PlantCrystals—Nanosized Plant Material for Improved Bioefficacy of Medical Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
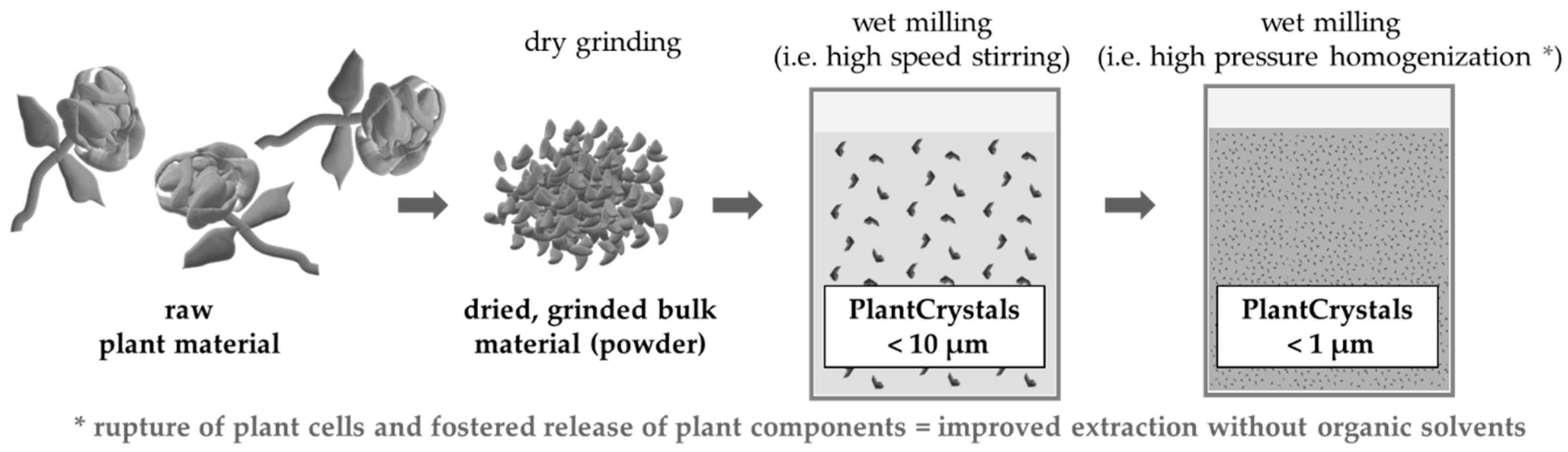
2.2.1. Production of PlantCrystals
2.2.2. Production of Plant Extracts
2.2.3. Characterization of PlantCrystals
