Synthesis and Characterization of PLA-Micro-structured Hydroxyapatite Composite Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Hydroxyapatite Particles
2.2. Synthesis of PLA/HA Composite Films
2.3. Characterization of Obtained Materials
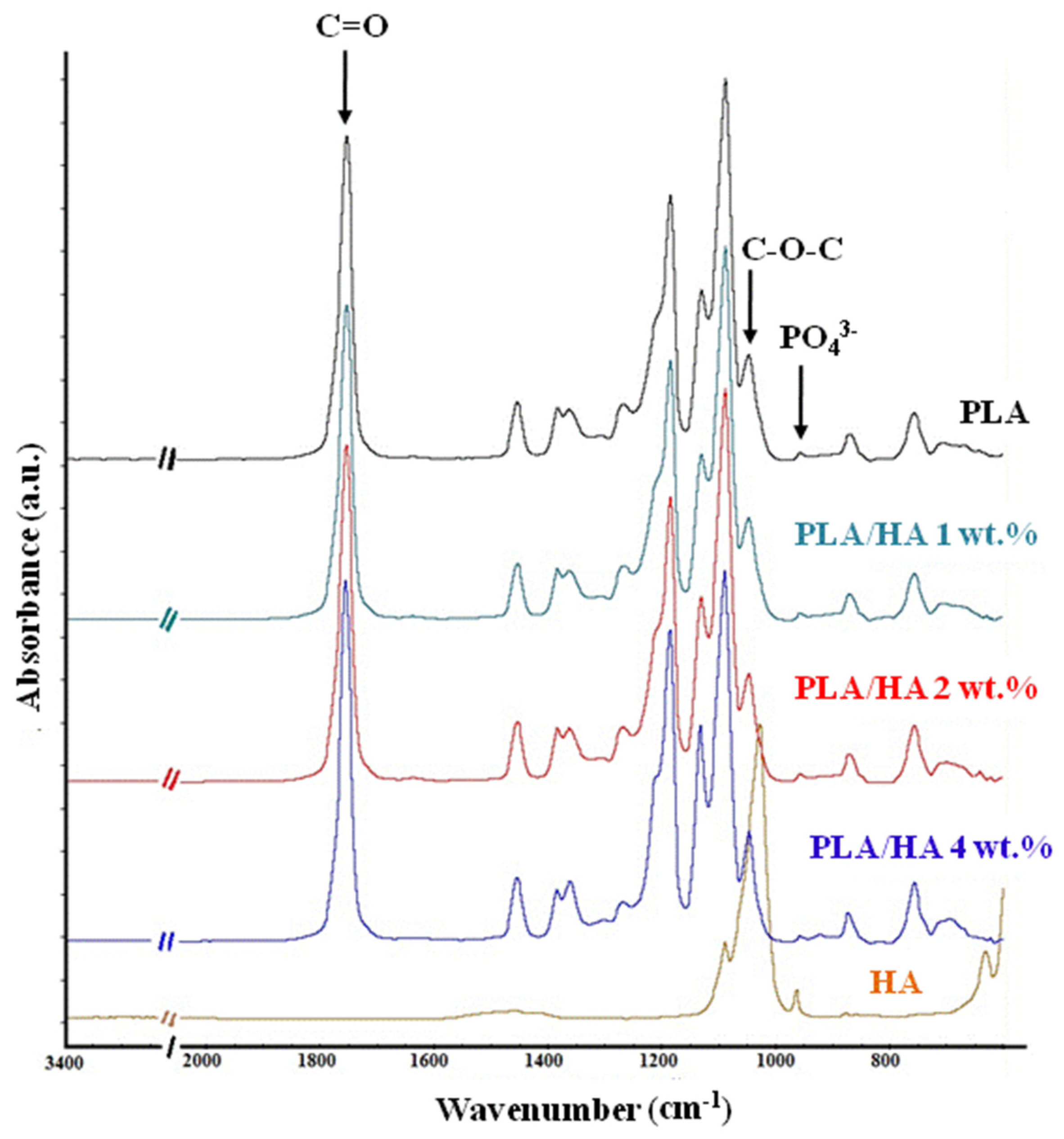
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thakur, V.K.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Miculescu, M.; Thakur, V.K.; Miculescu, F.; Voicu, S.I. Graphene-based polymer nanocomposite membranes: A review. Polym. Adv. Technol. 2016, 27, 844–859. [Google Scholar] [CrossRef]
- Ionita, M.; Crica, L.E.; Voicu, S.I.; Pandele, A.M.; Iovu, H. Fabrication of Cellulose Triacetate/Graphene Oxide Porous Membrane. Polym. Adv. Technol. 2016, 27, 350–357. [Google Scholar] [CrossRef]
- Ionita, M.; Vasile, E.; Crica, L.E.; Voicu, S.I.; Pandele, A.M.; Dinescu, S.; Predoiu, L.; Galateanu, B.; Hermenean, A.; Costache, M. Synthesis, characterization and in vitro studies of polysulfone/graphene oxide composite membranes. Compos. Part B Eng. 2015, 72, 108–115. [Google Scholar] [CrossRef]
- Neacsu, P.; Staras, A.I.; Voicu, S.I.; Ionascu, I.; Soare, T.; Uzun, S.; Cojocaru, V.D.; Pandele, A.M.; Croitoru, S.M.; Miculescu, F.; et al. Characterization and In Vitro and In Vivo Assessment of a Novel Cellulose Acetate-Coated Mg-Based Alloy for Orthopedic Applications. Materials 2017, 10, 686. [Google Scholar] [CrossRef] [PubMed]
- Pandele, A.M.; Neacsu, P.; Cimpean, A.; Staras, A.I.; Miculescu, F.; Iordache, A.; Voicu, S.I.; Thakur, V.K.; Toader, O.D. Cellulose acetate membranes functionalized with resveratrol by covalent immobilization for improved Osseointegration. Appl. Surf. Sci. 2018, 438, 2–13. [Google Scholar] [CrossRef]
- Voicu, S.I.; Condruz, R.M.; Mitran, V.; Cimpean, A.; Miculescu, F.; Andronescu, C.; Miculescu, M.; Thakur, V.K. Sericin Covalent Immobilization onto Cellulose Acetate Membranes. ACS Sustain. Chem. Eng. 2016, 4, 1765–1774. [Google Scholar] [CrossRef]
- Corobea, M.S.; Albu, M.G.; Ion, R.; Cimpean, A.; Miculescu, F.; Antoniac, I.V.; Raditoiu, V.; Sirbu, I.; Stoenescu, M.; Voicu, S.I.; et al. Advanced modification of titanium surface with collagen and doxycycline, a new approach in dental implants. J. Adhes. Sci. Technol. 2015, 29, 2537–2550. [Google Scholar] [CrossRef]
- Pandele, A.M.; Comanici, F.E.; Carp, C.A.; Miculescu, F.; Voicu, S.I.; Thakur, V.K.; Serban, B.C. Synthesis and characterization of cellulose acetate-hydroxyapatite micro and nano composites membranes for water purification and biomedical applications. Vacuum 2017, 146, 599–605. [Google Scholar] [CrossRef]
