Preparation of Chitosan/Polyacrylamide/Graphene Oxide Composite Membranes and Study of Their Methylene Blue Adsorption Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Preparation of CAG
2.3. Method for Experiment
2.3.1. Method for Measuring the Methylene Blue Concentration
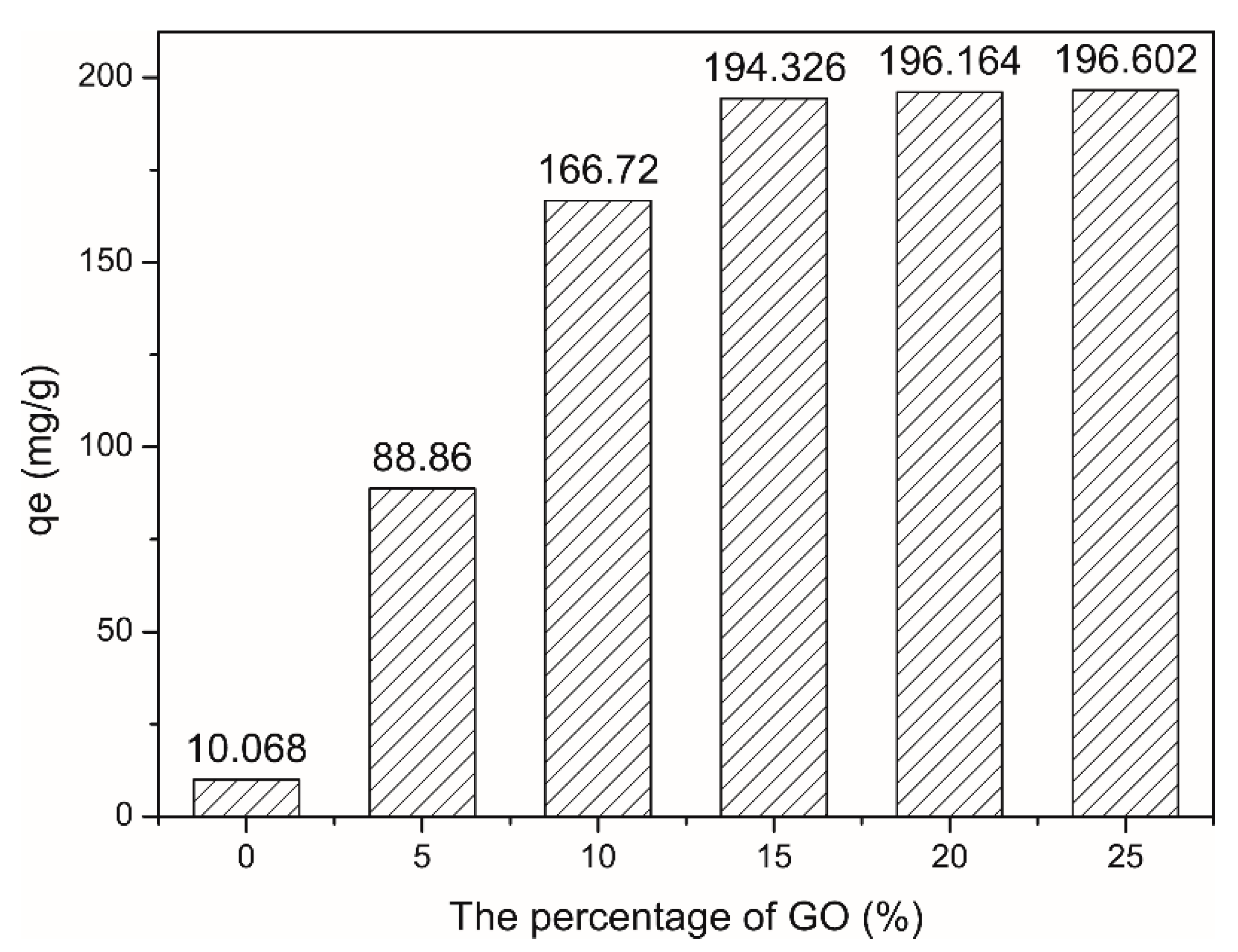
2.3.2. Influence of GO Amounts of CAG
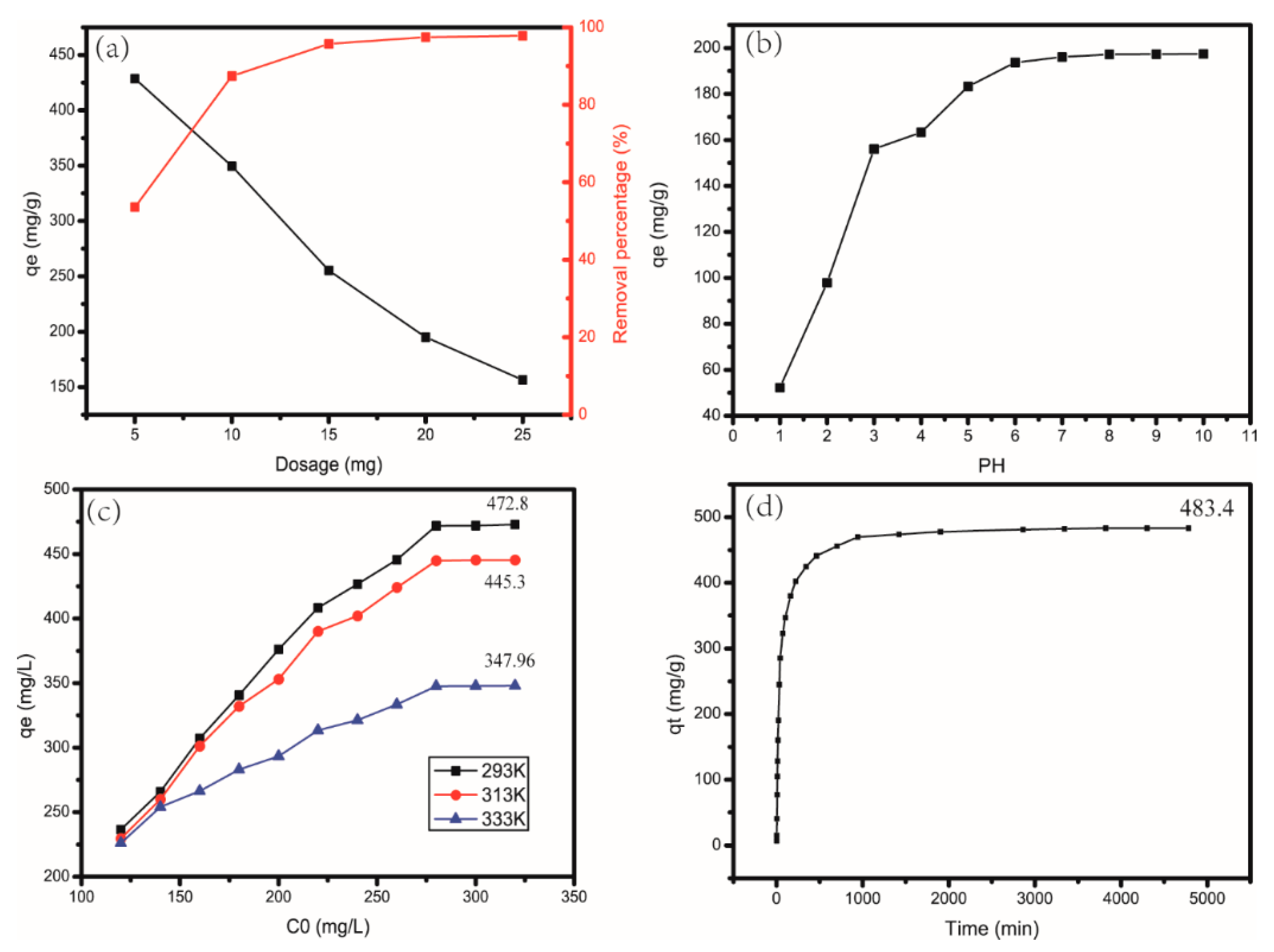
2.3.3. Influence of Different Quantities of CAG
2.3.4. Influence of the pH Value of MB Solution
2.3.5. Influence of the Temperature and Concentration of MB Solution
2.3.6. Influence of Adsorption Time
2.4. Characterization Method
3. Characterization of CAG Nanoparticles
3.1. SEM Analysis
3.2. XRD Analysis
3.3. FT-IR Analysis
3.4. BET Analysis
3.5. TGA Analysis
4. Methylene Blue Adsorption Properties
4.1. Influence of Different GO Proportions of CAG
4.2. Influence of Different Quantities of CAG
4.3. Influence of the pH Value of MB Solution
4.4. Influence of the Temperature and Concentration of MB Solution
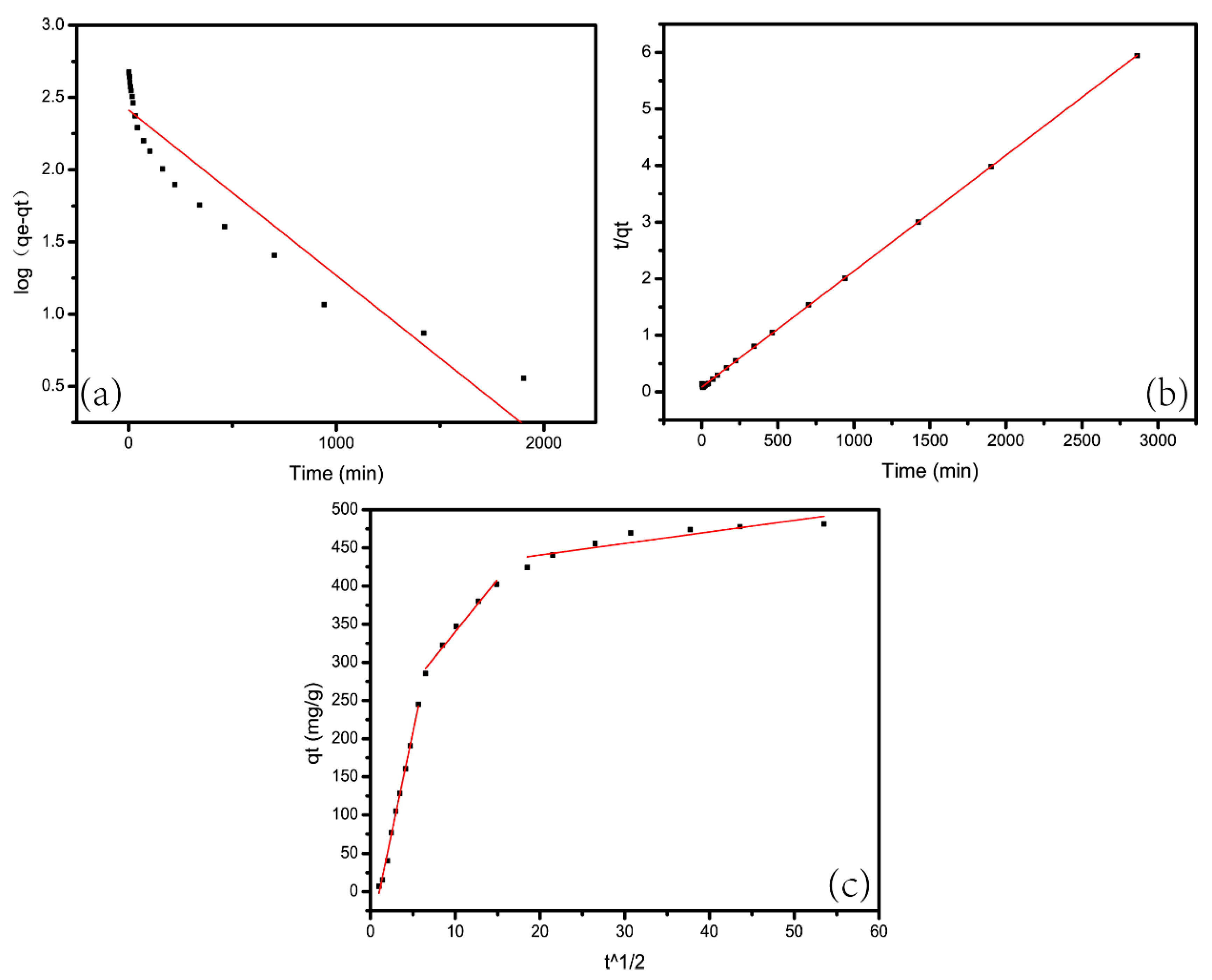
4.5. Influence of Adsorption Time
5. Adsorption Model Analysis
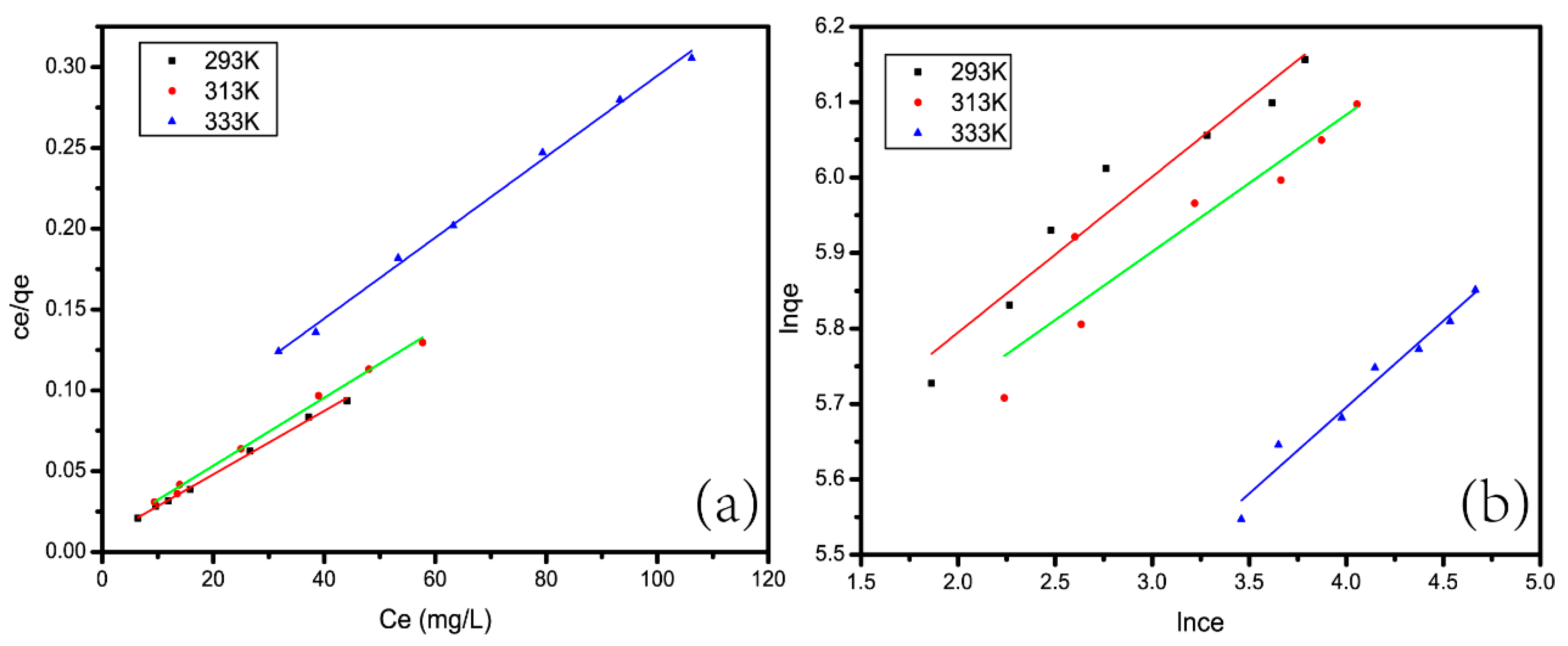
5.1. Adsorption Isotherm
5.2. Adsorption Kinetics
5.3. Adsorption Thermodynamics
6. Regeneration Research
7. Research Results
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Albano, G.; Aronica, L.A.; Biver, T.; Detti, R.; Pucci, A. Tris-Ethynylphenyl-amine Fluorophores: Synthesis, Characterisation and Test of Performances in Luminescent Solar Concentrators. Chemistryselect 2018, 3, 1749–1754. