High-Voltage Lithium-Ion Battery Using Substituted LiCoPO4: Electrochemical and Safety Performance of 1.2 Ah Pouch Cell
Abstract
:1. Introduction
2. Experimental
2.1. Full Cell Fabrication and Electrochemical Tests
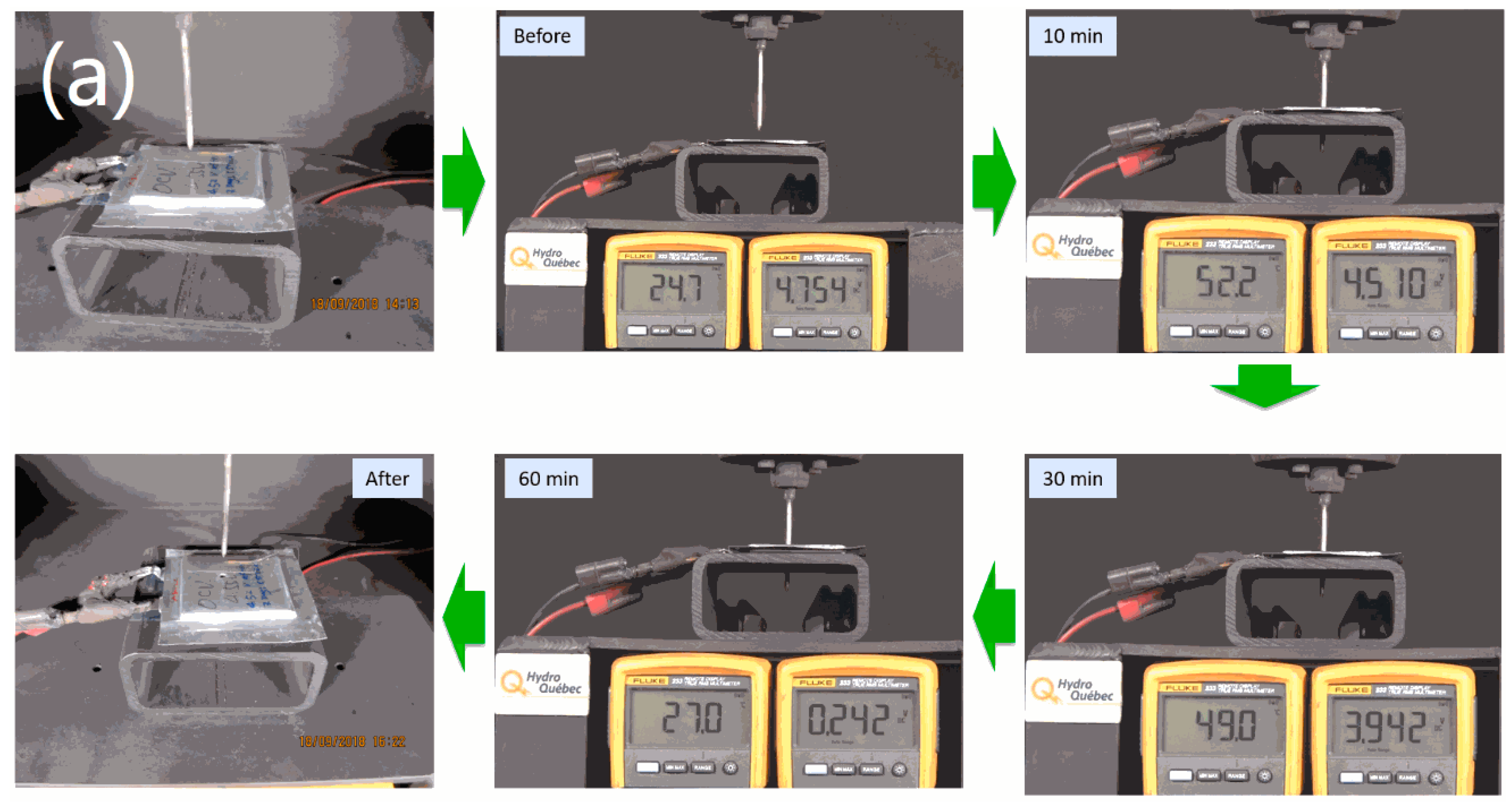
2.2. Safety Tests
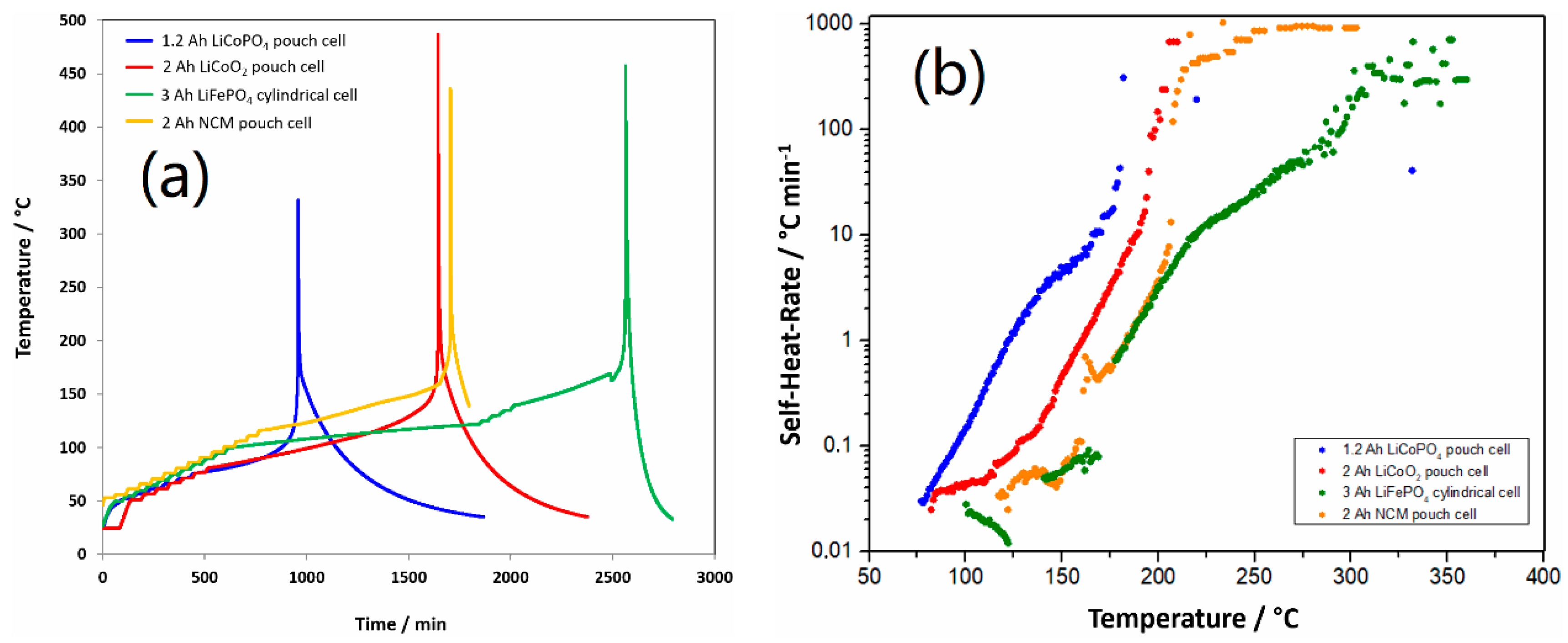
2.3. Accelerating Rate Calorimetry
2.4. Thermogravimetric Analysis and Differential Scanning Calorimetry
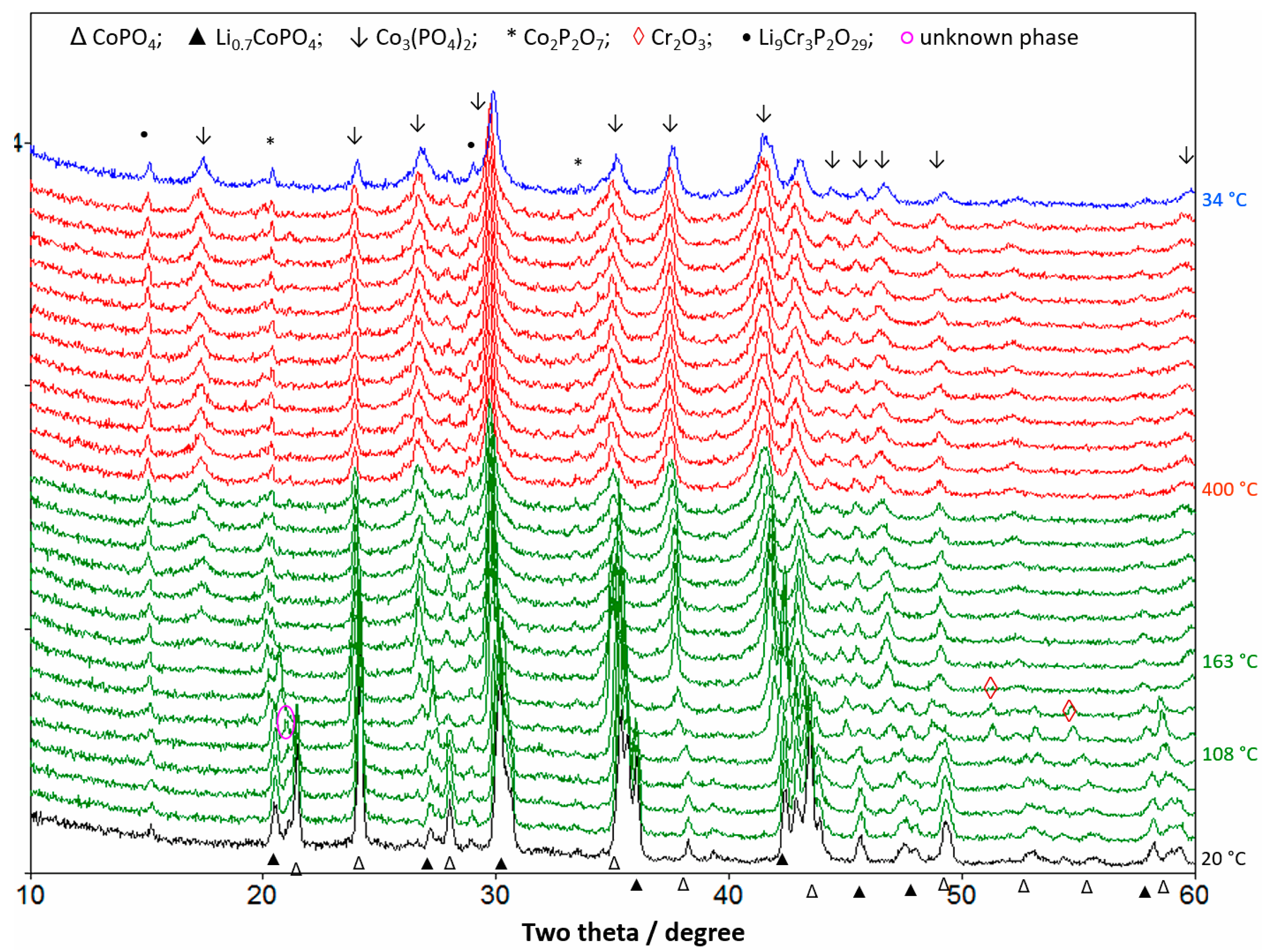
2.5. In Situ X-ray Diffraction Analysis
3. Results and Discussion
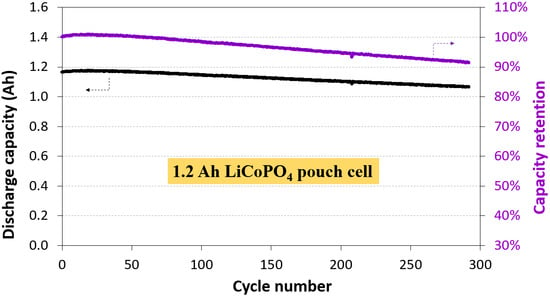
3.1. Electrochemical Performance of 1.2 Ah LCP Pouch Cell
3.2. Safety Studies of 1.2 Ah LCP Pouch Cell
3.3. Thermal Stability Studies of Substituted LCP Cathode Material
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amine, K.; Yasuda, H.; Yamachi, M. Olivine LiCoPO4 as 4.8 V Electrode Material for Lithium Batteries. Electrochem. Solid-State Lett. 2000, 3, 178–179. [Google Scholar] [CrossRef]
- Wolfenstine, J. Electrical conductivity of doped LiCoPO4. J. Power Sources 2006, 158, 1431–1435. [Google Scholar] [CrossRef]
- Markevich, E.; Sharabi, R.; Gottlieb, H.; Borgel, V.; Fridman, K.; Salitra, G.; Aurbach, D.; Semrau, G.; Schmidt, M.A.; Schall, N.; et al. Reasons for capacity fading of LiCoPO4 cathodes in LiPF6 containing electrolyte solutions. Electrochem. Commun. 2012, 15, 22–25. [Google Scholar] [CrossRef]
