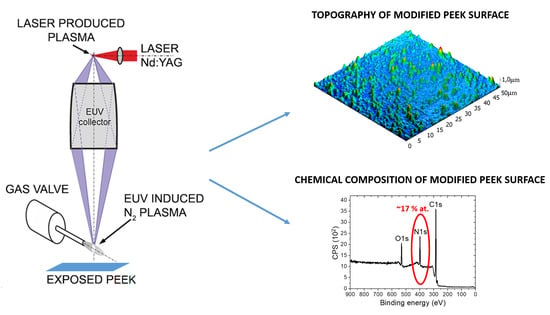
Physico-Chemical Surface Modifications of Polyetheretherketone (PEEK) Using Extreme Ultraviolet (EUV) Radiation and EUV-Induced Nitrogen Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Modifications of PEEK Films
2.2. Surface Analysis of Modified PEEK Surfaces
2.2.1. Atomic Force Microscopy (AFM) and Scanning Electron Microscopy (SEM)
2.2.2. X-ray Photoelectron Spectroscopy (XPS)
3. Results and Discussion
3.1. Morphological Changes on PEEK Surfaces
3.2. Chemical Analysis of Pristine and Modified PEEK Surfaces
- C*–COO (C4)—at ~285.4 eV (FWHM 1.3–1.4 eV)—corresponds to secondary chemical shifts. This is most likely a result of breaking the bonds between atoms marked as 3 and 2 (Figure 5), linking vicinal carbon rings.
- O–C*–O (C6)—at ~287.3 eV (FWHM 1.4–1.5 eV)—is most likely the effect of breaking the bond between the carbon marked as 3 (Figure 5) and the carbon ring. The empty bond is then filled with an oxygen atom.
- C*=O(OH) (C8)—at ~288.8–288.9 eV (FWHM ~1.5 eV)—is most likely formed as a result of breaking the bond between the atom marked as 3 (Figure 5) and the carbon ring and then attaching the OH group to the carbon atom marked as 3.
- C–O*H (O3)—at ~531.9 eV (FWHM 1.4–1.5 eV)—is most probably associated with the presence of OH groups within the chemical structure of the modified PEEK, as this peak corresponds to the peak C8 and fills the envelopes of the O1s band between O1 and O2 peaks modeled for the unmodified PEEK material.
- OH (water) (O5)—534.2–534.5 eV (FWHM 1.6–1.7 eV)—this peak most likely comes from OH groups of the water adsorbed on the sample surface after taking it out from the vacuum.
- C*–N (C5)—at 286.3–286.4 eV (FWHM ~1.5 eV)—peak related to the incorporation of nitrogen atoms into the PEEK structure as a result of breaking bonds of the carbon marked as 2 or 3 (Figure 5) and introducing nitrogen atoms in that place.
- N–C*=O (C7)—at 288.4–288.5 eV (FWHM 1.5 eV)—is formed most likely as a result of carbon bond breaking (carbon marked as 3—Figure 5) and filling it with a nitrogen atom.
- C*–OON (C9)—at 289.3–289.5 eV (FWHM 1.5 eV)—due to a huge chemical shift of this peak relative to the C1 peak, it was assumed that this structure most probably contains two oxygen atoms and a single nitrogen atom. The proof for the presence of this peak is a huge change in the shape of the C1s’ envelope around the binding energy values of 289.3–289.5 eV. Such change was not observed for the samples treated with EUV radiation only. Taking into account the location of this peak on the energy axis and the fact that the oxygen content in the samples modified with EUV irradiation in the presence of nitrogen does not exceed the content in the samples modified with EUV irradiation alone, there is a low probability that this peak represents structures containing oxygen only.