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Du, Q.; Sun, J.; Chen, L.; Hu, S.; Wang, Z.; Xia, Y.; Xia, L. Highly effective removal of basic fuchsin from aqueous solutions by anionic polyacrylamide/graphene oxide aerogels. J. Colloid Interface Sci. 2015, 453, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.P.; Chadwick, D.R.; Harrison, B.J.; Balasubramanian, S.P. Systematic review of intravenous methylene blue in parathyroid surgery. Br. J. Surg. 2012, 99, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- El-Zawahry, M.M.; Abdelghaffar, F.; Abdelghaffar, R.A.; Hassabo, A.G. Equilibrium and kinetic models on the adsorption of Reactive Black 5 from aqueous solution using Eichhomia crassipes/chitosan composite. Carbohydr. Polym. 2015, 1136, 507–515. [Google Scholar]
- Li, Y.; Sun, J.; Du, Q.; Zhang, L.; Yang, X.; Wu, S.; Xia, Y.; Wang, Z.; Xia, L.; Cao, A. Mechanical and dye adsorption properties of graphene oxide/chitosan composite fibers prepared by wet spinning. Carbohydr. Polym. 2014, 102, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Alemany, L.B.; Ci, L.; Ajayan, P.M. New insights into the structure and reduction of graphite oxide. Nat. Chem. 2009, 1, 403–408. [Google Scholar] [CrossRef]
- Seo, M.H.; Choi, S.M.; Kim, H.J.; Kim, W.B. The graphene-supported Pd and Pt catalysts for highly active oxygen reduction reaction in an alkaline condition. Electrochem. Commun. 2011, 13, 182–185. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Murali, S.; Cai, W.W.; Li, X.S.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- He, S.; Song, B.; Li, D.; Zhu, C.; Qi, W.; Wen, Y.; Wang, L.; Song, S.; Fang, H.; Fan, C. A Graphene Nanoprobe for Rapid, Sensitive, and Multicolor Fluorescent DNA Analysis. Adv. Funct. Mater. 2009, 20, 453–459. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Wang, M.; Niu, Z.; Wang, X.; Hou, B. Preparation and photocathodic protection property of ZnIn2S4/RGO/TiO2 composites for Q235 carbon steel under visible light. Nanotechnology 2018, 29, 435706. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, W.; Cui, X.; Li, Y.; Hou, B.; Zhang, X.; Wang, Y.; Cheng, L.; Zhang, P.; Li, J. AgInS2 and graphene co-sensitized TiO2 photoanodes for photocathodic protection of Q235 carbon steel under visible light. Nanotechnology 2020, 31, 305704. [Google Scholar] [CrossRef] [PubMed]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2010, 6, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.-H.; Zhang, X.-R.; Zeng, G.-M.; Gong, J.-L.; Niu, Q.-Y.; Liang, J. Simultaneous removal of Cd(II) and ionic dyes from aqueous solution using magnetic graphene oxide nanocomposite as an adsorbent. Chem. Eng. J. 2013, 226, 189–200. [Google Scholar] [CrossRef]
- Zhao, G.; Li, J.; Ren, X.; Chen, C.; Wang, X. Few-Layered Graphene Oxide Nanosheets As Superior Sorbents for Heavy Metal Ion Pollution Management. Environ. Sci. Technol. 2011, 45, 10454–10462. [Google Scholar] [CrossRef]
- Saeidi, N.; Parvini, M.; Niavarani, Z. High surface area and mesoporous graphene/activated carbon composite for adsorption of Pb(II) from wastewater. J. Environ. Chem. Eng. 2015, 3, 2697–2706. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Jiang, S.-J. Chitosan-functionalized graphene oxide: A novel adsorbent an efficient adsorption of arsenic from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 1698–1713. [Google Scholar] [CrossRef]
- Kaur, N.; Kaur, M.; Singh, D. Fabrication of mesoporous nanocomposite of graphene oxide with magnesium ferrite for efficient sequestration of Ni (II) and Pb (II) ions: Adsorption, thermodynamic and kinetic studies. Environ. Pollut. 2019, 253, 111–119. [Google Scholar] [CrossRef]
