Thermal Decomposition of Nanostructured Bismuth Subcarbonate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Bismuth Carbonate
2.3. Thermal Decomposition Kinetics Model
3. Results
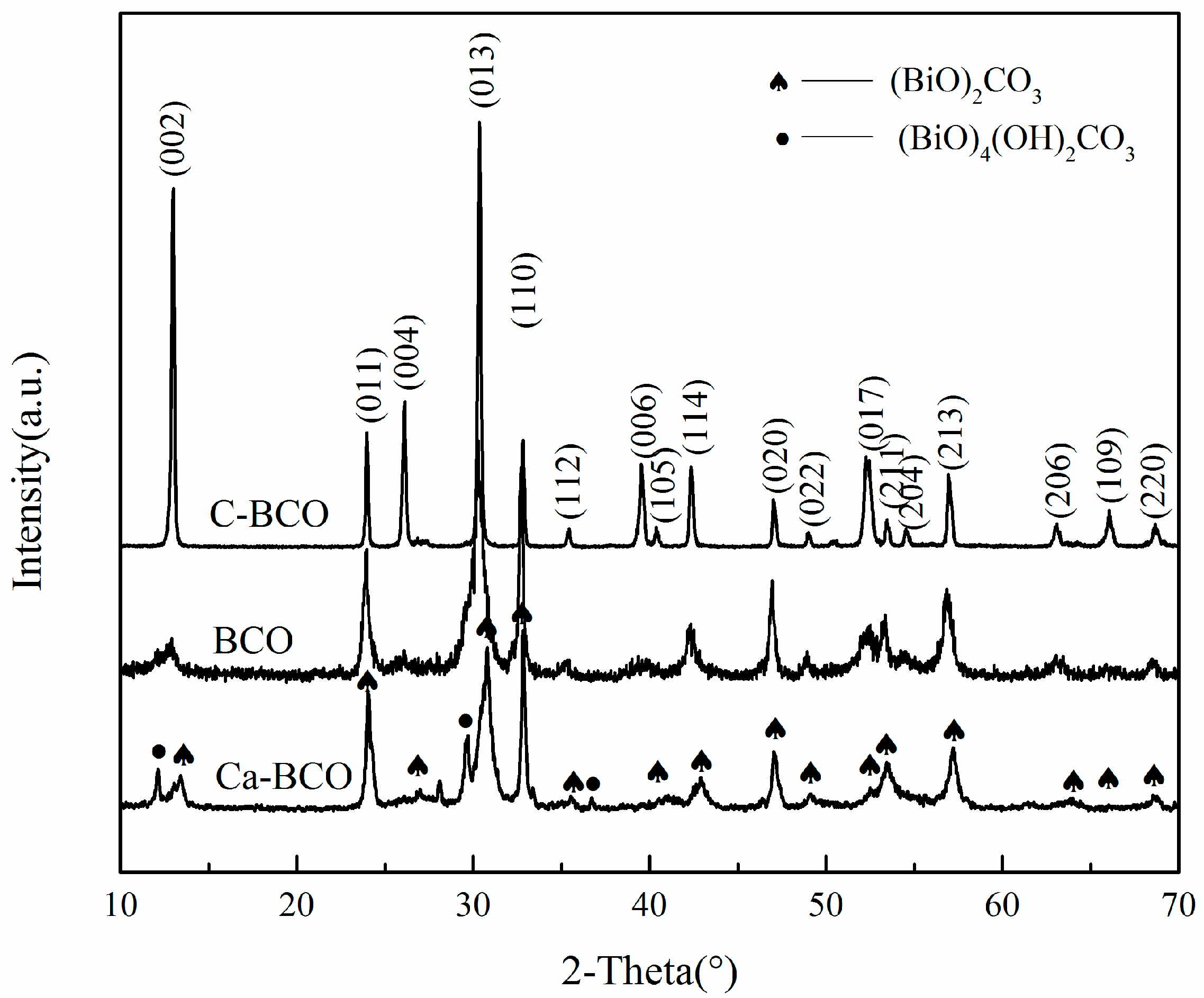
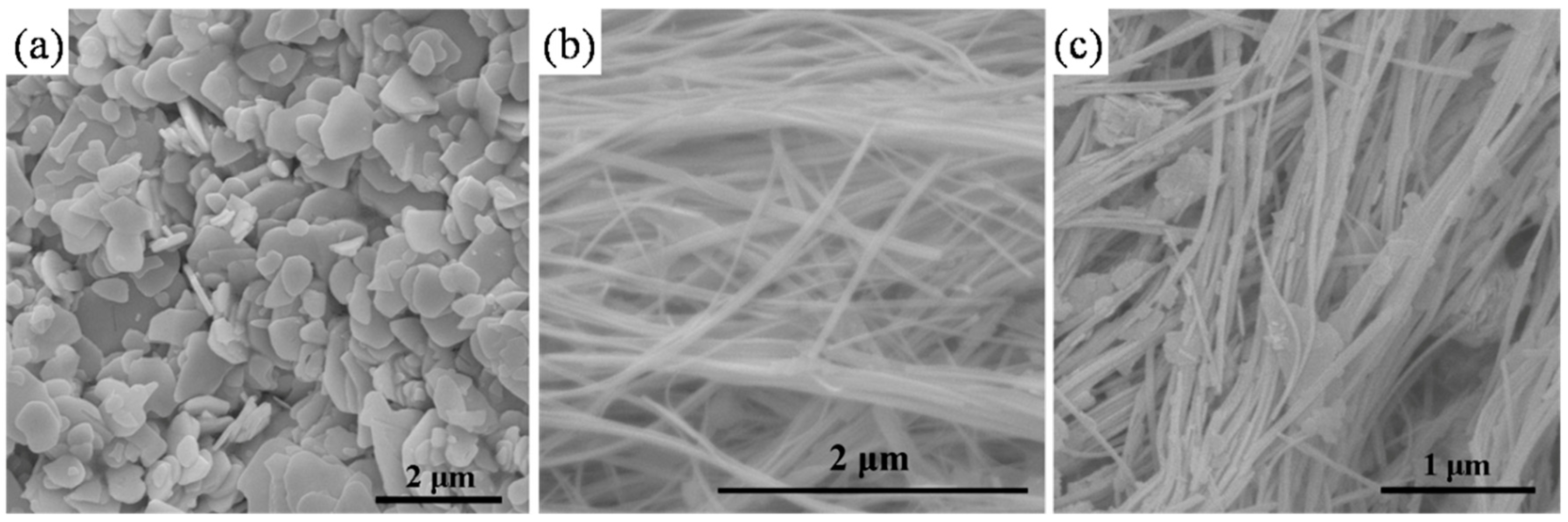
3.1. Characterization of (BiO)2CO3 Samples
3.2. Thermal Decomposition Characteristics of Nanostructured (BiO)2CO3
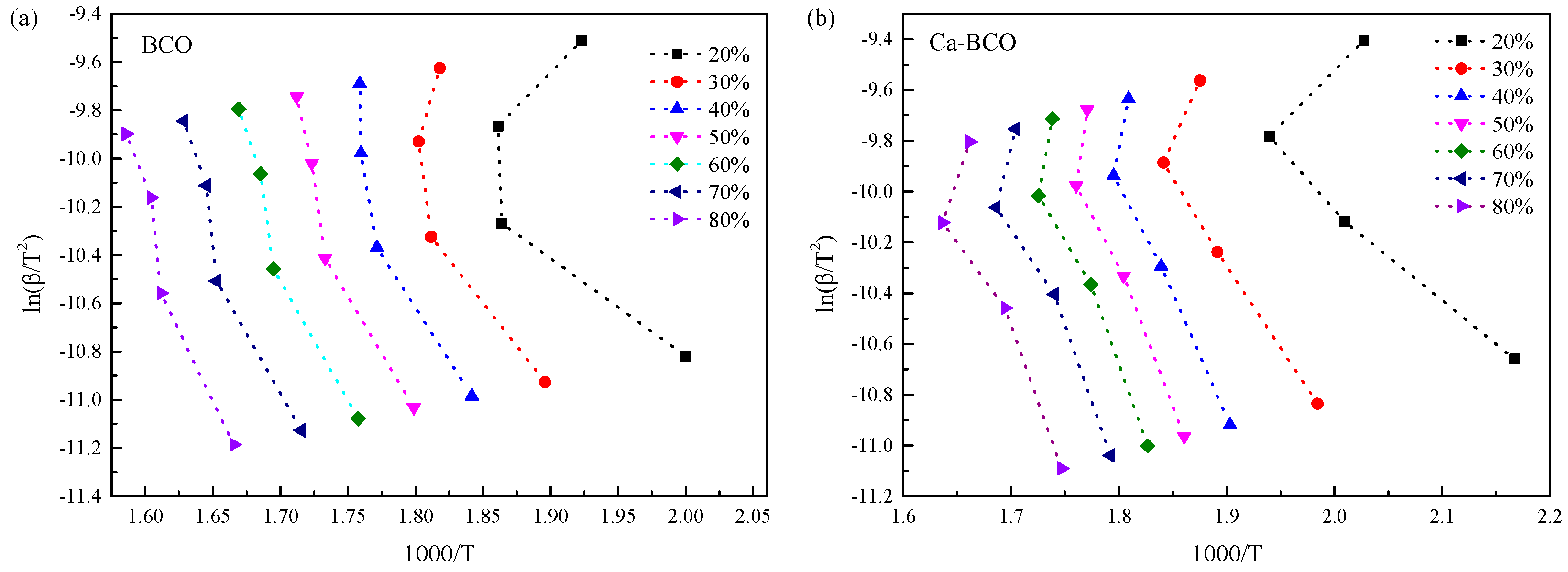
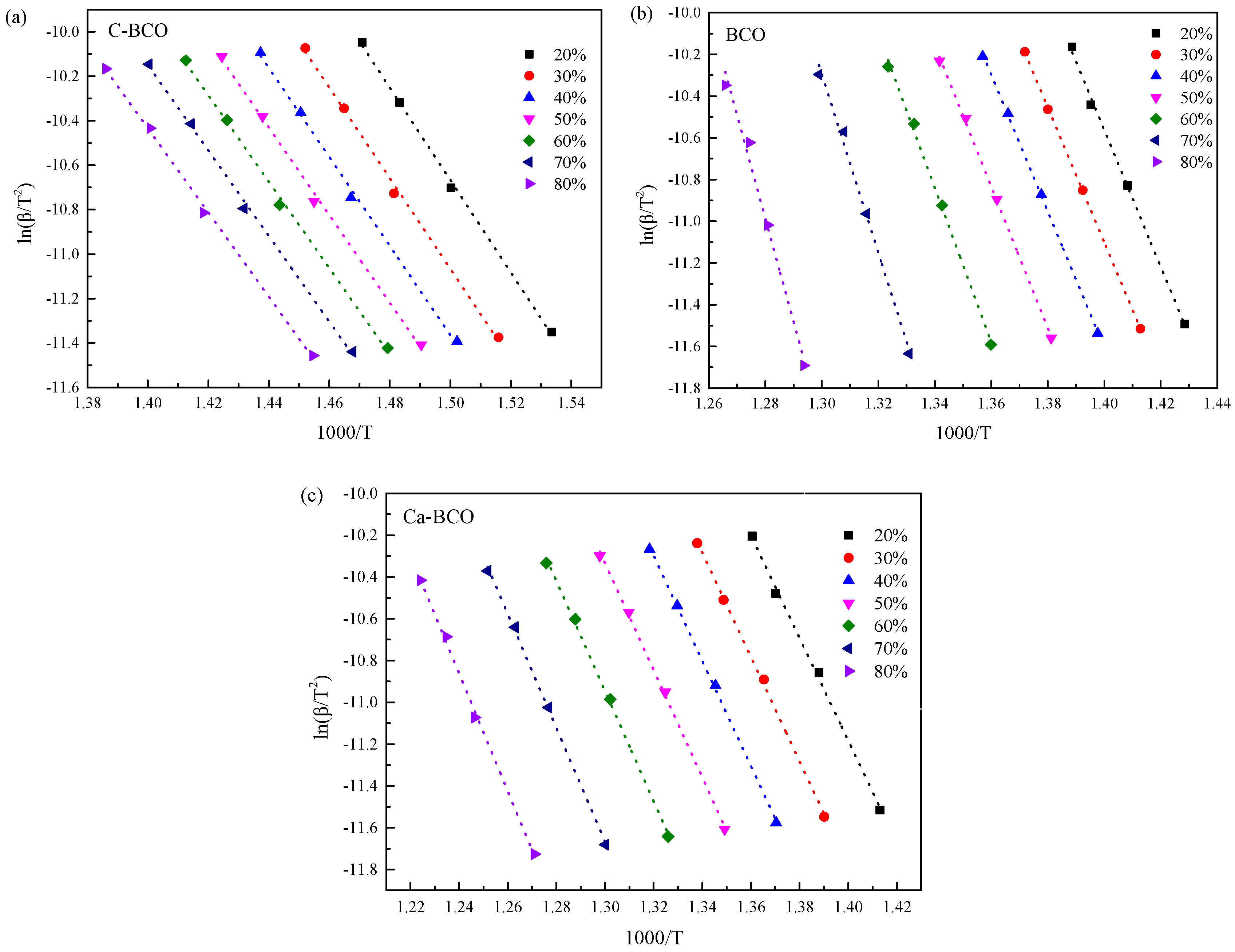
3.3. Thermal Decomposition Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.; Zhang, L.; Li, Y.; Yu, Y. Synthesis and internal electric field dependent photoreactivity of Bi3O4Cl single-crystalline nanosheets with high {001} facet exposure percentages. Nanoscale 2014, 6, 167–171. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, T.; Wang, X.; Wang, Y.; Fan, C.; Zhang, H. Facile composition-controlled preparation and photocatalytic application of BiOCl/Bi2O2CO3 nanosheets. Appl. Catal. B Environ. 2014, 150-151, 486–495. [Google Scholar] [CrossRef]
- Zhou, Y.; Grunwaldt, J.-D.; Krumeich, F.; Zheng, K.; Chen, G.; Stötzel, J.; Frahm, R.; Patzke, G.R. Hydrothermal Synthesis of Bi6S2O15 Nanowires: Structural, in situ EXAFS, and Humidity-Sensing Studies. Small 2010, 6, 1173–1179. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Huang, X.; Li, G. Hydrothermal synthesis of Bi2WO6 uniform hierarchical microspheres. Cryst. Growth Des. 2007, 7, 1350–1355. [Google Scholar] [CrossRef]
- Shimodaira, Y.; Kato, H.; Kobayashi, H.; Kudo, A. Photophysical properties and photocatalytic activities of bismuth molybdates under visible light irradiation. J. Phys. Chem. B 2006, 110, 17790–17797. [Google Scholar] [CrossRef]