- Miculescu, F.; Maidaniuc, A.; Voicu, S.I.; Thakur, V.K.; Stan, G.; Ciocan, L.T. Progress in Hydroxyapatite-Starch Based Sustainable Biomaterials for Biomedical Bone Substitution Applications. ACS Sustain. Chem. Eng. 2017, 5, 8491–8512. [Google Scholar] [CrossRef]
- Voicu, S.I.; Pandele, M.A.; Vasile, E.; Rughinis, R.; Crica, L.; Pilan, L.; Ionita, M. The impact of sonication time through polysulfone graphene oxide composite films properties. Dig. J. Nanomater. Biostructures 2013, 8, 1389–1394. [Google Scholar]
- Tábi, T.; Sajó, I.E.; Szabó, F.; Luyt, A.S.; Kovács, J.G. Crystalline structure of annealed polylactic acid and its relation to processing. Express Polym. Lett. 2010, 4, 659–668. [Google Scholar] [CrossRef]
- Voicu, S.I.; Ninciuleanu, C.M.; Muhulet, O.; Miculescu, M. Cellulose acetate membranes with controlled porosity and their use for the separation of amino acids and proteins. J. Optoelectron. Adv. Mater. 2014, 16, 903–908. [Google Scholar]
- Miculescu, M.; Muhulet, A.; Nedelcu, A.; Voicu, S.I. Synthesis and characterization of polysulfone-carbon nanotubes -polyethylene imine composite membranes. Optoelectron. Adv. Mater. Rapid Commun. 2014, 8, 1072–1076. [Google Scholar]
- Russias, J.; Saiz, E.; Nalla, R.K.; Gryn, K.; Ritchie, R.O.; Tomsia, A.P. Fabrication and mechanical properties of PLA/HA composites: A study of in vitro degradation. Mater. Sci. Eng. C 2006, 26, 1289–1295. [Google Scholar] [CrossRef]
- Gong, M.; Zhao, Q.; Dai, L.; Li, Y.; Jiang, T. Fabrication of polylactic acid/hydroxyapatite/graphene oxide composite and their thermal stability, hydrophobic and mechanical properties. J. Asian Ceram. Soc. 2017, 5, 160–168. [Google Scholar] [CrossRef]
- Ma, H.; Su, W.; Tai, Z.; Sun, D.; Yan, X.; Liu, B.; Xue, Q. Preparation and cytocompatibility of polylactic acid/hydroxyapatite/graphene oxide nanocomposite fibrous membrane. Chin. Sci. Bull. 2012, 57, 3051–3058. [Google Scholar] [CrossRef]
- Dumitriu, C.; Voicu, S.I.; Muhulet, A.; Nechifor, G.; Popescu, S.; Ungureanu, C.; Carja, A.; Miculescu, F.; Trusca, R.; Pirvu, C. Cellulose acetate-titanium dioxide nanotubes membrane fraxiparinized through polydopamine. Carbohydr. Polym. 2018, 181, 215–223. [Google Scholar] [CrossRef]
- Muhulet, A.; Miculescu, F.; Voicu, S.I.; Schütt, F.; Thakur, V.K.; Mishra, Y.K. Fundamentals and Scopes of Doped Carbon Nanotubes towards Energy and Biosensing Applications. Mater. Today Energy 2018, 9, 154–186. [Google Scholar] [CrossRef]
- Rakmae, S.; Ruksakulpiwat, Y.; Sutapun, W.; Suppakarn, N. Physical properties and cytotoxicity of surface-modified bovine bone-based hydroxyapatite/poly (lactic acid) composites. J. Compos. Mater. 2011, 45, 1259–1269. [Google Scholar] [CrossRef]