- Oostinga, J.B.; Heersche, H.B.; Liu, X.; Morpurgo, A.F.; Vandersypen, L.M.K. Gate-induced insulating state in bilayer graphene devices. Nat. Mater. 2008, 7, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, H.; Yu, J.; Wang, Y.; Zhu, J.; Hu, Z. Mechanically strong and pH-responsive carboxymethyl chitosan/graphene oxide/polyacrylamide nanocomposite hydrogels with fast recoverability. J. Biomater. Sci. -Polym. Ed. 2017, 28, 1899–1917. [Google Scholar] [CrossRef]
- Justin, R.; Chen, B. Characterisation and drug release performance of biodegradable chitosan-graphene oxide nanocomposites. Carbohydr. Polym. 2013, 103, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Liu, Y.-T.; Xie, X.-M. Self-healable, super tough graphene oxide-poly(acrylic acid) nanocomposite hydrogels facilitated by dual cross-linking effects through dynamic ionic interactions. J. Mater. Chem. B 2015, 3, 4001–4008. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Farag, R.K.; Mohamed, R.R. Synthesis and Characterization of Carboxymethyl Chitosan Nanogels for Swelling Studies and Antimicrobial Activity. Molecules 2013, 18, 190–203. [Google Scholar] [CrossRef]
- Wei, Q.-B.; Luo, Y.-L.; Fu, F.; Zhang, Y.-Q.; Ma, R.-X. Synthesis, characterization, and swelling kinetics of pH-responsive and temperature-responsive carboxymethyl chitosan/polyacrylamide hydrogels. J. Appl. Polym. Sci. 2013, 129, 806–814. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.L.; Zhao, X.S.; Wu, J. Graphene/Polyaniline Nanofiber Composites as Supercapacitor Electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Shen, Y.; Fang, Q.; Chen, B. Environmental Applications of Three-Dimensional Graphene-Based Macrostructures: Adsorption, Transformation, and Detection. Environ. Sci. Technol. 2015, 49, 67–84. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Chem. Phys. 2015, 40, 1361–1413. [Google Scholar] [CrossRef] [Green Version]