- Oh, S.M.; Myung, S.T.; Sun, Y.K. Olivine LiCoPO4–carbon composite showing high rechargeable capacity. J. Mater. Chem. 2012, 22, 14932–14937. [Google Scholar] [CrossRef]
- Li, H.H.; Jin, J.; Wei, J.P.; Zhou, Z.; Yan, J. Fast synthesis of core-shell LiCoPO4/C nanocomposite via microwave heating and its electrochemical Li intercalation performances. Electrochem. Commun. 2009, 11, 95–98. [Google Scholar] [CrossRef]
- Liu, J.; Conry, T.E.; Song, X.; Yang, L.; Doeff, M.M.; Richardson, T.J. Spherical nanoporous LiCoPO4/C composites as high performance cathode materials for rechargeable lithium-ion batteries. J. Mater. Chem. 2011, 21, 9984–9987. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Read, J.; Allen, J.L. Effect of carbon on the electronic conductivity and discharge capacity LiCoPO4. J. Power Sources 2007, 163, 1070–1073. [Google Scholar] [CrossRef]
- Zhang, M.; Garcia-Araez, N.; Hector, A.L. Understanding and development of olivine LiCoPO4 cathode materials for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 14483–14517. [Google Scholar] [CrossRef]
- Allen, J.L.; Allen, J.L.; Thompson, T.; Delp, S.A.; Wolfenstine, J.; Jow, T.R. Cr and Si Substituted-LiCo0.9Fe0.1PO4: Structure, full and half Li-ion cell performance. J. Power Sources 2016, 327, 229–234. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, W.; Kim, C.; Cho, M.; Guerfi, A.; Delp, S.A.; Allen, J.L.; Jow, T.R.; Zaghib, K. High-energy lithium-ion battery using substituted LiCoPO4: From coin type to 1 Ah cell. J. Power Sources 2018, 388, 52–56. [Google Scholar] [CrossRef]
- Guerfi, A.; Dontigny, M.; Kobayashi, Y.; Vijh, A.; Zaghib, K. Investigations on some electrochemical aspects of lithium-ion ionic liquid/gel polymer battery systems. J. Solid State Electrochem. 2009, 12, 1003–1014. [Google Scholar] [CrossRef]
- Xu, J.; Chou, S.-L.; Avdeev, M.; Sale, M.; Liu, H.-K.; Dou, S.-X. Lithium rich and deficient effects in LixCoPO4 (x = 0.90, 0.95, 1, 1.05) as cathode material for lithium-ion batteries. Electrochim Acta 2013, 88, 865–870. [Google Scholar] [CrossRef]
- Lecce, D.D.; Brutti, S.; Panero, S.; Hassoun, J. A new Sn-C/LiFe0.1Co0.9PO4 full lithium-ion cell with ionic liquid-based electrolyte. Mater. Lett. 2015, 139, 329–332. [Google Scholar] [CrossRef]
- Balducci, A. Ionic Liquids in Lithium-Ion Batteries. Top Curr. Chem. (Z) 2017, 375, 20. [Google Scholar] [CrossRef]