- Azzaoui, K.; Mejdoubi, E.; Lamhamdi, A.; Hammouti, B.; Akartasse, N.; Berrabah, M.; Elidrissi, A.; Jodeh, S.; Hamed, O.; Abidi, N. Novel Tricomponenets composites Films from Polylactic Acid/ Hydroxyapatite/ Poly- Caprolactone Suitable For Biomedical Applications. J. Mater. Environ. Sci. 2016, 7, 761–769. [Google Scholar]
- Monmaturapoj, N.; Srion, A.; Chalermkarnon, P.; Buchatip, S.; Petchsuk, A.; Noppakunmongkolchai, W.; Mai-Ngam, K. Properties of poly(lactic acid)/hydroxyapatite composite through the use of epoxy functional compatibilizers for biomedical application. J. Biomater. Appl. 2017, 32, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chan, K.W.; Shen, J.; Wong, H.M.; Yeung, K.W.K.; Tjong, S.C. Melt-compounded polylactic acid composite hybrids with hydroxyapatite nanorods and silver nanoparticles: Biodegradation, antibacterial ability, bioactivity and cytotoxicity. RSC Adv. 2015, 5, 72288–72299. [Google Scholar] [CrossRef]
- Sanchez-Arevalo, F.M.; Munoz-Ramırez, L.D.; Alvarez-Camacho, M.; Rivera-Torres, F.; Maciel-Cerda, A.; Montiel-Campos, R.; Vera-Graziano, R. Macro- and micromechanical behaviours of poly(lactic acid)–hydroxyapatite electrospun composite scaffolds. J. Mater. Sci. 2017, 52, 3353–3367. [Google Scholar] [CrossRef]
- Salerno, A.; Fernandez-Gutierrez, M.; San Roman del Barrio, J.; Pascual, C.D. Macroporous and nanometre scale fibrous PLA and PLA–HA composite scaffolds fabricated by a biosafe strategy. RSC Adv. 2014, 4, 61491. [Google Scholar] [CrossRef]
- Jeong, S.I.; Ko, E.K.; Yum, J.; Jung, C.H.; Lee, Y.M.; Shin, H. Nanofibrous Poly(lactic acid)/Hydroxyapatite Composite Scaffolds for Guided Tissue Regeneration. Macromol. Biosci. 2008, 8, 328–338. [Google Scholar] [CrossRef]
- Miculescu, F.; Mocanu, A.C.; Dascalu, C.A.; Maidaniuc, A.; Batalu, D.; Berbecaru, A.; Voicu, S.I.; Miculescu, M.; Thakur, V.K.; Ciocan, L.T. Facile synthesis and characterization of hydroxyapatite particles for high value nanocomposites and biomaterials. Vacuum 2017, 146, 614–622. [Google Scholar] [CrossRef]
- Maidaniuc, A.; Miculescu, F.; Voicu, S.I.; Andronescu, C.; Miculescu, M.; Matei, E.; Mocanu, A.C.; Pencea, I.; Csaki, I.; Machedon-Pisu, T.; et al. Induced wettability and surface-volume correlation of composition for bovine bone derived hydroxyapatite particles. Appl. Surf. Sci. 2018, 438, 147–157. [Google Scholar] [CrossRef]