- Cui, P.; Wang, J.; Wang, Z.; Chen, J.; Xing, X.; Wang, L.; Yu, R. Bismuth oxychloride hollow microspheres with high visible light photocatalytic activity. Nano Res. 2016, 9, 593–601. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Manser, J.S.; Kamat, P.V. All solution-processed lead halide perovskite-BiVO4 tandem assembly for photolytic solar fuels production. J. Am. Chem. Soc. 2015, 137, 974–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grice, J.D. A solution to the crystal structures of bismutite and beyerite. Can. Mineral 2002, 40, 693–698. [Google Scholar] [CrossRef]
- Peng, S.; Li, L.; Tan, H.; Wu, Y.; Cai, R.; Yu, H.; Huang, X.; Zhu, P.; Ramakrishna, S.; Srinivasan, M.; et al. Monodispersed Ag nanoparticles loaded on the PVP-assisted synthetic Bi2O2CO3 microspheres with enhanced photocatalytic and supercapacitive performances. J. Mater. Chem. A 2013, 1, 7630–7638. [Google Scholar] [CrossRef]
- Dong, F.; Zheng, A.; Sun, Y.; Fu, M.; Jiang, B.; Wing-Kei, H.; Lee, S.C.; Wu, Z. One-pot template-free synthesis, growth mechanism and enhanced photocatalytic activity of monodisperse (BiO)2CO3 hierarchical hollow microspheres self-assembled with single-crystalline nanosheets. Cryst. Eng. Comm. 2012, 14, 3534–3544. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Z.; Wang, F.; Cao, K.; Dmitry, E.; Doronkin, D.E.; Dong, F.; Grunwaldt, J.D. Facile synthesis of surface N-doped Bi2O2CO3: Origin of visible light photocatalytic activity and in situ DRIFTS studies. J. Hazard. Mater. 