- Lapping, J.G.; Borkiewicz, O.J.; Wiaderek, K.M.; Allen, J.L.; Jow, T.R.; Cabana, J. Structural Changes and Reversibility Upon Deintercalation of Li from LiCoPO4 Derivatives. ACS Appl. Mater. Interfaces 2020, 12, 20570–20578. [Google Scholar] [CrossRef]
- Guerfi, A.; Duchesne, S.; Kobayashi, Y.; Vijh, A.; Zaghib, K. LiFePO4 and graphite electrodes with ionic liquids based on bis(fluorosulfonyl)imide (FSI)− for Li-ion batteries. J. Powers Sources 2008, 175, 866–873. [Google Scholar] [CrossRef]
- Hou, Y.; Chang, K.; Li, B.; Tang, H.; Wang, Z.; Zou, J.; Yuan, H.; Lu, Z.; Chang, Z. Highly [010]-oriented self-assembled LiCoPO4/C nanoflakes as high-performance cathode for lithium ion batterie. Nano Res. 2018, 11, 2424–2435. [Google Scholar] [CrossRef]
- Manzi, J.; Brutti, S. Surface chemistry on LiCoPO4 electrodes in lithium cells: SEI formation and self-discharge. Electrochim. Acta 2016, 222, 1839–1846. [Google Scholar] [CrossRef]
- Okita, N.; Kisu, K.; Iwama, E.; Sakai, Y.; Lim, Y.; Takami, Y.; Sougrati, M.T.; Brousse, T.; Rozier, P.; Simon, P.; et al. Stabilizing the Structure of LiCoPO4 Nanocrystals via Addition of Fe3+: Formation of Fe3+ Surface Layer, Creation of Diffusion-Enhancing Vacancies, and Enabling High-Voltage Battery Operation. Chem. Mater. 2018, 30, 6675–6683. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, C.-Y.; Tang, X. Cycling degradation of an automotive LiFePO4 lithium-ion battery. J. Power Sources 2011, 196, 1513–1520. [Google Scholar] [CrossRef]