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2016, 307, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Zhang, D.; Huang, H.; Jin, Z.; Peng, X. Ionic polyacrylamide hydrogel improved by graphene oxide for efficient adsorption of methylene blue. Res. Chem. Intermed. 2019, 45, 1545–1563. [Google Scholar] [CrossRef]
- He, Q.; Li, Z.; Fu, C. Preparation of Graphene Oxide/Diatomite Composites and Their Application in the Removal of Methylene Blue in Wastewater. Chem. World 2017, 58, 268–274. [Google Scholar]
- Wang, C.B.; Zhou, J.W.; Chu, L.L. Chlorine-functionalized reduced graphene oxide for methylene blue removal. RSC Adv. 2015, 5, 52466–52472. [Google Scholar] [CrossRef]
- Li, Y.; Du, Q.; Liu, T.; Sun, J.; Wang, Y.; Wu, S.; Wang, Z.; Xia, Y.; Xia, L. Methylene blue adsorption on graphene oxide/calcium alginate composites. Carbohydr. Polym. 2013, 95, 501–507. [Google Scholar] [CrossRef]
- Gao, L.; Wang, Y.; Yan, L.; Cui, L.; Hu, L.; Wei, Q.; Du, B. A novel magnetic polysaccharide-graphene oxide composite for removal of cationic dyes from aqueous solution. New J. Chem. 2015, 39, 2908–2916. [Google Scholar] [CrossRef]
- Yang, S.-T.; Luo, J.; Liu, J.-H.; Zhou, Q.; Wan, J.; Ma, C.; Liao, R.; Wang, H.; Liu, Y. Graphene Oxide/Chitosan Composite for Methylene Blue Adsorption. Nanosci. Nanotechnol. Lett. 2013, 5, 372–376. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.V.; Bui, L.T.; Dinh, T.T.; Le, D.H.; Huynh, C.D.; Trinh, A.X. Graphene oxide/Fe3O4/chitosan nanocomposite: A recoverable and recyclable adsorbent for organic dyes removal. Application to methylene blue. Mater. Res. Express 2017, 4, 9. [Google Scholar] [CrossRef]