2016, 307, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; So, M.H.; Yang, J.; Deng, F.; Che, C.M.; Sun, H. Fabrication of bismuth subcarbonate nanotube arrays from bismuth citrate. Chem. Commun. 2006, 21, 2265–2267. [Google Scholar] [CrossRef]
- Umar, A.; Ahmad, R.; Kumar, R.; Ahmed, A.; Ibrahim, A.A.; Baskoutas, S. Bi2O2CO3 nanoplates: Fabrication and characterization of highly sensitive and selective cholesterol biosensor. J. Alloy. Compd. 2016, 683, 433–438. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.; Sheng, M.; Zhang, Q.; Zhao, Z.; Lin, Y.; Liu, H.; Greta, R.; Patzke, G.R. Environmentally friendly room temperature synthesis and humidity sensing applications of nanostructured Bi2O2CO3. Sens. Actuators B Chem. 2013, 188, 1312–1318. [Google Scholar] [CrossRef]
- Selvamani, T.; Asiri, A.M.; Al-Youbi, A.O.; Anandan, S. Emergent synthesis of bismuth subcarbonate nanomaterials with various morphologies towards photocatalytic activities—An overview. Mater. Sci. Forum 2013, 764, 169–193. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Huang, B.; Yang, K.; Zhang, X.; Qin, X.; Dai, Y. Preparation, electronic structure, and photocatalytic properties of Preparation, electronic structure, and photocatalytic properties of Bi2O2CO3 nanosheet. Appl. Surf. Sci. 2010, 257, 172–175. [Google Scholar] [CrossRef]
- Zhao, T.; Zai, J.; Xu, M.; Zhou, Q.; Su, Y.; Wang, K.; Qian, X. Hierarchical Bi2O2CO3 microspheres with improved visible-light-driven photocatalytic activity. CrystEng Comm 2011, 13, 4010–4017. [Google Scholar] [CrossRef]