- N*–C (N1)—at 399.2–399.4 eV (FWHM 1.9–2.1 eV)—peak corresponds to C5
- N*–C=O (N2)—at 400.0–400.2 eV (FWHM 1.8–1.9 eV)—peak corresponds to C7
- N*–x (N3)—at 400.8–400.1 eV (FWHM 1.8–2.1 eV)—it is difficult to interpret this peak unambiguously. However, taking into account the measurements of the reference polymers containing nitrogen atoms, such as PU, Kapton, nylon, confronted with the data taken from literature, it can be assumed that these are nitrogen atoms bonded with carbon atoms which form chemical bonds with at least two oxygen atoms.
- N–C=O* (O4)—at 532.1–532.2 eV (FWHM ~1.5–1.6 eV)—peak corresponds to C7.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, R.; Tang, S.; Tan, H.; Qian, J.; Lin, W.; Wang, Y.; Liu, C.; Wei, J.; Tang, T. Preparation, characterization, in vitro bioactivity, and cellular responses to a polyetheretherketone bioactive composite containing nanocalcium silicate for bone repair. ASC Appl. Mater. Interfaces 2014, 6, 12214–12225. [Google Scholar] [CrossRef]
- Kobayashi, T.; Rikukawa, M.; Sanui, K.; Ogata, N. Proton-conducting polymers derived from poly(ether-etherketone) and poly(4-phenoxybenzoyl-1,4-phenylene). Solid State Ion. 1998, 106, 219–225. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [Green Version]
- Toth, J.M.; Wang, M.; Estes, B.T.; Scifert, J.L.; Seim, H.B.; Turner, A.S. Poletheretherketone as a biomaterial for spinal applications. Biomaterials 2006, 27, 324–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reisnger, B.; Fahrner, M.; Frischauf, I.; Yakunin, S.; Švorčík, V.; Fiedorowicz, H.; Bartnik, A.; Romanin, C.; Heitz, J. EUV micropatterning for biocompatibility control of PET. Appl. Phys. A 2010, 100, 511–516. [Google Scholar] [CrossRef]
- Jacobs, T.; Declercq, H.; De Geyter, N.; Cornelissen, R.; Dubruel, P.; Leys, C.; Beaurain, A.; Payen, E.; Morent, R. Plasma surface modification of polylactic acid to promote interaction with fibroblasts. J. Mater. Sci.: Mater. Med. 2013, 24, 469–478. [Google Scholar] [CrossRef]
- Slepička, P.; Slepičková Kasálková, N.; Pinkner, A.; Sajdl, P.; Kolská, Z.; Švorčík, V. Plasma induced cytocompatibility of stabilized poly-L-lactic acid doped with graphene nanoplatelets. React. Funct. Polym. 2018, 131, 266–275. [Google Scholar] [CrossRef]
- Ul Ahad, I.; Butruk, B.; Ayele, M.; Budner, B.; Bartnik, A.; Fiedorowicz, H.; Ciach, T.; Brabazon, D. Extreme ultraviolet (EUV) surface modification of polytetrafluoroethylene (PTFE) for control of biocompatibility. Nucl. Instrum. Methods Phys. Res. B 2015, 364, 98–107. [Google Scholar] [CrossRef] [Green Version]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurf. Biotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef] [Green Version]
- Czwartos, J.; Budner, B.; Bartnik, A.; Kasprzycka, W.; Fiedorowicz, H. Effect of photoionized plasma and EUV induced surface modification on physico-chemical properties and cytocompatibility of PLLA. Express Polym. Lett. 2020, 14, 1063–1077. [Google Scholar] [CrossRef]