2.2.4. Determination of AOC, Phenolic, Flavonoid and Carotenoid Content
Determination of AOC with DPPH Assay
Determination of AOC with ORAC Assay
Determination of Total Polyphenol Content
Determination of Flavonoid Content
Determination of Carotenoid Content
2.2.5. Statistical Analysis
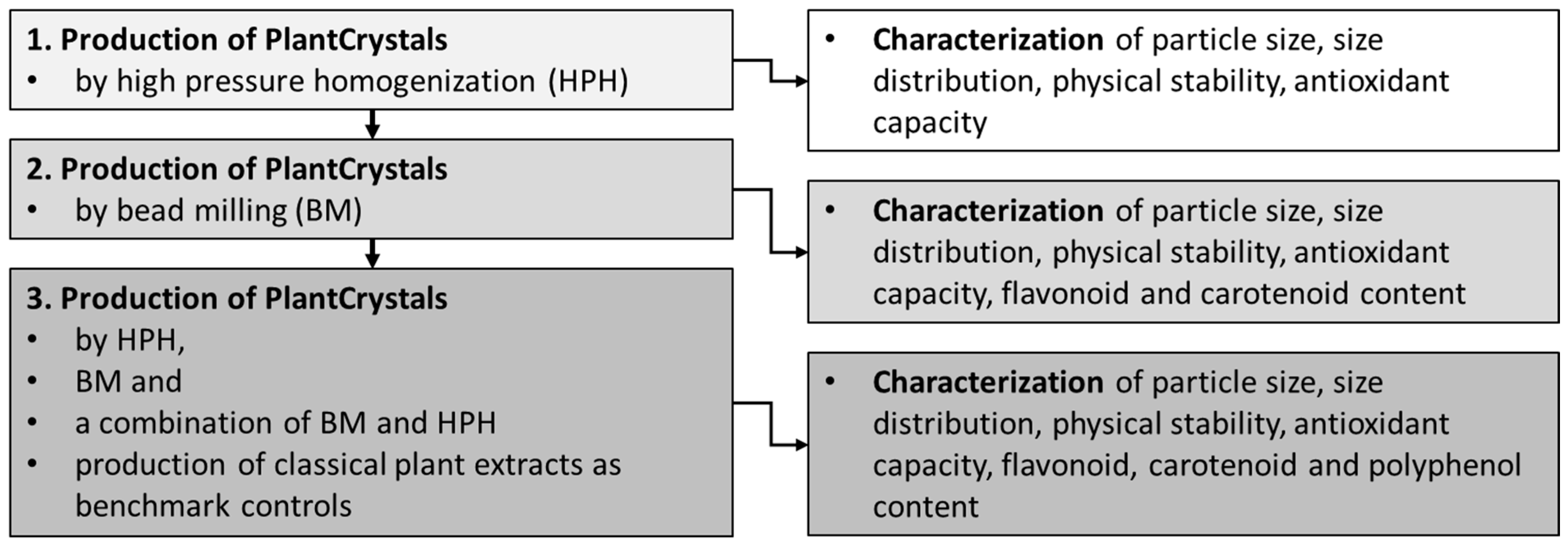
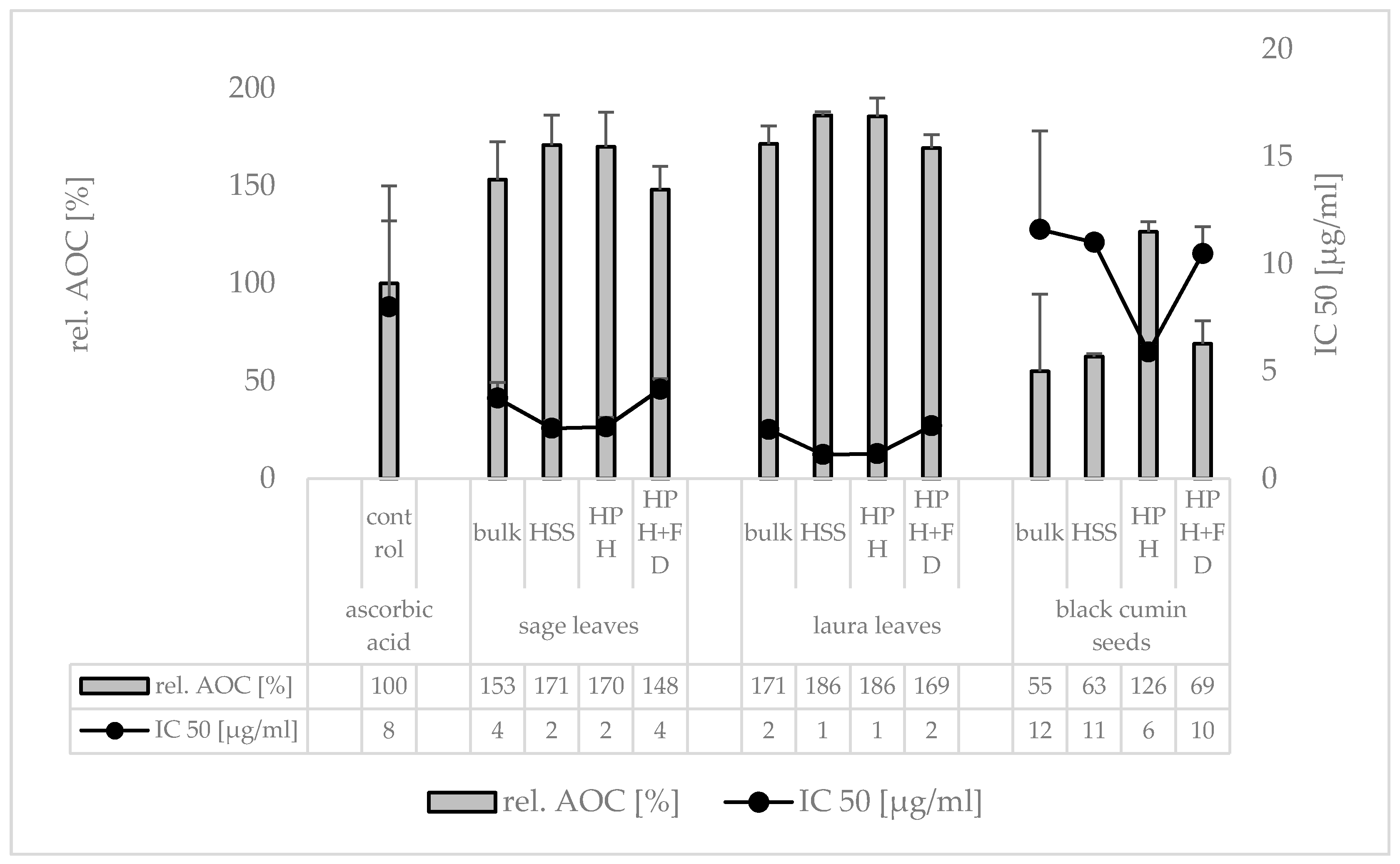
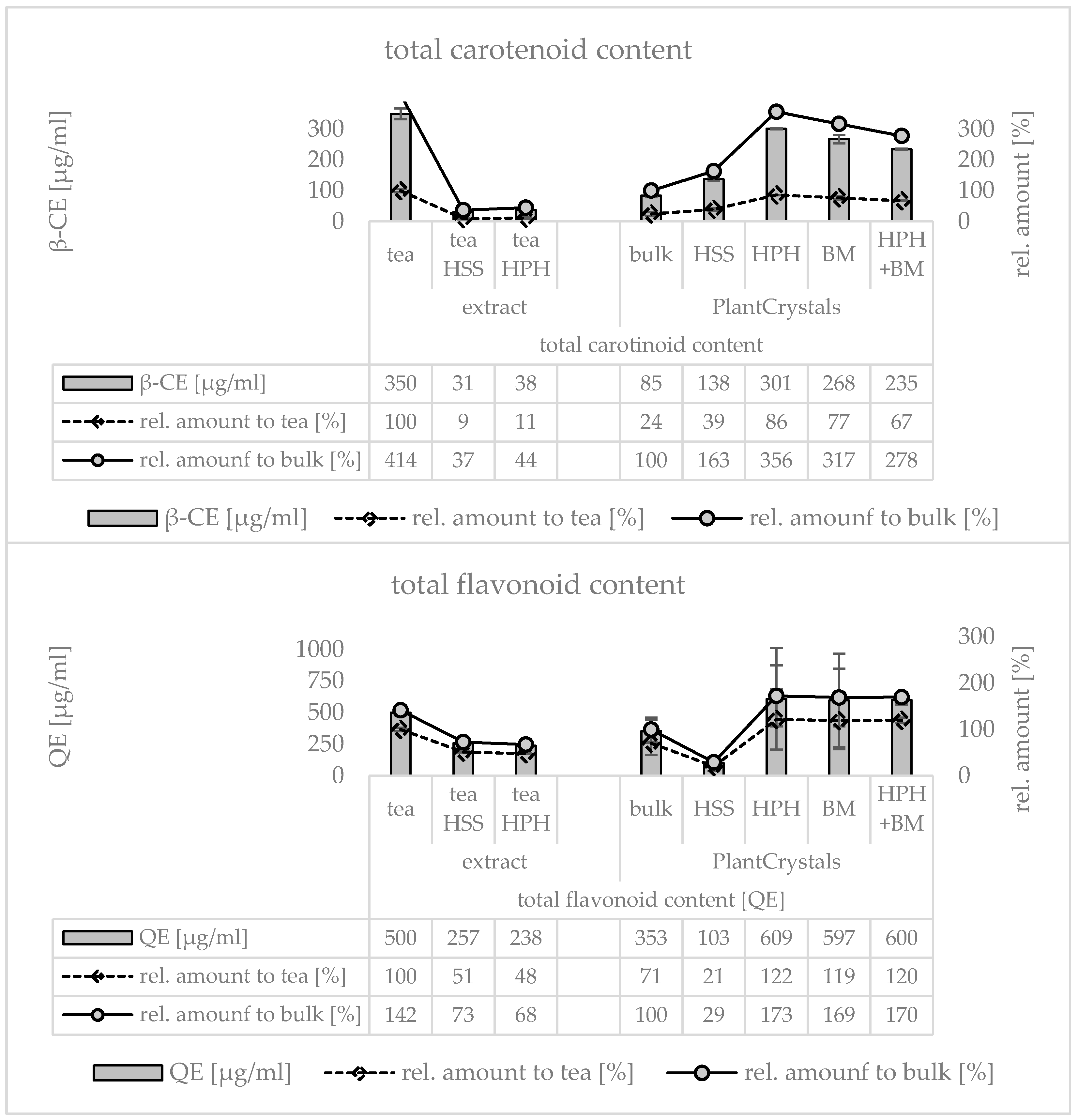
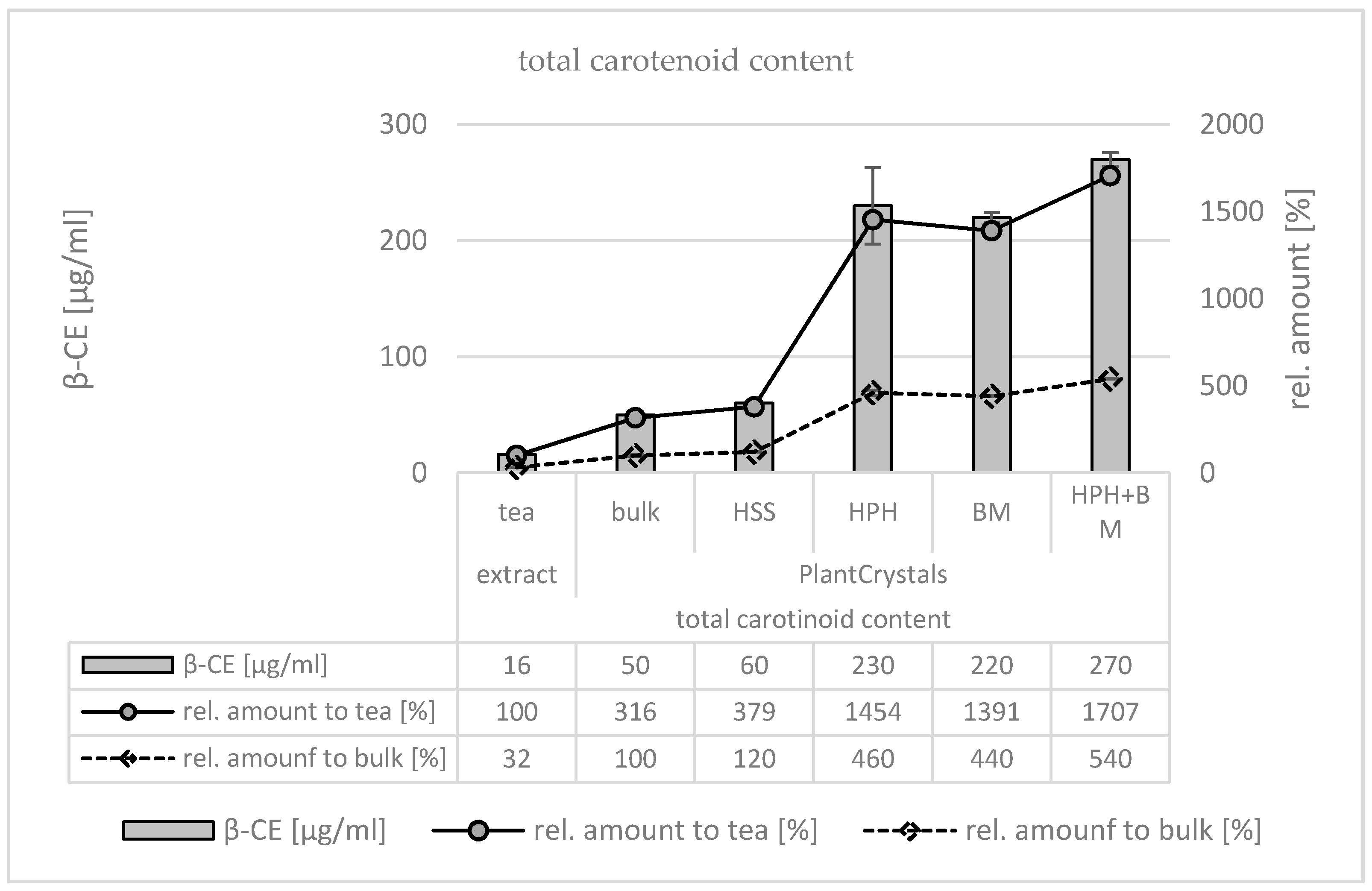
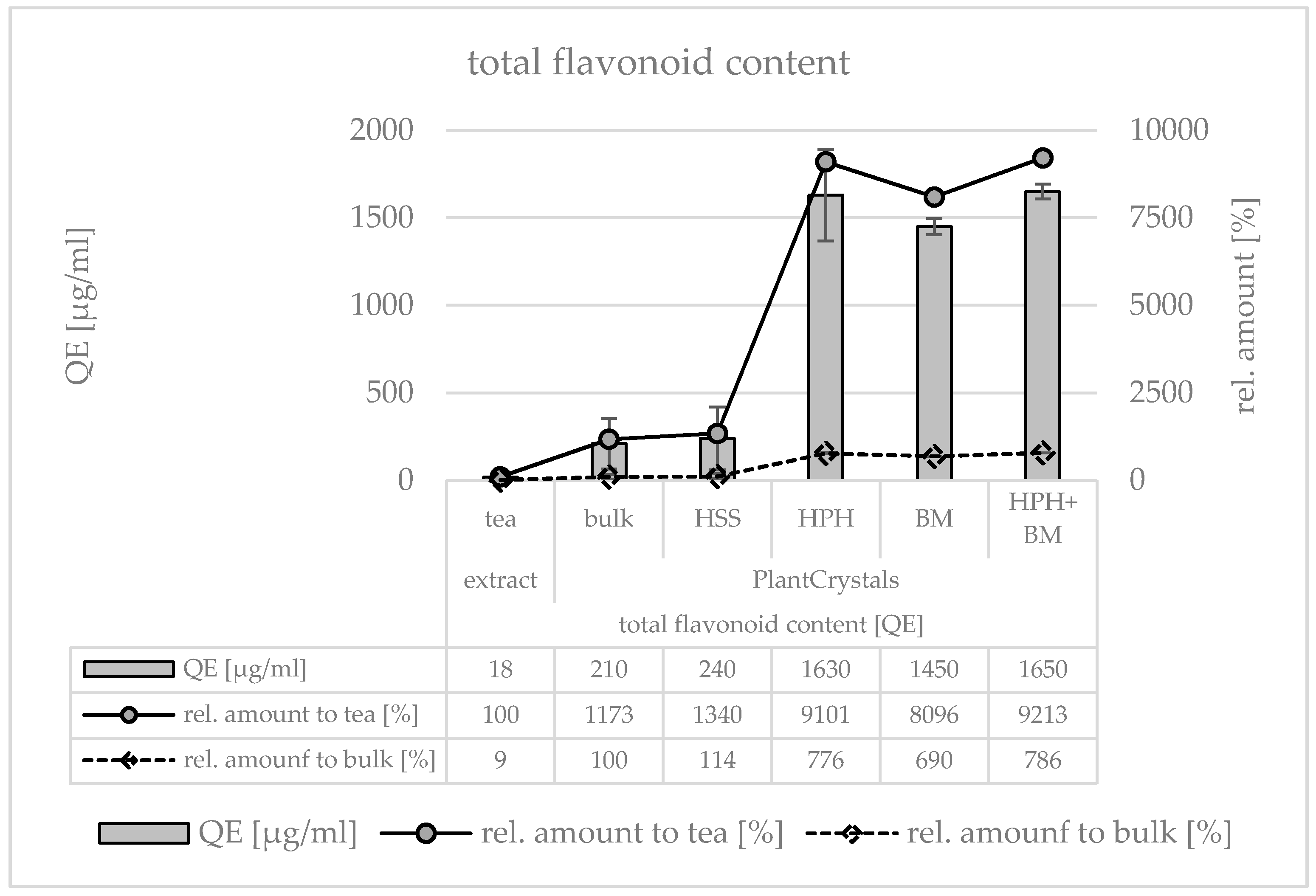
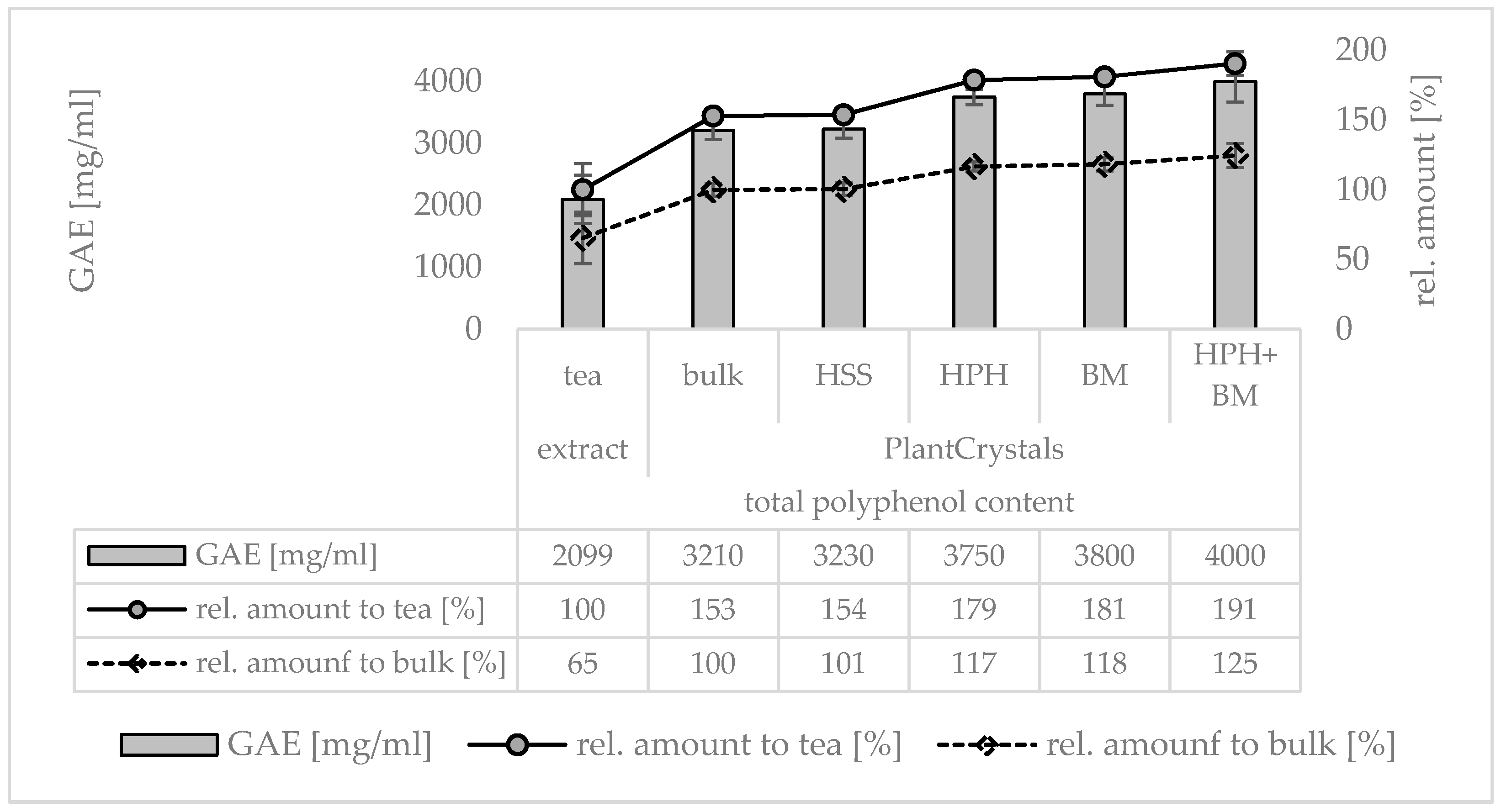
3. Results
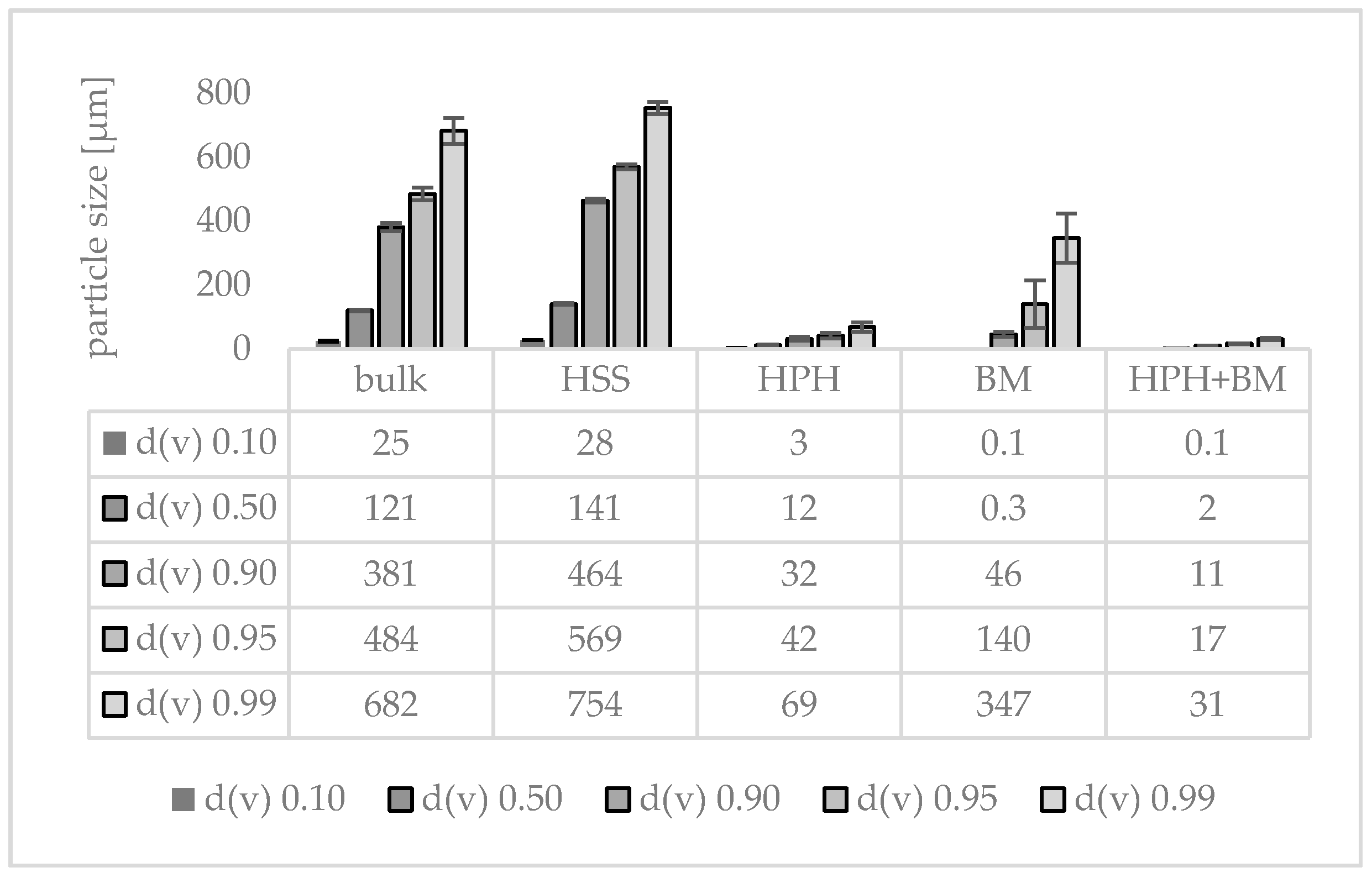