- Savag, T.; Rao, A.M. Thermal properties of nanomaterials and nanocomposites. In Thermal Conductivity; Physics of Solids and Liquids; Tritt, T.M., Ed.; Springer: Boston, MA, USA, 2004. [Google Scholar]
- Bian, Y.; Ma, Y.; Shang, Y.; Tan, P.; Pan, J. Self-integrated β-Bi2O3/Bi2O2.33@Bi2O2CO3 ternary composites: Formation mechanism and visible light photocatalytic activity. Appl. Surf. Sci. 2018, 430, 613–624. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, W.; Zhang, Q.; Cao, J.; Huang, R.J.; Ho, W.; Lee, S.C. In situ fabrication of α-Bi2O3/(BiO)2CO3 nanoplate heterojunctions with tunable optical property and photocatalytic activity. Sci. Rep. 2016, 6, 23435. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.; Lian, J.; Hojamberdiev, M.; Que, W. Facile fabrication of porous Bi2O3 microspheres by thermal treatment of Bi2O2CO3 microspheres and its photocatalysis properties. J. Clust. Sci. 2013, 24, 829–841. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta. 2011, 520, 1–19. [Google Scholar]
- Kissigner, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar]
- Cheng, H.K.F.; Chong, M.F.; Liu, E.; Zhou, K.; Li, L. Thermal decomposition kinetics of multiwalled carbon nanotube/polypropylene nanocomposites. J. Therm. Anal. Calorim. 2014, 117, 63–71. [Google Scholar]
- Kissigner, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar]
- Akahira, T.; Sunose, T. Method of determining activation deterioration constant of electrical insulation materials. Res. Report Chiba Inst. Technol Sci. Technol. 1971, 16, 22–31. [Google Scholar]