- Wu, X.; Meledina, M.; Barthel, J.; Liu, Z.; Tempel, H.; Kungl, H.; Mayer, J.; Eichel, R.-A. Investigation of the Li–Co antisite exchange in Fe-substituted LiCoPO4 cathode for high-voltage lithium ion batteries. Energy Storage Mater. 2019, 22, 138–146. [Google Scholar] [CrossRef]
- Zhou, Q.; Henderson, W.A.; Appetecchi, G.B.; Montanino, M.; Passerini, S. Physical and Electrochemical Properties of N-Alkyl-N-methylpyrrolidinium Bis(fluorosulfonyl)imide Ionic Liquids: PY13FSI and PY14FSI. J. Phys. Chem. B 2008, 112, 13577–13580. [Google Scholar] [CrossRef] [PubMed]
- Perea, A.; Paolella, A.; Dubé, J.; Champagne, D.; Mauger, A.; Zaghib, K. State of charge influence on thermal reactions and abuse tests in commercial lithium-ion cells. J. Power Source 2018, 399, 392–397. [Google Scholar] [CrossRef]
- Zaghib, K.; Dontigny, M.; Perret, P.; Guerfi, A.; Ramanathan, M.; Prakash, J.; Mauger, A.; Julien, C.M. Electrochemical and thermal characterization of lithium titanate spinel anode in C–LiFePO4//C–Li4Ti5O12 cells at sub-zero temperatures. J. Power Sources 2014, 248, 1050–1057. [Google Scholar] [CrossRef]