- Ghorai, S.; Sarkar, A.; Raoufi, M.; Panda, A.B.; Schonherr, H.; Pal, S. Enhanced Removal of Methylene Blue and Methyl Violet Dyes from Aqueous Solution Using a Nanocomposite of Hydrolyzed Polyacrylamide Grafted Xanthan Gum and Incorporated Nanosilica. ACS Appl. Mater. Interfaces 2014, 6, 4766–4777. [Google Scholar] [CrossRef]
- Nguyen Thi My, H.; Pham Thi Thuy, T.; Nguyen Minh, D.; Nguyen Huu, H. Synthesis of Chitosan/Graphene Oxide Nanocomposites for Methylene Blue Adsorption. In Proceedings of the International Conference on Chemical Engineering, Food and Biotechnology, Ho Chi Minh City, Vietnam, 12–13 October 2017; Phung, L.T.K., Hoang, H.A., Viet, T.T., Eds.; Volume 1878. [Google Scholar]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. A 1906, 57, 385–470. [Google Scholar]
- Dogan, M.; Alkan, M.; Demirbas, O.; Ozdemir, Y.; Ozmetin, C. Adsorption kinetics of maxilon blue GRL onto sepiolite from aqueous solutions. Chem. Eng. J. 2006, 124, 89–101. [Google Scholar] [CrossRef]
- Ho, Y.S. Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods. Water Res. 2005, 40, 119–125. [Google Scholar] [CrossRef]
- Chiou, M.S.; Li, H.Y. Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads. Chemosphere 2003, 50, 1095–1105. [Google Scholar] [CrossRef]
- Kannan, N.; Sundaram, M.M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons-a comparative study. Dyes Pigment. 2001, 51, 25–40. [Google Scholar] [CrossRef]