- Taylor, P.; Sunder, S.; Lopata, V.J. Structure, spectra, and stability of solid bismuth carbonates. Can. J. Chem. 1984, 62, 2863–2873. [Google Scholar]
- Valencia, K.; López, A.; Hernández-Gordillo, A.; Zanella, R.; Rodil, S.E. Stabilized β-Bi2O3 nanoparticles from (BiO)4CO3(OH)2 precursor and their photocatalytic properties under blue light. Ceram. Int. 2018, 44, 22329–22338. [Google Scholar]
- Cai, G.; Xu, L.; Wei, B.; Che, J.; Gao, H.; Sun, W. Facile synthesis of β-Bi2O3/Bi2O2CO3 nanocomposite with high visible-light photocatalytic activity. Mater. Lett. 2014, 120, 1–4. [Google Scholar]
- Matskevich, N.I.; Wolf, T.; Adelmann, P.; Semerikova, A.N.; Gelfond, N.V.; Zolotova, E.S.; Matskevich, M.Y. Enthalpy of formation and lattice energy of bismuth perrhenate doped by neodymium and indium oxides. Thermochim. Acta 2017, 658, 63–67. [Google Scholar]

| Sample | β/°C·min−1 | 1st Mass Loss Zone/°C | Peak Temperature/°C | Mass Loss/% | 2nd Mass Loss Zone/°C | Peak Temperature/°C | Mass Loss/% |
|---|---|---|---|---|---|---|---|
| C-BCO | 5 | 320.1–514.6 | 400.0 | 8.26 | |||
| 10 | 324.9–543.9 | 417.9 | 8.33 | ||||
| 15 | 327.5–551.0 | 432.0 | 8.36 | ||||
| 20 | 328.0–563.5 | 436.0 | 8.40 | ||||
| BCO | 5 | 193.3–367.8 | 276.3 | 1.47 | 367.8–624.8 | 434.8 | 4.23 |
| 10 | 205.9–383.8 | 288.6 | 1.39 | 383.8–625.9 | 447.7 | 4.04 | |
| 15 | 211.2–387.2 | 296.2 | 1.41 | 387.2–635.2 | 453.7 | 4.10 | |
| 20 | 215.4–394.9 | 315.4 | 1.43 | 394.9–641.9 | 460.4 | 4.07 | |
| Ca-BCO | 5 | 209.3–341.3 | 271.8 | 0.80 | 341.3–621.8 | 451.3 | 5.10 |
| 10 | 211.5–355.5 | 285.6 | 0.81 | 355.5–637.0 | 464.6 | 5.13 | |