- Bai, J.; Ding, R.; Wang, Y.; Chen, T.; Xu, Q.; Feng, B.; Duan, K.; Zhi, W.; Weng, J.; Wang, J. Surface modification of polyetheretherketone by grafting amino groups to improve its hydrophilicity and cytocompatibility. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Wang, W.; Luo, C.J.; Huang, J.; Edirisinghe, M. PEEK surface modification by fast ambient-temperature sulfonation for bone implant applications. J. R. Soc. Interface 2019, 16. [Google Scholar] [CrossRef] [Green Version]
- Riveiro, A.; Soto, R.; Comesana, R.; Boutinguiza, M.; del Val, J.; Quintero, F.; Lusquinos, F.; Pou, J. Laser surface modification of PEEK. Appl. Surf. Sci. 2012, 258, 9437–9442. [Google Scholar] [CrossRef]
- Wilson, A.; Jones, I.; Salmat-Zadeh, F.; Watts, J.F. Laser surface modification of poly(etheretherketone) to enhance surface free energy, wettability and adhesion. Int. J. Adhes. Adhes. 2015, 62, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Deng, Y.; Yang, L.; Shi, X.; Yang, W.; Chen, Z. Enhanced antibacterial property and osteo-differentiation activity on plasma treated porous polyetheretherketone with hierarchical micro/nano-topography. J. Biomater. Sci. Polym. Ed. 2018, 29, 520–542. [Google Scholar] [CrossRef]
- Briem, D.; Strametz, S.; Schröder, K.; Meenen, N.M.; Lehmann, W.; Linhart, W.; Ohl, A.; Rueger, M.J. Response of primary fibroblasts and osteoblasts to plasma treated polyetheretherketone (PEEK) surfaces. J. Mater. Sci.: Mater. Med. 2005, 16, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Waser-Althaus, J.; Salamon, A.; Waser, M.; Podeste, C.; Kreutzer, M.; Pieles, U.; Müller, B.; Peters, K. Differentiation of human mesenchymal stem cells on plasma-treated polyetheretherketone. J. Mater. Sci.: Mater. Med. 2014, 25, 515–525. [Google Scholar] [CrossRef]
- Novotna, Z.; Reznickova, A.; Rimpelova, S.; Vesely, M.; Kolska, Z.; Svorcik, V. Tailoring of PEEK bioactivity for improved cell interaction: Plasma treatment in action. RSC Adv. 2015, 5, 41428–41436. [Google Scholar] [CrossRef] [Green Version]
- Wiącek, A.E.; Terpiłowski, K.; Jurak, M.; Worzakowska, M. Effect of low-temperature plasma on chitosan-coated PEEK polymer characteristics. Eur. Polym. J. 2016, 78, 1–13. [Google Scholar] [CrossRef]
- Gravis, D.; Poncin-Epaillard, F.; Coulon, J.-F. Correlation between the surface chemistry, the surface free energy and the adhesion of metallic coatings onto plasma-treated Poly(ether ether ketone). Appl. Surf. Sci. 2020, 501. [Google Scholar] [CrossRef]
- Dupuis, A.; Ho, T.H.; Fahs, A.; Lafabrier, A.; Louarn, G.; Bacharouche, J.; Airoudj, A.; Aragon, E.; Chailan, J.-F. Improving adhesion of powder coating on PEEK composite: Influence of atmospheric plasma parameters. Appl. Surf. Sci. 2015, 357, 1196–1204. [Google Scholar] [CrossRef]