- Neghlani, P.K.; Rafizadeh, M.; Taromi, F.A. Preparation of aminated-polyacrylonitrile nanofiber membranes for the adsorption of metal ions: Comparison with microfibers. J. Hazard. Mater. 2011, 186, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wan, Y.; Xie, Y.; Zhai, R.; Zhang, B.; Liu, J. The removal of dye from aqueous solution using alginate-halloysite nanotube beads. Chem. Eng. J. 2012, 187, 210–216. [Google Scholar] [CrossRef]
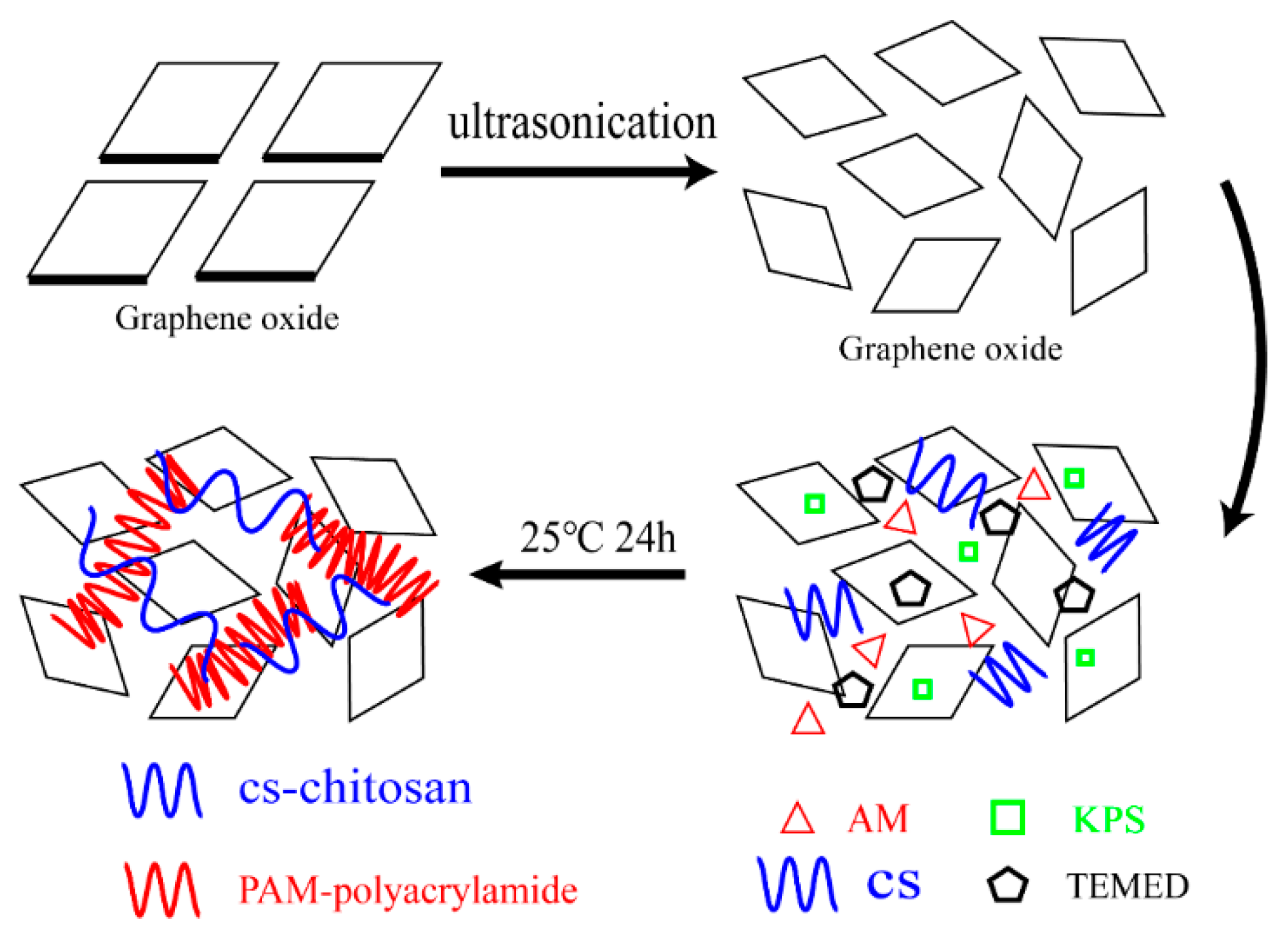






| Number | AM (g) | GO (mg) | CS (g) | KPS (mg) | TEMED (mL) | H2O (mL) | GO % |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 0 | 0.03 | 0.01 | 0.5 | 10 | 0% |
| 2 | 1 | 54 | 0.03 | 0.01 | 0.5 | 10 | 5% |
| 3 | 1 | 114 | 0.03 | 0.01 | 0.5 | 10 | 10% |
| 4 | 1 | 184 | 0.03 | 0.01 | 0.5 | 10 | 15% |
| 5 | 1 | 260 | 0.03 | 0.01 | 0.5 | 10 | 20% |
| 6 | 1 | 325 | 0.03 | 0.01 | 0.5 | 10 | 25% |
| T/K | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| - | qmax | kL | R2 | RL | kF | 1/n | R2 |
| - | (mg/g) | (L/mg) | - | - | (L/mg) | - | - |
| 293 K | 510.204 | 0.2253 | 0.9975 | 0.017–0.027 | 217.02 | 0.2064 | 0.9337 |
| 313 K | 476.19 | 0.1871 | 0.9953 | 0.019–0.323 | 210.61 | 0.1815 | 0.8522 |
| 333 K | 400.28 | 0.0564 | 0.9967 | 0.059–0.099 | 119.11 | 0.2289 | 0.9613 |
| Number | Sample | Maximum Adsorption Capacity | References |
|---|---|---|---|
| 1 | Activated lignin-chitosan | 36.25 mg/g | [29] |
| 2 | polyacrylamide/GO hydrogel | 255.48 mg/g | [30] |
| 3 | Graphene Oxide/Diatomite Composites | 125 mg/g | [31] |
| 4 | Chlorine-functionalized reduced graphene oxide | 221.4 mg/g | [32] |
| 5 | graphene oxide/calcium alginate composites | 181.81 mg/g | [33] |
| 6 | polysaccharide-graphene oxide composite | 358.4 mg/g | [34] |
| 7 | Graphene Oxide/Chitosan | 468 mg/g | [35] |
| 8 | Graphene oxide/Fe3O4/chitosan nanocomposite | 30.10 mg/g | [36] |
