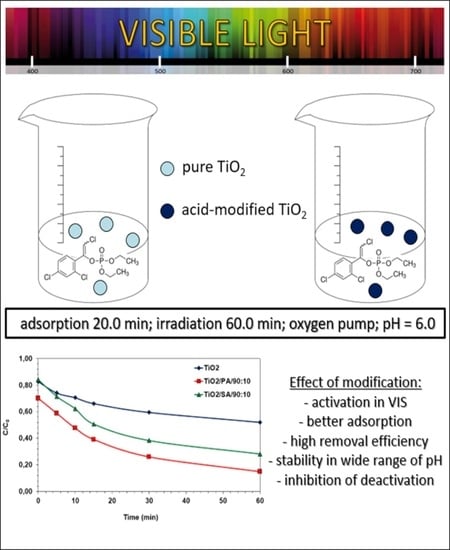
TiO2 Modified with Organic Acids for the Decomposition of Chlorfenvinphos under the Influence of Visible Light: Activity, Performance, Adsorption, and Kinetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Model Solution
2.3. Instrumental Analysis
2.4. Synthesis of Photocatalyst Samples
2.5. Experimental Procedures
2.5.1. Characterization of Photocatalysts Samples
2.5.2. Effect of Different Dose of Catalysts
2.5.3. Optimal Modification of the Catalysts
2.5.4. Optimal Adsorption Time
2.5.5. Effect of pH
2.5.6. Deactivation Tests
2.5.7. Influence of Oxygen
2.5.8. Radical Scavenger Test
2.6. Kinetics
- r—oxidation rate of pollutants, mg min/L
- C—concentration of pollutants, mg/L
- k—reaction rate constant, min−1
- K—constant balance
- t—contact time, min
3. Results of Tests and Their Discussion
3.1. UV-DRS and SEM Test
3.2. Effect of Catalyst Dosage
3.3. Modification of the Catalyst
3.4. Optimal Adsorption Time
3.5. Effect of pH on the Chlorfenvinphos Degradation
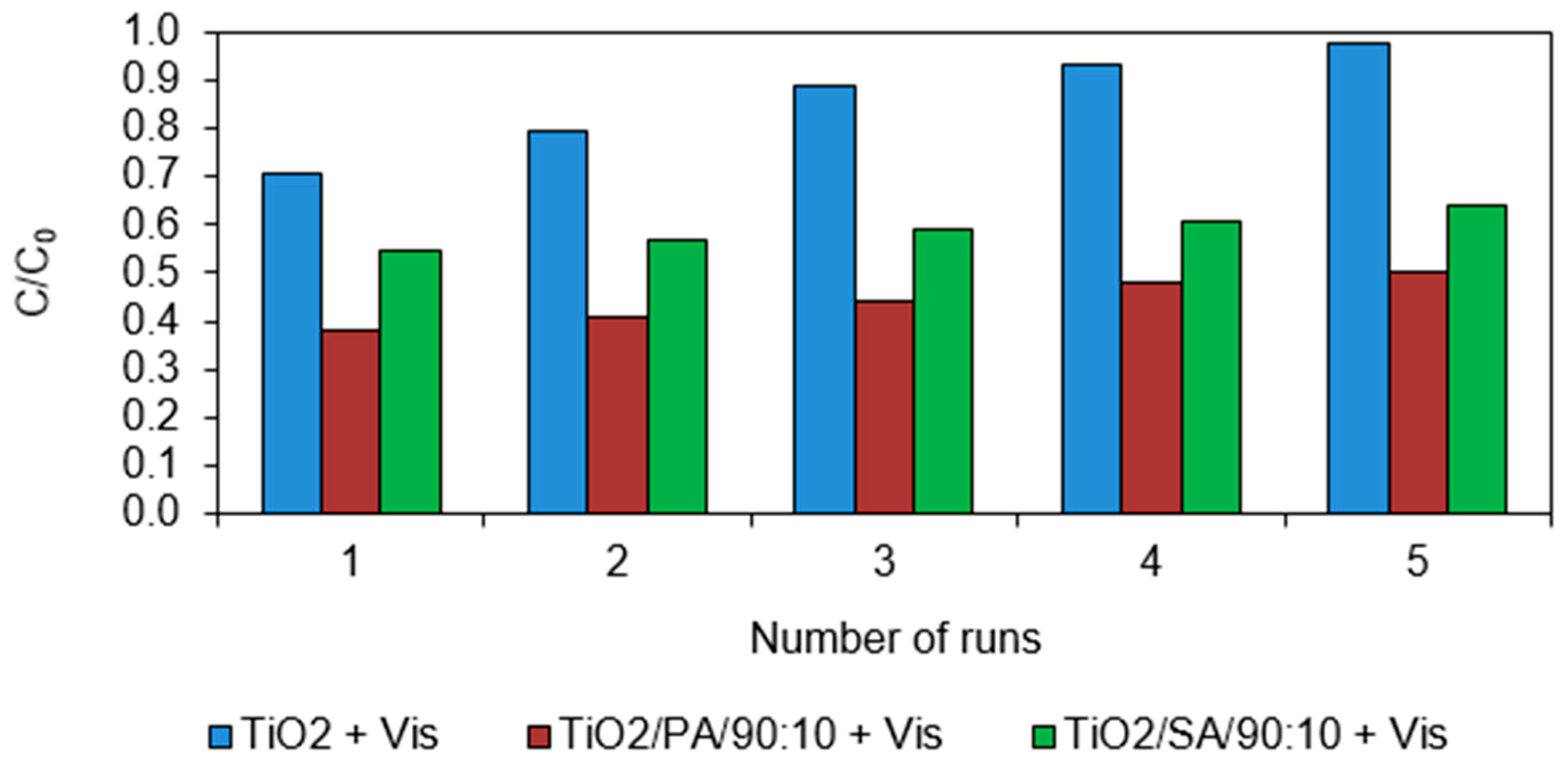
3.6. Lifetime of the Catalysts
3.7. Effect of Oxygen
3.8. Radical Scavengers Test
4. Summary
Funding
Conflicts of Interest
References
- Ravoet, J.; Reybroeck, W.; de Graaf, D.C. Pesticides for apicultural and/or agricultural application found in belgian honey bee wax combs. Bull Env. Contam. Toxicol. 2015, 94, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Laws, E.A. (Ed.) Environmental Toxicology: Selected Entries from the Encyclopedia of Sustainability Science and Technology; Springer-Verlag: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Mahdy, F.M.; El-Maghraby, S.I. Effect of processing on 14C-Chlorfenvinphos residues in maize oil and bioavailability of its cake residues on rats. Bull. Environ. Contam. Toxicol. 2010, 84, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Environment Agency. Welsh Steep Dip Monitoring Programme 1999; Environment Agency Wales: Cardiff, UK, 2000.