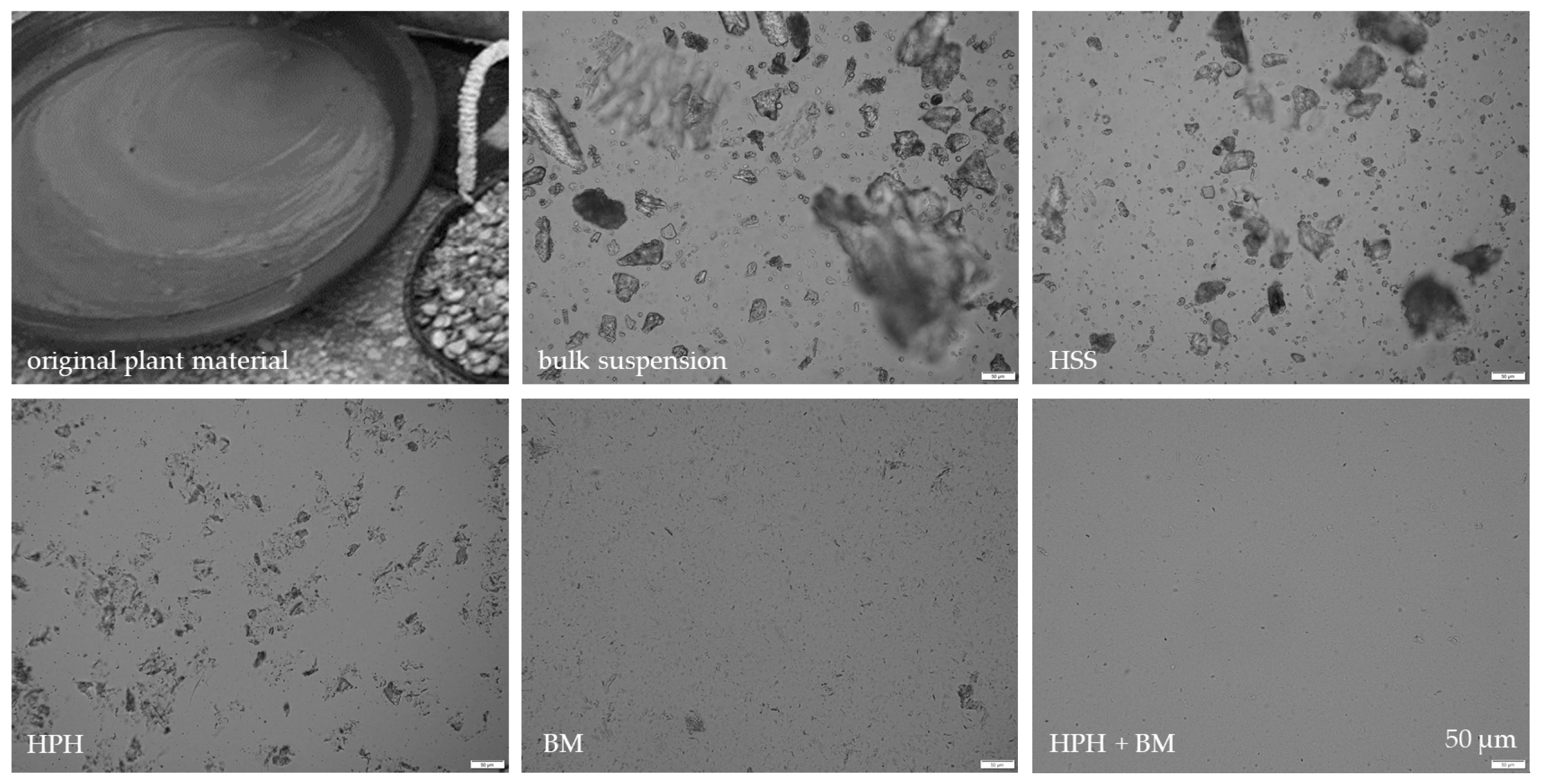
3.1. Production and Characterization of Plantcrystals Produced by High Pressure Homogenization
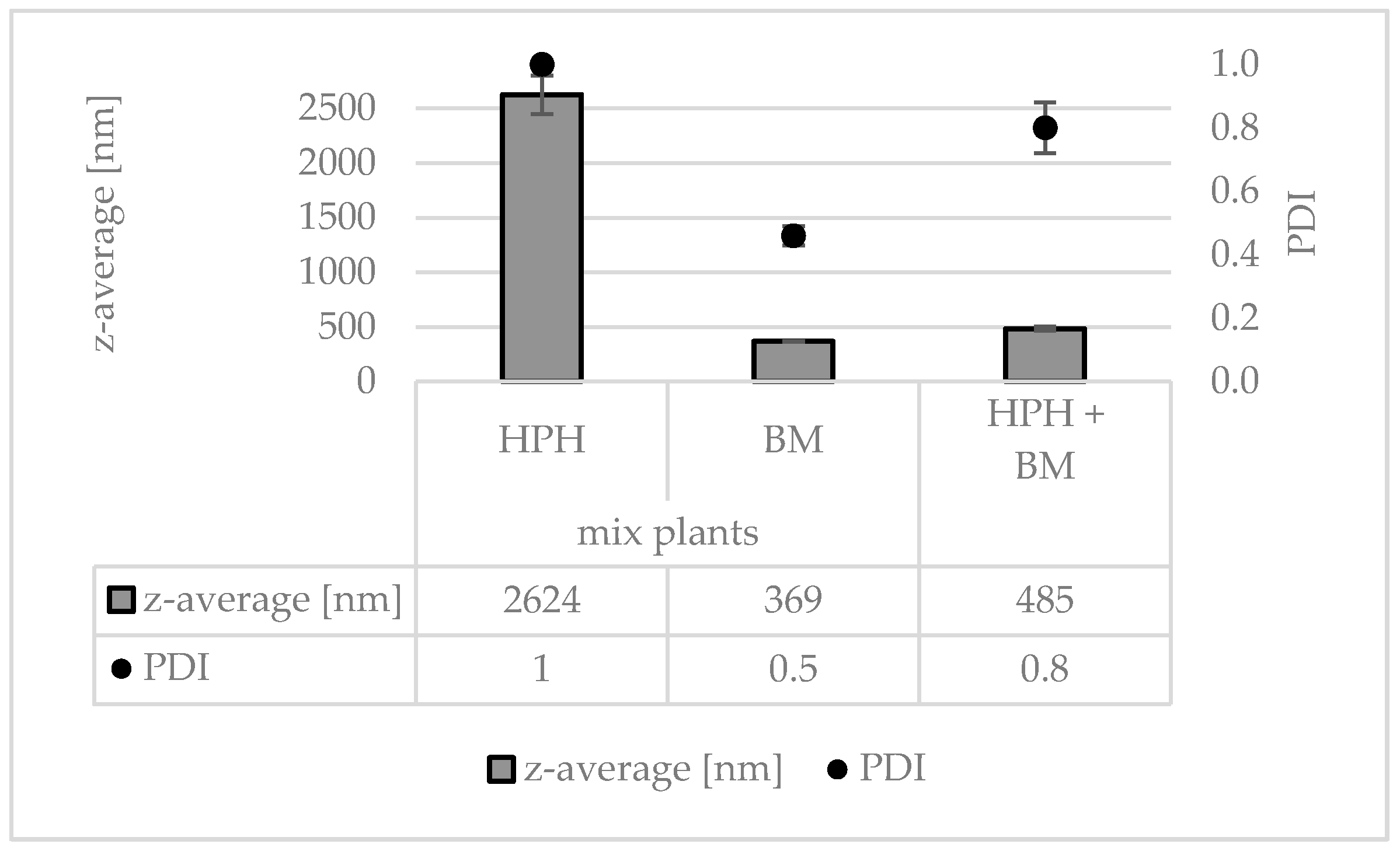
3.2. Production and Characterization of Plantcrystals Produced by Bead Milling
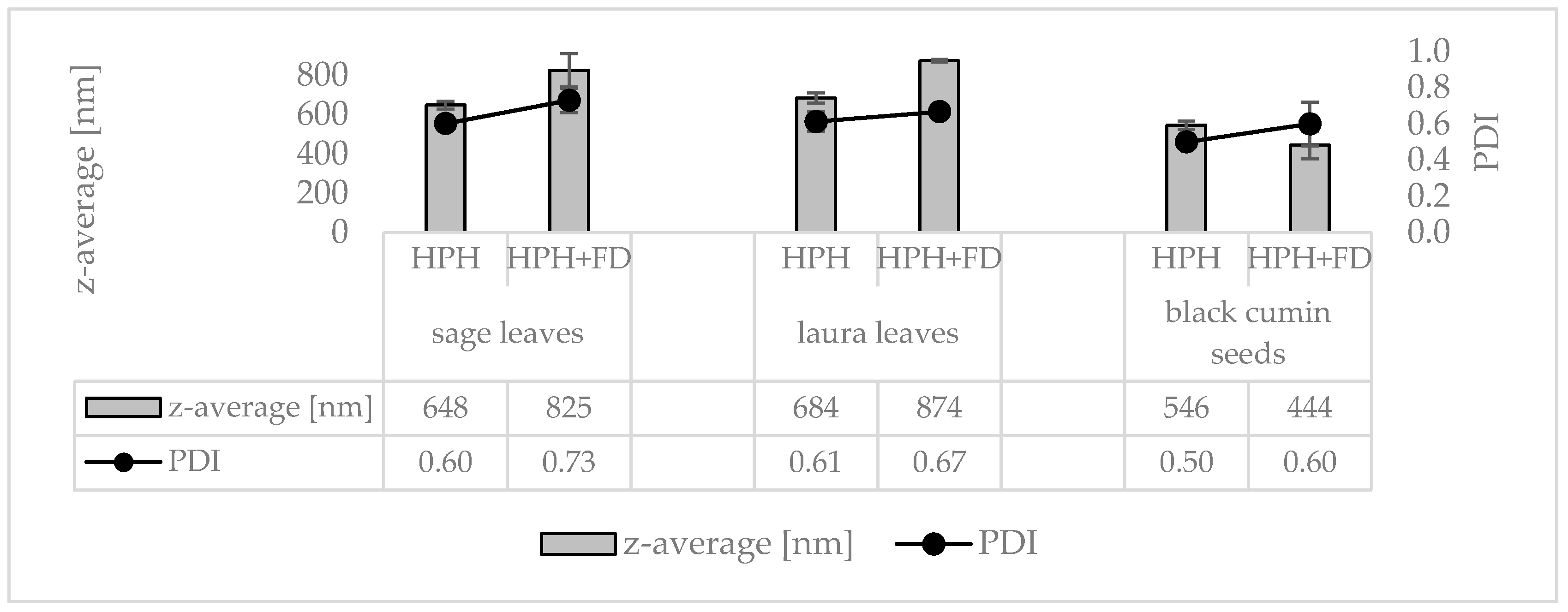