- Maidaniuc, A.; Miculescu, M.; Voicu, S.I.; Ciocan, L.T.; Niculescu, M.; Corobea, M.C.; Rada, M.E.; Miculescu, F. Effect of micron sized silver particles concentration on the adhesion induced by sintering and antibacterial properties of hydroxyapatite microcomposites. J. Adhes. Sci. Technol. 2016, 30, 1829–1841. [Google Scholar] [CrossRef]
- Voicu, S.I.; Dobrica, A.; Sava, S.; Ivan, A.; Naftanaila, L. Cationic surfactants-controlled geometry and dimensions of polymeric membrane pores. J. Optoelectron. Adv. Mater. 2012, 14, 923–928. [Google Scholar]
- Persson, M.; Lorite, G.S.; Cho, S.W.; Tuukkanen, J.; O Skrifvars, M. Melt Spinning of Poly(lactic acid) and Hydroxyapatite Composite Fibers: Influence of Filler Content on the Fiber Properties. ACS Appl. Mater. Interfaces 2013, 5, 6864–6872. [Google Scholar] [CrossRef] [PubMed]
- Cukrowski, I.; Popović, L.; Barnard, W.; Paul, S.O.; van Rooyen, P.H.; Liles, D.C. Modeling and spectroscopic studies of bisphosphonate–bone interactions. The Raman, NMR and crystallographic investigations of Ca–HEDP complexes. Bone 2007, 41, 668–678. [Google Scholar] [CrossRef]
- Bikiaris, D. Can nanoparticles really enhance thermalstability of polymers? Part II: An overview on thermal decomposition of polycondensation polymers. Thermochim. Acta 2011, 523, 25–45. [Google Scholar] [CrossRef]
- Senatov, F.S.; Niaza, K.V.; Zadorozhnyy, M.Y.; Maksimkin, A.V.; Kaloshkin, S.D.; Estrin, Y.Z. Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. J. Mech. Behav. Biomed. Mater. 2016, 57, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Corobea, M.C.; Muhulet, O.; Miculescu, F.; Antoniac, I.V.; Vuluga, Z.; Florea, D.; Vuluga, D.M.; Butnaru, M.; Ivanov, D.; Voicu, S.I.; et al. Novel Nanocomposite Membranes from Cellulose Acetate and Clay-Silica Nanowires. Polym. Adv. Technol. 2016, 27, 1586–1595. [Google Scholar] [CrossRef]
- Satulu, V.; Mitu, B.; Pandele, A.M.; Voicu, S.I.; Kravets, L.; Dinescu, G. Composite polyethylene terephthalate track membranes with thin teflon-likelayers: Preparation and surface properties. Appl. Surf. Sci. 2019, 476, 452–459. [Google Scholar] [CrossRef]
- Miculescu, F.; Ciocan, L.; Miculescu, M.; Ernuteanu, A. Effect of heating process on micro structure level of cortical bone prepared for compositional analysis. Dig. J. Nanomater. Biostruct. 2011, 6, 225–233. [Google Scholar]
- Miculescu, F.; Jepu, I.; Porosnicu, C.; Lungu, C.P.; Miculescu, M.; Burhala, B. A Study on the Influence of the Primary Electron Beam on Nanodimensional Layers Analysis. Dig. J. Nanomater. Biostruct. 2011, 6, 307–317. [Google Scholar]