- Ouyang, Z.-W.; Chen, E.-C.; Wu, T.-M. Thermal Stability and Magnetic Properties of Polyvinylidene Fluoride/Magnetite Nanocomposites. Materials 2015, 8, 4553–4564. [Google Scholar] [CrossRef]
- Bramnik, N.N.; Nikolowski, K.; Trots, D.M.; Ehrenberg, H. Thermal Stability of LiCoPO4 Cathodes. Electrochem. Solid-State Lett. 2008, 11, A89–A93. [Google Scholar] [CrossRef]
- Cottrell, T.L. The Strengths of Chemical Bonds, 2nd ed.; Butterworth: London, UK, 1958. [Google Scholar]
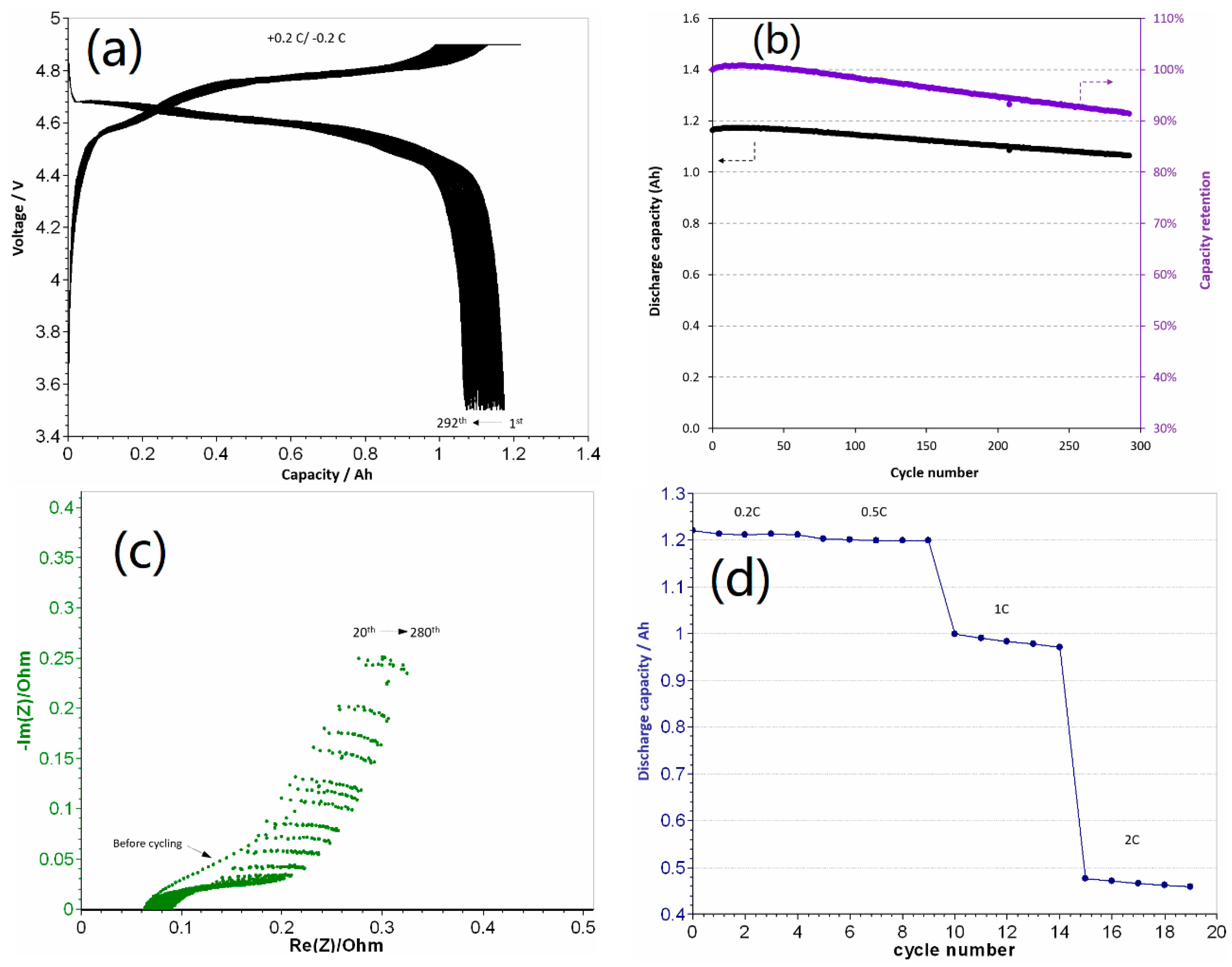



| Sn-C/Doped-LCP | Gr/Doped-LCP | LTO/LCP | Gr/Doped-LCP | |
|---|---|---|---|---|
| Electrolyte | 0.2 mol Kg−1 LiTFSI/Pyr12TFSI | 1.2 M LiPF6/EC-EMC | 1.0 M LiPF6/EC-DMC-DEC | 1.2 M LiFSI/Py13FSI |
| Cell type | Coin cell | Coin cell | Coin cell | Pouch cell |
| C-rate | 0.05 | 0.33 | 0.1 | 0.2 |
| Specific capacity (mAh g−1) | 90 | 120 | 144 | 120 |
| Cycle number | 7 | 250 | 100 | 290 |
| Capacity retention (%) | 83 | >80 | 93.1 | 91 |
| Ref | 13 | 9 | 18 | This work |
| Cell | Onset Temp. (°C) | Thermal Runaway Temp. (°C) | Max. Temp. (°C) |
|---|---|---|---|
| 1.2 Ah LCP | 77 | 168 | 330 |
| 2 Ah LCO | 82 | 185 | 485 |
| 2 Ah NCM | 117 | 204 | 436 |