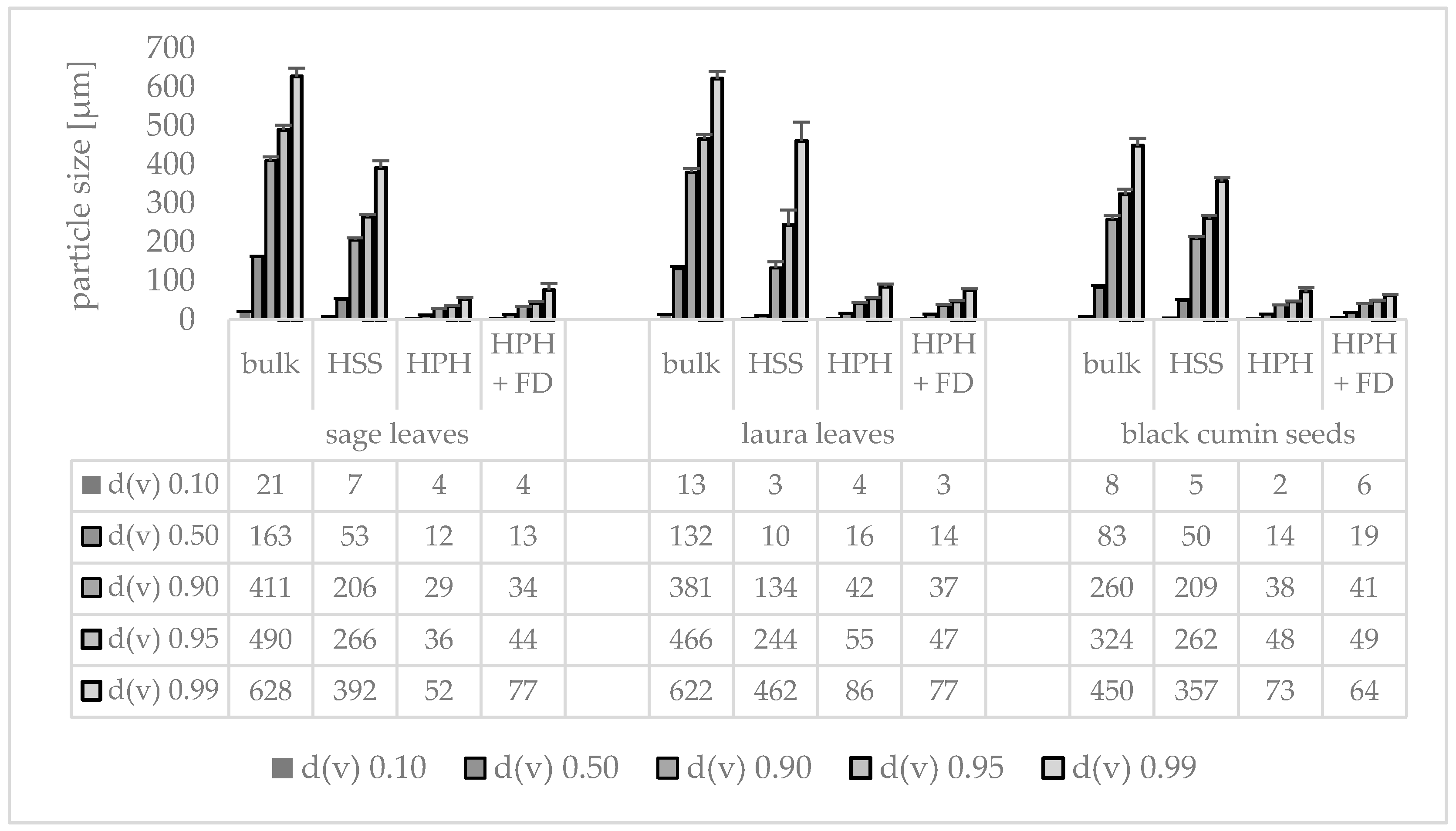
3.3. Production and Characterization of Plantcrystals Produced by Combined Milling Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- MarketsandMarkets. Plant Extracts Market by Type (Phytomedicines & Herbal Extracts, Essential Oils, Spices, Flavors & Fragrances), Application (Pharmaceuticals & Dietary Supplements, Food & Beverages, Cosmetics), Source, and Region—Global Forecast to 2025—REPORT CODE FB 1399. Available online: https://www.marketsandmarkets.com/Market-Reports/plant-extracts-market-942.html (accessed on 20 August 2020).