- Mi, H.Y.; Palumbo, S.; Jiang, S.; Turng, L.S.; Li, W.J.; Peng, X.F. Thermoplastic polyurethane/hydroxyapatite electrospun scaffolds for bone tissue engineering: Effects of polymer properties and particle size. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1434–1444. [Google Scholar] [CrossRef]
- Yuan, Q.; Qin, C.; Wu, J.; Xu, A.; Zhang, Z.; Liao, J.; Lin, S.; Ren, X.; Zhang, P. Synthesis and characterization of Cerium-doped hydroxyapatite/polylactic acid composite coatings on metal substrates. Mater. Chem. Phys. 2016, 182, 365–371. [Google Scholar] [CrossRef]
- Morsi, M.A.; Hezma, A.E.M. Effect of iron doped hydroxyapatite nanoparticles on the structural, morphological, mechanical and magnetic properties of polylactic acid polymer. J. Mater. Res. Technol. 2019, 8, 2098–2106. [Google Scholar] [CrossRef]
- Yuan, Q.; Wu, J.; Qin, C.; Xu, A.; Zhang, Z.; Lin, S.; Ren, X.; Zhang, P. Spin-coating synthesis and characterization of Zn-doped hydroxyapatite/ polylactic acid composite coatings. Surf. Coat. Technol. 2016, 307, 461–469. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Chen, T.; Zhang, N.; Wei, Q.; Tian, J.; Wang, Y.; Ma, C.; Lu, Y. Hydroxyapatite/silver electrospun fibers for anti-infection and osteoinduction. J. Adv. Res. 2020, 21, 91–102. [Google Scholar] [CrossRef]
- Mondal, S.; Nguyen, T.P.; Pham, V.H.; Hoang, G.; Manivasagan, P.; Kim, M.H.; Nam, S.Y.; Oh, J. Hydroxyapatite nano bioceramics optimized 3D printed poly lactic acid scaffold for bone tissue engineering application. Ceram. Int. 2019. [Google Scholar] [CrossRef]
- Cai, X.; Tong, H.; Shen, X.; Chen, W.; Yan, J.; Hu, J. Preparation and characterization of homogeneous chitosan–polylactic acid/hydroxyapatite nanocomposite for bone tissue engineering and evaluation of its mechanical properties. Acta Biomater. 2009, 5, 2693–2703. [Google Scholar] [CrossRef] [PubMed]
- Pietrzykowska, E.; Mukhovskyi, R.; Chodara, A.; Wojnarowicz, J.; Koltsov, I.; Chudoba, T.; Łojkowski, W. Composites of polylactide and nano-hydroxyapatite created by cryomilling and warm isostatic pressing for bone implants applications. Mater. Lett. 2019, 236, 625–628. [Google Scholar] [CrossRef]
- Lopresti, F.; Pavia, F.C.; Vitrano, I.; Kersaudy-Kerhoas, M.; Brucato, V.; La Carrubba, V. Effect of hydroxyapatite concentration and size on morpho-mechanical properties of PLA-based randomly oriented and aligned electrospun nanofibrous mats. J. Mech. Behav. Biomed. Mater. 2020, 101, 103449. [Google Scholar] [CrossRef]
- Prasad, A.; Bhasney, S.M.; Sankar, M.R.; Katiyar, V. Fish Scale Derived Hydroxyapatite reinforced Poly (Lactic acid) Polymeric Bio-films: Possibilities for Sealing/locking the Internal Fixation Devices. Mater. Today Proc. 2017, 4, 1340–1349. [Google Scholar] [CrossRef]