| 3 Ah LFP | 106 | 219 | 455 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Kim, C.; Perea, A.; Joël, D.; Zhu, W.; Collin-Martin, S.; Forand, A.; Dontigny, M.; Gagnon, C.; Demers, H.; et al. High-Voltage Lithium-Ion Battery Using Substituted LiCoPO4: Electrochemical and Safety Performance of 1.2 Ah Pouch Cell. Materials 2020, 13, 4450. https://doi.org/10.3390/ma13194450
Liu D, Kim C, Perea A, Joël D, Zhu W, Collin-Martin S, Forand A, Dontigny M, Gagnon C, Demers H, et al. High-Voltage Lithium-Ion Battery Using Substituted LiCoPO4: Electrochemical and Safety Performance of 1.2 Ah Pouch Cell. Materials. 2020; 13(19):4450. https://doi.org/10.3390/ma13194450
Chicago/Turabian StyleLiu, Dongqiang, Chisu Kim, Alexis Perea, Dubé Joël, Wen Zhu, Steve Collin-Martin, Amélie Forand, Martin Dontigny, Catherine Gagnon, Hendrix Demers, and et al. 2020. "High-Voltage Lithium-Ion Battery Using Substituted LiCoPO4: Electrochemical and Safety Performance of 1.2 Ah Pouch Cell" Materials 13, no. 19: 4450. https://doi.org/10.3390/ma13194450
APA StyleLiu, D., Kim, C., Perea, A., Joël, D., Zhu, W., Collin-Martin, S., Forand, A., Dontigny, M., Gagnon, C., Demers, H., Delp, S., Allen, J., Jow, R., & Zaghib, K. (2020). High-Voltage Lithium-Ion Battery Using Substituted LiCoPO4: Electrochemical and Safety Performance of 1.2 Ah Pouch Cell. Materials, 13(19), 4450. https://doi.org/10.3390/ma13194450