- Fedel, M.; Micheli, V.; Thaler, M.; Awaja, F. Effect of nitrogen plasma treatment on the crystallinity and self-bonding of polyetheretherketone (PEEK) for biomedical applications. Polym. Adv. Technol. 2020, 31, 240–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartnik, A.; Fiedorowicz, H.; Jarocki, R.; Kostecki, J.; Szczurek, M.; Wachulak, P.W. Laser-plasma EUV source dedicated for surface processing of polymers. Nucl. Instrum. Methoods Phys. Res. A 2011, 647, 125–131. [Google Scholar] [CrossRef]
- Bartnik, A.; Skrzeczanowski, W.; Fiedorowicz, H.; Wachulak, P.; Fok, T. Low-temperature plasmas induced in nitrogen by extreme ultraviolet (EUV) pulses. Laser Part. Beams 2018, 36, 76–83. [Google Scholar] [CrossRef]
- Bartnik, A.; Lisowski, W.; Sobczak, J.; Wachulak, P.; Budner, B.; Korczyc, B.; Fiedorowicz, H. Simultaneous treatment of polymer surface by EUV radiation and ionized nitrogen. Appl. Phys. A 2012, 109, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Bäerle, D. Laser Processing and Chemistry, 4th ed.; Springer: Berlin, Germany, 2011. [Google Scholar]
- Lippert, T.; Dickinson, T. Chemical and Spectroscopic Aspects of Polymer Ablation: Special Features and Novel Directions. Chem. Rev. 2003, 103, 453–485. [Google Scholar] [CrossRef]
- Murthy, N.S.; Prabhu, R.D.; Martin, J.J.; Zhou, L.; Headrick, R.L. Self-assembled and etched cones on laser ablated polymer surfaces. J. Appl. Phys. 2006, 100, 023538:1–023538:12. [Google Scholar] [CrossRef]
- Bartnik, A.; Fiedorowicz, H.; Jarocki, R.; Kostecki, J.; Rakowski, R.; Szczurek, A.; Szczurek, M. Micro- and Nanoprocessing of Polymers Using a Laser Plasma Extreme Ultraviolet Source. Acta Phys. Pol. A 2010, 117, 384–390. [Google Scholar] [CrossRef]
- Bartnik, A.; Fiedorowicz, H.; Jarocki, R.; Kostecki, J.; Szczurek, M. PMMA and FEP surface modifications induced with EUV pulses in two selected wavelength ranges. Appl. Phys. A 2010, 98, 61–65. [Google Scholar] [CrossRef]
- Bartnik, A.; Fiedorowicz, H.; Jarocki, R.; Kostecki, J.; Szczurek, M.; Bilinski, A.; Chernyayeva, O.; Sobczak, J.W. Physical and chemical modifications of PET surface using a laser-plasma EUV source. Appl. Phys. A 2010, 99, 831–836. [Google Scholar] [CrossRef]
- Bartnik, A.; Fiedorowicz, H.; Burdyńska, S.; Jarocki, R.; Kostecki, J.; Szczurek, M. Combined effect of EUV irradiation and acetone treatment on PET surface. Appl. Phys. A 2011, 103, 173–178. [Google Scholar] [CrossRef]
- Hopp, B.; Bor, Z.; Homolya, E.; Mihalik, E. Investigation of conical structures created by ArF excimer laser irradiation of polycarbonate. Appl. Surf. Sci. 1997, 109, 232–235. [Google Scholar] [CrossRef]








| Symbol of the Peak | Chemical Group | Position (eV) | FWHM (eV) | PEEK (at.%) | PEEK EUV40 (at.%) | PEEK EUV150 (at.%) | PEEK EUV40 N2 300 µs (at.%) | PEEK EUV150 N2 300 µs (at.%) | PEEK EUV40 N2 350 µs (at.%) | PEEK EUV150 N2 350 µs (at.%) |