- Briskin, D.P. Medicinal plants and phytomedicines. Linking plant biochemistry and physiology to human health. Plant Physiol. 2000, 124, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Ebokaiwe, A.P.; Osawe, S.; Griffin, S.; Keck, C.M.; Olusanya, O.; Ehiri, R.C. Loranthus micranthus nanoparticles abates streptozotocin-instigated testicular dysfunction in Wistar rats: Involvement of glucose metabolism enzymes, oxido-inflammatory stress, steroidogenic enzymes/protein and Nrf2 pathway. Andrologia 2020, e13749. [Google Scholar] [CrossRef] [PubMed]
- Estevam, E.C.; Griffin, S.; Nasim, M.J.; Denezhkin, P.; Schneider, R.; Lilischkis, R.; Dominguez-Alvarez, E.; Witek, K.; Latacz, G.; Keck, C.; et al. Natural selenium particles from Staphylococcus carnosus: Hazards or particles with particular promise? J. Hazard. Mater. 2017, 324, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.; Masood, M.I.; Nasim, M.J.; Sarfraz, M.; Ebokaiwe, A.P.; Schäfer, K.-H.; Keck, C.M.; Jacob, C. Natural Nanoparticles: A Particular Matter Inspired by Nature. Antioxidants 2017, 7, 3. [Google Scholar] [CrossRef]
- Griffin, S.; Sarfraz, M.; Farida, V.; Nasim, M.J.; Ebokaiwe, A.P.; Keck, C.M.; Jacob, C. No time to waste organic waste: Nanosizing converts remains of food processing into refined materials. J. Environ. Manag. 2018, 210, 114–121. [Google Scholar] [CrossRef]
- Griffin, S.; Sarfraz, M.; Hartmann, S.F.; Pinnapireddy, S.R.; Nasim, M.J.; Bakowsky, U.; Keck, C.M.; Jacob, C. Resuspendable Powders of Lyophilized Chalcogen Particles with Activity against Microorganisms. Antioxidants 2018, 7, 23. [Google Scholar] [CrossRef]
- Griffin, S.; Tittikpina, N.K.; Al-marby, A.; Alkhayer, R.; Denezhkin, P.; Witek, K.; Gbogbo, K.A.; Batawila, K.; Duval, R.E.; Nasim, M.J.; et al. Turning Waste into Value: Nanosized Natural Plant Materials of Solanum incanum L. and Pterocarpus erinaceus Poir with Promising Antimicrobial Activities. Pharmaceutics 2016, 8, 11. [Google Scholar] [CrossRef]
- Sarfraz, M.; Griffin, S.; Gabour Sad, T.; Alhasan, R.; Nasim, M.J.; Irfan Masood, M.; Schäfer, K.H.; Ejike, C.E.C.C.; Keck, C.M.; Jacob, C.; et al. Milling the Mistletoe: Nanotechnological Conversion of African Mistletoe (Loranthus micranthus) Intoantimicrobial Materials. Antioxidants 2018, 7, 60. [Google Scholar] [CrossRef]
- Scholz, P.; Keck, C.M. Nanocrystals: From Raw Material to the Final Formulated Oral Dosage Form—A Review. Curr. Pharm. Des. 2015, 21, 4217–4228. [Google Scholar] [CrossRef]
- Rachmawati, H.; Rahma, A.; Al Shaal, L.; Müller, R.H.; Keck, C.M. Destabilization Mechanism of Ionic Surfactant on Curcumin Nanocrystal against Electrolytes. Sci. Pharm. 2016, 84, 685–693. [Google Scholar] [CrossRef]
- Rachmawati, H.; Al Shaal, L.; Müller, R.H.; Keck, C.M. Development of curcumin nanocrystal: Physical aspects. J. Pharm. Sci. 2013, 102, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Keck, C.M.; Müller, R.H. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur. J. Pharm. Biopharm. 2006, 62, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Gohla, S.; Keck, C.M. State of the art of nanocrystals—Special features, production, nanotoxicology aspects and intracellular delivery. Eur. J. Pharm. Biopharm. 2011, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Romero, G.B.; Keck, C.M.; Müller, R.H. Simple low-cost miniaturization approach for pharmaceutical nanocrystals production. Int. J. Pharm. 2016, 501, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Heinen, M. Kräuterapotheke—Schönheitstee der Chinesischen Kaiserinnen. Available online: https://kraeuter-apotheke.eu/kaiserinnentee.htm (accessed on 20 August 2020).
- Chang, R.C.-C.; So, K.-F. Lycium Barbarum and Human Health; Springer: Dordrecht, The Netherlands, 2015; ISBN 9789401796576. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Katsube, T.; Tabata, H.; Ohta, Y.; Yamasaki, Y.; Anuurad, E.; Shiwaku, K.; Yamane, Y. Screening for antioxidant activity in edible plant products: Comparison of low-density lipoprotein oxidation assay, DPPH radical scavenging assay, and Folin-Ciocalteu assay. J. Agric. Food Chem. 2004, 52, 2391–2396. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- Ordonez, A.; Gomez, J.; Vattuone, M.; Isla, M. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001; ISBN 9781578810727. [Google Scholar]
- Salazar, J.; Ghanem, A.; Müller, R.H.; Möschwitzer, J.P. Nanocrystals: Comparison of the size reduction effectiveness of a novel combinative method with conventional top-down approaches. Eur. J. Pharm. Biopharm. 2012, 81, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.; Robinson, C.W. Disruption of Saccharomyces cerevisiae using enzymatic lysis combined with high-pressure homogenization. Biotechnol. Tech. 1990, 4, 329–334. [Google Scholar] [CrossRef]
- Scholz, P.; Arntjen, A.; Müller, R.H.; Keck, C.M. ARTcrystal process for industrial nanocrystal production—Optimization of the ART MICCRA pre-milling step. Int. J. Pharm. 2014, 465, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, X.; Zhang, X.; Liu, J.; Hao, Y.; Yang, X.; Wang, Y. Comparative evaluation of the antioxidant effects of the natural vitamin C analog 2-O-β-D-glucopyranosyl-L-ascorbic acid isolated from Goji berry fruit. Arch. Pharm. Res. 2011, 34, 801–810. [Google Scholar] [CrossRef]
- Wojdyło, A.; Carbonell-Barrachina, Á.A.; Legua, P.; Hernández, F. Phenolic composition, ascorbic acid content, and antioxidant capacity of Spanish jujube (Ziziphus jujube Mill.) fruits. Food Chem. 2016, 201, 307–314. [Google Scholar] [CrossRef]
- Heaton, J.W.; Marangoni, A.G. Chlorophyll degradation in processed foods and senescent plant tissues. Trends Food Sci. Technol. 1996, 7, 8–15. [Google Scholar] [CrossRef]
- Scheer, H. Chlorophylls; CRC Press: Boca Raton, FL, USA, 1991; ISBN 0849368421. [Google Scholar]
- Monfalouti, H.E.; Guillaume, D.; Denhez, C.; Charrouf, Z. Therapeutic potential of argan oil: A review. J. Pharm. Pharmacol. 2010, 62, 1669–1675. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin-Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Jiménez-Jiménez, I.; Sánchez-Moreno, C.; Saura-Calixto, F. Evaluation of free radical scavenging of dietary carotenoids by the stable radical 2,2-diphenyl-1-picrylhydrazyl. J. Sci. Food Agric. 2000, 80, 1686–1690. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Lester, G.E.; Lewers, K.S.; Medina, M.B.; Saftner, R.A. Comparative analysis of strawberry total phenolics via Fast Blue BB vs. Folin–Ciocalteu: Assay interference by ascorbic acid. J. Food Compos. Anal. 2012, 27, 102–107. [Google Scholar] [CrossRef]
- Süß, A.; Danner, M.; Obster, C.; Locherer, M.; Hank, T.; Richter, K. Measuring Leaf Chlorophyll Content with the Konica Minolta SPAD-502Plus; EnMAP: Potsdam, Germany, 2015; Available online: https://gfzpublic.gfz-potsdam.de/rest/items/item_1388302/component/file_1388303/content (accessed on 30 September 2020).