- Tverdokhlebov, S.I.; Bolbasov, E.N.; Shesterikov, E.V.; Antonova, L.V.; Golovkin, A.S.; Matveeva, V.G.; Petlin, D.G.; Anissimov, Y.G. Modification of polylactic acid surface using RF plasma discharge with sputter deposition of a hydroxyapatite target for increased biocompatibility. Appl. Surf. Sci. 2015, 329, 32–39. [Google Scholar] [CrossRef]
- Ma, B.; Han, J.; Zhang, S.; Liu, F.; Wang, S.; Duan, J.; Sang, Y.; Jiang, H.; Li, D.; Ge, S.; et al. Hydroxyapatite nanobelt/polylactic acid Janus membrane with osteoinduction/barrier dual functions for precise bone defect repair. Acta Biomater. 2018, 71, 108–117. [Google Scholar] [CrossRef]
- Talal, A.; Waheed, N.; Al-Masri, M.; McKay, I.J.; Tanner, K.E.; Hughes, F.J. Absorption and release of protein from hydroxyapatite-polylactic acid (HA-PLA) membranes. J. Dent. 2009, 37, 820–826. [Google Scholar] [CrossRef] [PubMed]






| Sample Name | wt.% | Td10% (°C) | Tmax (°C) | Tc | Tm1 | Tm2 | ΔHc (J/g) | ΔHm (J/g) | χc (%) |
|---|---|---|---|---|---|---|---|---|---|
| PLA | 100 | 221 | 352 | 106.9 | 141.4 | 148.4 | 18.99 | 21.82 | 3.02 |
| PLA/HA 1 wt.% | 99 | 226 | 350 | 103.9 | 142.6 | 150.3 | 22.63 | 23.3 | 0.72 |
| PLA/HA 2 wt.% | 98 | 274 | 351 | 104.2 | 142.6 | 150.6 | 22.63 | 23.78 | 1.23 |
| PLA/HA 4 wt.% | 96 | 254 | 351 | 106 | 141.8 | 149.5 | 24.75 | 26.37 | 1.73 |
| Sample Name | Young’s Modulus (MPa) |
|---|---|
| PLA | 17 ± 0.59 |
| PLA/HA 1 wt.% | 17 ± 0.55 |
| PLA/HA 2 wt.% | 15 ± 0.14 |
| PLA/HA 4 wt.% | 14 ± 1.44 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandele, A.M.; Constantinescu, A.; Radu, I.C.; Miculescu, F.; Ioan Voicu, S.; Ciocan, L.T. Synthesis and Characterization of PLA-Micro-structured Hydroxyapatite Composite Films. Materials 2020, 13, 274. https://doi.org/10.3390/ma13020274
Pandele AM, Constantinescu A, Radu IC, Miculescu F, Ioan Voicu S, Ciocan LT. Synthesis and Characterization of PLA-Micro-structured Hydroxyapatite Composite Films. Materials. 2020; 13(2):274. https://doi.org/10.3390/ma13020274
Chicago/Turabian StylePandele, Andreea Madalina, Andreea Constantinescu, Ionut Cristian Radu, Florin Miculescu, Stefan Ioan Voicu, and Lucian Toma Ciocan. 2020. "Synthesis and Characterization of PLA-Micro-structured Hydroxyapatite Composite Films" Materials 13, no. 2: 274. https://doi.org/10.3390/ma13020274
APA StylePandele, A. M., Constantinescu, A., Radu, I. C., Miculescu, F., Ioan Voicu, S., & Ciocan, L. T. (2020). Synthesis and Characterization of PLA-Micro-structured Hydroxyapatite Composite Films. Materials, 13(2), 274. https://doi.org/10.3390/ma13020274








