominas, C.; Graule, T. Development of UV-curable ZrO2 slurries for additive manufacturing (LCM-DLP) technology. J. Eur. Ceram. Soc. 2019, 39, 3797–3803. [Google Scholar] [CrossRef]
- Borlaf, M.; Szubra, N.; Serra-Capdevila, A.; Kubiak, W.W.; Graule, T. Fabrication of ZrO2 and ATZ materials via UV-LCM-DLP additive manufacturing technology. J. Eur. Ceram. Soc. 2020, 40, 1574–1581. [Google Scholar] [CrossRef]
- Conti, L.; Bienenstein, D.; Borlaf, M.; Graule, T. Effects of the layer height and exposure energy on the lateral resolution of zirconia parts printed by lithography-based additive manufacturing. Materials 2020, 13, 1317. [Google Scholar] [CrossRef]
- Alm, B.; Knitter, R.; Haußelt, J. Development of a ceramic micro heat exchanger design, construction, and testing. Chem. Eng. Technol. 2005, 28, 1554–1560. [Google Scholar] [CrossRef]
- Ashman, S.; Kandlikar, S.G. A Review on Manufacturing Processes for Microchannel Heat Exchanger Fabrication. In Proceedings of the ASME 4th International Conference on Nanochannels, Microchannels, and Minichannels, Parts A and B, Limerick, Ireland, 19–21 June 2006. [Google Scholar]
- Ożóg, P.; Kata, D.; Graule, T. Tape casting of UV-curable aluminum nitride-based slurries. Ceram. Int. 2018, 44, 22800–22807. [Google Scholar] [CrossRef]
- Aluminum Nitride, AlN Ceramic Properties [Internet]. Available online: https://www.accuratus.com/alumni.html (accessed on 1 March 2019).
- Griffith, M.L.; Halloran, J.W. Stereolithography of Ceramics. In Proceedings of the 27th International SAMPE Technical Conference, Albuquerque, New Mexico, 9–12 October 1995. [Google Scholar]
- Griffith, M.L.; Halloran, J.W. Ultraviolet Curing of Highly Loaded Ceramic Suspensions for Stereolithography of Ceramics. In Proceedings of the 5th Symposium, Solid Freeform Fabrication, Austin, TX, USA, 8–10 August 1994. [Google Scholar]
- Griffith, M.L.; Halloran, J.W. Freeform Fabrication of Ceramics via Stereolithography. J. Am. Ceram. Soc. 1996, 79, 2601–2608. [Google Scholar] [CrossRef]
- Huang, R.J.; Jiang, Q.G.; Wu, H.D.; Li, Y.H.; Liu, W.Y.; Lu, X.X.; Wu, S.H. Fabrication of complex shaped ceramic parts with surface-oxidized Si3N4 powder via digital light processing based stereolithography method. Ceram. Int. 2019, 45, 5158–5162. [Google Scholar] [CrossRef]
- Altun, A.A.; Prochaska, T.; Konegger, T.; Schwentenwein, M. Dense, Strong, and Precise Silicon Nitride-Based Ceramic Parts by Lithography-Based Ceramic Manufacturing. Appl. Sci. 2020, 10, 996. [Google Scholar] [CrossRef]
- Chartier, T.; Dupas, C.; Geffroy, P.M.; Pateloup, V.; Colas, M.; Cornette, J.; Guillemet-Fritsch, S. Influence of irradiation parameters on the polymerization of ceramic reactive suspensions for stereolithography. J. Eur. Ceram. Soc. 2017, 37, 4431–4436. [Google Scholar] [CrossRef]
- Wu, K.C.; Halloran, J.W. Photopolymerization monitoring of ceramic stereolithography resins by FTIR methods. J. Mater. Sci. 2005, 40, 71–76. [Google Scholar] [CrossRef]
- Schwarzer, E.; Holtzhausen, S.; Scheithauer, U.; Ortmann, C.; Oberbach, T.; Moritz, T.; Michaelis, A. Process development for additive manufacturing of functionally graded alumina toughened zirconia components intended for medical implant application. J. Eur. Ceram. Soc. 2019, 39, 522–530. [Google Scholar] [CrossRef]
- Schwarzer, E.; Götz, M.; Markova, D.; Stafford, D.; Scheithauer, U.; Moritz, T. Lithography-based ceramic manufacturing (LCM)—Viscosity and cleaning as two quality influencing steps in the process chain of printing green parts. J. Eur. Ceram. Soc. 2017, 37, 5329–5338. [Google Scholar] [CrossRef]
- Mitteramskogler, G.; Gmeiner, R.; Felzmann, R.; Gruber, S.; Hofstetter, C.; Stampfl, J.; Ebert, J.; Wachter, W.; Laubersheimer, J. Light curing strategies for lithography-based additive manufacturing of customized ceramics. Addit. Manuf. 2014, 1, 110–118. [Google Scholar] [CrossRef]











| Printing Parameters | PP-A | PP-B | PP-C | PP-D |
|---|---|---|---|---|
| Layer Thickness (μm) | 25 | 25 | 25 | 10 |
| Exposure Energy (mJ∙cm−2) | 66 | 38 | 47 | 47 |
| Heating Rate (min−1) | Temperature (°C) | Dwell Time (min) |
|---|---|---|
| - | 25 | - |
| 0.1 | 120 | 360 |
| 0.5 | 360 | 960 |
| 0.5 | 460 | 360 |
| Free cooling | 25 | 0 |
| Material | 0.5 wt % GTO | d10 (µm) | d50 (µm) | d90 (µm) |
|---|---|---|---|---|
| AlN | − | 1.90 | 4.10 | 7.40 |
| AlN | + | 1.20 | 2.80 | 5.50 |
| Al2O3 | − | 6.70 | 35.50 | 131.00 |
| Al2O3 | + | 0.60 | 0.90 | 1.20 |
| Y2O3 | − | 7.40 | 30.00 | 51.00 |
| Y2O3 | + | 0.44 | 0.70 | 1.50 |
| Shaping Method | ρth (g∙cm−3) | ρapp (g∙cm−3) | ρrel (%) | λ25 °C (W∙m−1∙K−1) |
|---|---|---|---|---|
| UV-LCM | 3.39 | 2.84 | 83.71 | 4.34 |
| Pressing | 3.39 | 3.27 | 96.38 | 75.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ożóg, P.; Rutkowski, P.; Kata, D.; Graule, T. Ultraviolet Lithography-Based Ceramic Manufacturing (UV-LCM) of the Aluminum Nitride (AlN)-Based Photocurable Dispersions. Materials 2020, 13, 4219. https://doi.org/10.3390/ma13194219
Ożóg P, Rutkowski P, Kata D, Graule T. Ultraviolet Lithography-Based Ceramic Manufacturing (UV-LCM) of the Aluminum Nitride (AlN)-Based Photocurable Dispersions. Materials. 2020; 13(19):4219. https://doi.org/10.3390/ma13194219
Chicago/Turabian StyleOżóg, Paulina, Paweł Rutkowski, Dariusz Kata, and Thomas Graule. 2020. "Ultraviolet Lithography-Based Ceramic Manufacturing (UV-LCM) of the Aluminum Nitride (AlN)-Based Photocurable Dispersions" Materials 13, no. 19: 4219. https://doi.org/10.3390/ma13194219
APA StyleOżóg, P., Rutkowski, P., Kata, D., & Graule, T. (2020). Ultraviolet Lithography-Based Ceramic Manufacturing (UV-LCM) of the Aluminum Nitride (AlN)-Based Photocurable Dispersions. Materials, 13(19), 4219. https://doi.org/10.3390/ma13194219