|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C-C=C | 284.8 | 1.3 | 62.3 | 49.8 | 50.0 | 19.6 | 21.3 | 23.2 | 22.5 |
| C2 | C*–O–C* | 286.4 | 1.6–1.7 | 19.9 | 14.6 | 10.5 | 9.9 | 7.4 | 8.5 | 5.0 |
| C3 | C=O | 286.7 | 1.7–1.8 | 4.0 | 2.3 | 1.1 | 0.9 | 1.2 | 2.1 | 2.0 |
| C4 | C*–COO | 285.4 | 1.3–1.4 | - | 17.2 | 22.8 | 46.0 | 37.7 | 26.8 | 22.2 |
| C5 | C*–N | 286.3–286.4 | 1.5 | - | - | - | 1.8 | 5.5 | 7.1 | 8.2 |
| C6 | O–C*–O | 287.3 | 1.4–1.5 | - | 3.0 | 3.8 | 5.3 | 6.6 | 6.3 | 7.0 |
| C7 | N–C*=O | 288.4–288.5 | 1.5 | - | - | - | 1.0 | 1.7 | 2.2 | 3.8 |
| C8 | C*=O(OH) | 288.8–288.9 | 1.5 | - | 1.5 | 2.0 | 1.6 | 0.9 | 1.3 | 0.5 |
| C9 | C*–OON | 289.3–289.5 | 1.5 | - | - | - | 0.0 | 0.6 | 0.9 | 2.2 |
| N1 | N*–C | 399.2–399.4 | 1.9–2.1 | - | - | - | 1.8 | 5.5 | 7.1 | 8.2 |
| N2 | N*–C=O | 400.0–400.2 | 1.8–1.9 | - | - | - | 1.0 | 1.7 | 2.2 | 3.8 |
| N3 | N*–x | 400.8–401.0 | 1.8–2.1 | - | - | - | 0.8 | 1.4 | 1.6 | 5.2 |
| O1 | O*=C | 531.1 | 1.5 | 4.0 | 2.3 | 1.1 | 0.9 | 1.2 | 2.1 | 2.0 |
| O2 | C–O*–C | 533.2 | 1.6 | 9.9 | 7.3 | 5.3 | 5.0 | 3.7 | 4.3 | 2.5 |
| O3 | C–O*H | 531.9 | 1.4–1.5 | - | 1.5 | 2.0 | 1.6 | 1.0 | 1.3 | 0.5 |
| O4 | N–C=O* | 532.1–532.2 | 1.5–1.6 | - | - | - | 1.0 | 1.7 | 2.2 | 3.8 |
| O5 | OH(water) | 534.2–534.5 | 1.6–1.7 | 0.6 | 1.4 | 2.0 | 1.0 | 0.9 | 0.7 |
| Elements | PEEK (at.%) | PEEK EUV40 (at.%) | PEEK EUV150 (at.%) | PEEK EUV40 N2 300 µs (at.%) | PEEK EUV150 N2 300 µs (at.%) | PEEK EUV40 N2 350 µs (at.%) | PEEK EUV150 N2 350 µs (at.%) |
|---|---|---|---|---|---|---|---|
| C | 86.1 | 88.3 | 90.2 | 86.0 | 82.9 | 78.4 | 73.3 |
| N | - | - | - | 3.5 | 8.6 | 10.9 | 17.2 |
| O | 13.9 | 11.7 | 9.8 | 10.5 | 8.5 | 10.8 | 9.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czwartos, J.; Budner, B.; Bartnik, A.; Wachulak, P.; Fiedorowicz, H.; Mierczyk, Z. Physico-Chemical Surface Modifications of Polyetheretherketone (PEEK) Using Extreme Ultraviolet (EUV) Radiation and EUV-Induced Nitrogen Plasma. Materials 2020, 13, 4466. https://doi.org/10.3390/ma13194466
Czwartos J, Budner B, Bartnik A, Wachulak P, Fiedorowicz H, Mierczyk Z. Physico-Chemical Surface Modifications of Polyetheretherketone (PEEK) Using Extreme Ultraviolet (EUV) Radiation and EUV-Induced Nitrogen Plasma. Materials. 2020; 13(19):4466. https://doi.org/10.3390/ma13194466
Chicago/Turabian StyleCzwartos, Joanna, Bogusław Budner, Andrzej Bartnik, Przemysław Wachulak, Henryk Fiedorowicz, and Zygmunt Mierczyk. 2020. "Physico-Chemical Surface Modifications of Polyetheretherketone (PEEK) Using Extreme Ultraviolet (EUV) Radiation and EUV-Induced Nitrogen Plasma" Materials 13, no. 19: 4466. https://doi.org/10.3390/ma13194466
APA StyleCzwartos, J., Budner, B., Bartnik, A., Wachulak, P., Fiedorowicz, H., & Mierczyk, Z. (2020). Physico-Chemical Surface Modifications of Polyetheretherketone (PEEK) Using Extreme Ultraviolet (EUV) Radiation and EUV-Induced Nitrogen Plasma. Materials, 13(19), 4466. https://doi.org/10.3390/ma13194466