| 9 | Hydrolyzed Polyacrylamide Grafted Xanthan Gum and Incorporated Nanosilica | 378.8 mg/g | [37] |
| 10 | Chitosan/Graphene Oxide Nanocomposites | 662.25 mg/g | [38] |
| C0 (mg/L) | 100 | |
|---|---|---|
| Pseudo-first-order model | k1 (min−1) | 0.023 |
| qe (mg/g) | 258.22 | |
| R2 | 0.8868 | |
| Pseudo-second-order model | k2 (g/mg min) | 4.65 × 10−5 |
| qe (mg/g) | 500 | |
| R2 | 0.9998 | |
| Intraparticle diffusion model | kid1 | 52.539 |
| c | −54.51 | |
| R12 | 0.9952 | |
| kid2 | 13.73 | |
| R22 | 0.9819 | |
| c | 202.7 | |
| kid3 | 1.513 | |
| R32 | 0.7621 | |
| c | 410.43 | |
| T/K | ΔG 0 (kJ/mol) | ΔH 0 (kJ/mol) | ΔS 0 (J/mol) |
|---|---|---|---|
| 293 K | −5.94 | −33.2 | −93.01 |
| 313 K | −4.05 | ||
| 333 K | −2.23 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhang, G.; Li, Y. Preparation of Chitosan/Polyacrylamide/Graphene Oxide Composite Membranes and Study of Their Methylene Blue Adsorption Properties. Materials 2020, 13, 4407. https://doi.org/10.3390/ma13194407
Wang Z, Zhang G, Li Y. Preparation of Chitosan/Polyacrylamide/Graphene Oxide Composite Membranes and Study of Their Methylene Blue Adsorption Properties. Materials. 2020; 13(19):4407. https://doi.org/10.3390/ma13194407
Chicago/Turabian StyleWang, Zheqi, Guohua Zhang, and Yanhui Li. 2020. "Preparation of Chitosan/Polyacrylamide/Graphene Oxide Composite Membranes and Study of Their Methylene Blue Adsorption Properties" Materials 13, no. 19: 4407. https://doi.org/10.3390/ma13194407
APA StyleWang, Z., Zhang, G., & Li, Y. (2020). Preparation of Chitosan/Polyacrylamide/Graphene Oxide Composite Membranes and Study of Their Methylene Blue Adsorption Properties. Materials, 13(19), 4407. https://doi.org/10.3390/ma13194407