| Name | Binomial Name | Part of Plant | Source |
|---|---|---|---|
| sage | Salvia officinalis L. | leave | local market Palestine * |
| laurel | Laurus nobilis L. | leave | local market Palestine * |
| black cumin | Nigella sativa L. | seed | local market Palestine * |
| grape | Vitis vinifera L. | leave | private garden, Marburg, Germany |
| female ginseng | Angelica sinensis Diels | root | IndigoHerbs, UK |
| jujube-red dates | Ziziphus jujube Mill. | fruit | Jonnic Food Co., China |
| goji berries | Lycium chinense Mill. | fruit | BioJoy GmbH, Germany |
| jasmine tea | Jasminum L. | flower, leave | Sonnentor GmbH, Austria |
| argan | Argania spinosa L. | seed | local market Morocco ** |
| Name | Latin Name | Amount |
|---|---|---|
| female ginseng | Angelica sinensis | 10.0 g |
| jujube-red dates | Ziziphus jujuba | 5.0 g |
| goji berries | Lycium chinense Mill. | 3 pieces |
| jasmine tea | Jasminum L. | 5.0 g |
| water | Aqua purificata | 300 mL |
| Size & AOC Parameters | Bulk | BM | |||||
|---|---|---|---|---|---|---|---|
| LD Data | d(v) 0.10 (µm) | 30 | ± | 2 | 0.14 | ± | 0.01 |
| d(v) 0.50 (µm) | 226 | ± | 6 | 0.96 | ± | 0.6 | |
| d(v) 0.90 (µm) | 402 | ± | 14 | 15 | ± | 2.8 | |
| d(v) 0.95 (µm) | 449 | ± | 16 | 26 | ± | 4 | |
| d(v) 0.99 (µm) | 523 | ± | 23 | 52 | ± | 13 | |
| DLS Data | z-average (nm) | n.a. | 485 | ± | 11 | ||
| PDI | n.a. | 0.5 | ± | 0.03 | |||
| ZP (Mv) | n.a. | −19 | ± | 0.8 | |||
| AOC | IC50 (µg/mL) | 3.1 | ± | 0.7 | 2.1 | ± | 0.5 |
| rel. AOC (%) | 162 | ± | 23 | 174 | ± | 23 | |
| Argan Seeds | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size Parameters | Bulk | HSS | HPH | BM | HPH + BM | |||||||||||
| LD Data | d(v) 0.10 (µm) | 40 | ± | 7 | 112 | ± | 10 | 3 | ± | 0 | 0.03 | ± | 0 | 0.02 | ± | 0 |
| d(v) 0.50 (µm) | 199 | ± | 4 | 282 | ± | 3 | 12 | ± | 7 | 0.10 | ± | 0 | 0.07 | ± | 0 | |
| d(v) 0.90 (µm) | 322 | ± | 5 | 464 | ± | 4 | 33 | ± | 11 | 5 | ± | 2 | 0.27 | ± | 0 | |
| d(v) 0.95 (µm) | 355 | ± | 5 | 514 | ± | 4 | 43 | ± | 17 | 75 | ± | 16 | 128 | ± | 14 | |
| d(v) 0.99 (µm) | 406 | ± | 5 | 593 | ± | 5 | 166 | ± | 70 | 324 | ± | 54 | 364 | ± | 25 | |
| DLS Data | z-average (nm) | n.a. | n.a. | 901 | ± | 122 | 202 | ± | 4 | 153 | ± | 2 | ||||
| - | PDI | n.a. | n.a. | 0.7 | ± | 0 | 0.3 | ± | 0 | 0.3 | ± | 0 | ||||
| AOC-Value | Tea | Bulk | HSS | HPH | BM | HPH + BM | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (µg/mL) | 4 | ± | 2 | 2 | ± | 0 | 2 | ± | 0 | 3 | ± | 0 | 3 | ± | 0 | 3 | ± | 0 |
| Rel. AOCDPPH to Bulk (%) | 171 | ± | 58 | 100 | ± | 6 | 93 | ± | 6 | 144 | ± | 12 | 105 | ± | 4 | 115 | ± | 7 |
| ORAC-Value (µmol/µL) | 110 | ± | 9 | 118 | ± | 31 | 214 | ± | 13 | 204 | ± | 8 | 215 | ± | 7 | 179 | ± | 3 |
| Rel. AOCORAC to Bulk (%) | 93 | ± | 8 | 100 | ± | 27 | 182 | ± | 6 | 174 | ± | 4 | 183 | ± | 3 | 152 | ± | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abraham, A.M.; Alnemari, R.M.; Jacob, C.; Keck, C.M. PlantCrystals—Nanosized Plant Material for Improved Bioefficacy of Medical Plants. Materials 2020, 13, 4368. https://doi.org/10.3390/ma13194368
Abraham AM, Alnemari RM, Jacob C, Keck CM. PlantCrystals—Nanosized Plant Material for Improved Bioefficacy of Medical Plants. Materials. 2020; 13(19):4368. https://doi.org/10.3390/ma13194368
Chicago/Turabian StyleAbraham, Abraham M., Reem M. Alnemari, Claus Jacob, and Cornelia M. Keck. 2020. "PlantCrystals—Nanosized Plant Material for Improved Bioefficacy of Medical Plants" Materials 13, no. 19: 4368. https://doi.org/10.3390/ma13194368
APA StyleAbraham, A. M., Alnemari, R. M., Jacob, C., & Keck, C. M. (2020). PlantCrystals—Nanosized Plant Material for Improved Bioefficacy of Medical Plants. Materials, 13(19), 4368. https://doi.org/10.3390/ma13194368