| 15 | 217.4–379.4 | 293.9 | 1.03 | 379.4–641.9 | 472.9 | 5.03 | |
| 20 | 213.6–368.6 | 297.6 | 0.69 | 368.6–650.6 | 479.1 | 5.07 |
| Samples | 1st Weight-Loss Zone | 2nd Weight-Loss Zone | ||
|---|---|---|---|---|
| Ea/kJ/mol | R2 | Ea/kJ/mol | R2 | |
| C-BCO | 159.48 | 0.99123 | ||
| BCO | 118.69 | 0.9773 | 226.77 | 0.99363 |
| Ca-BCO | 122.84 | 0.9970 | 213.89 | 0.99928 |
| Conversion Rate/% | C-BCO | BCO | Ca-BCO | |||
|---|---|---|---|---|---|---|
| Ea/kJ/mol | R2 | Ea/kJ/mol | R2 | Ea/kJ/mol | R2 | |
| 20 | 178.49 | 0.99794 | 246.71 | 0.99848 | 204.02 | 0.99699 |
| 30 | 169.32 | 0.9965 | 249.88 | 0.99914 | 207.59 | 0.99864 |
| 40 | 166.37 | 0.99656 | 253.81 | 0.99797 | 209.37 | 0.99905 |
| 50 | 163.72 | 0.99692 | 262.43 | 0.99618 | 213.22 | 0.9982 |
| 60 | 161.53 | 0.99729 | 276.72 | 0.99457 | 219.15 | 0.9977 |
| 70 | 159.92 | 0.99749 | 293.11 | 0.99522 | 226.79 | 0.99846 |
| 80 | 157.42 | 0.99784 | 334.49 | 0.99375 | 234.47 | 0.9975 |
| Average | 164.39 ± 4.94 | 257.91 ± 10.78 * | 216.37 ± 6.84 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, S.; Jin, S.; Cui, K. Thermal Decomposition of Nanostructured Bismuth Subcarbonate. Materials 2020, 13, 4287. https://doi.org/10.3390/ma13194287
Sheng S, Jin S, Cui K. Thermal Decomposition of Nanostructured Bismuth Subcarbonate. Materials. 2020; 13(19):4287. https://doi.org/10.3390/ma13194287
Chicago/Turabian StyleSheng, Su, Shengming Jin, and Kuixin Cui. 2020. "Thermal Decomposition of Nanostructured Bismuth Subcarbonate" Materials 13, no. 19: 4287. https://doi.org/10.3390/ma13194287