- Boxall, A.B.A.; Fogg, L.A.; Blackwell, P.A.; Blackwell, P.; Kay, P.; Pemberton, E.J.; Croxford, A. Veterinary Medicines in the Environment. In Reviews of Environmental Contamination and Toxicology; Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2004; pp. 1–91. [Google Scholar] [CrossRef]
- State Environmental Monitoring, Results of Surface Water Tests-Rivers. 2016. Available online: http://www.katowice.wios.gov.pl/monitoring/informacje/stan2016/wody_pow/rzeki.pdf (accessed on 5 November 2019).
- Ismael, M. Highly effective ruthenium-doped TiO2 nanoparticles photocatalyst for visible-light-driven photocatalytic hydrogen production. New J. Chem. 2019, 43, 9596–9605. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Seery, M.K.; George, R.; Floris, P.; Pillai, S.C. Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J. Photochem. Photobiol. A Chem. 2007, 189, 258–263. [Google Scholar] [CrossRef]
- Xing, B.; Shi, C.; Zhang, C.; Yi, G.; Chen, L.; Guo, H.; Huang, G.; Cao, J. Preparation of TiO2/Activated Carbon Composites for Photocatalytic Degradation of RhB under UV Light Irradiation. Available online: https://www.hindawi.com/journals/jnm/2016/8393648/ (accessed on 20 November 2019).
- Reddy, P.A.L.; Reddy, P.V.L.; Kwon, E.; Kim, K.-H.; Akter, T.; Kalagara, S. Recent advances in photocatalytic treatment of pollutants in aqueous media. Environ. Int. 2016, 91, 94–103. [Google Scholar] [CrossRef]
- Zawadzki, P.; Kudlek, E.; Dudziak, M. Influence of the photocatalyst type and pH of solution on photocatalytic decomposition efficiency of bisphenol A. Proc. ECOpole 2017, 11, 661–669. [Google Scholar] [CrossRef]
- Mert, E.H.; Yalçın, Y.; Kılıç, M.; San, N.; Çınar, Z. Surface modification of TiO2 with ascorbic acid for heterogeneous photocatalysis: theory and experiment. J. Adv. Oxid. Technol. 2016, 11, 199–207. [Google Scholar] [CrossRef]
- D’Amato, C.A.; Giovannetti, R.; Zannotti, M.; Rommozzi, E.; Minicucci, M.; Gunnella, R.; Di Cicco, A. Band gap implications on Nano-TiO2 surface modification with ascorbic acid for visible light-active polypropylene coated photocatalyst. Nanomaterials 2018, 8, 599. [Google Scholar] [CrossRef]
- Li, H.; Liu, B.; Yin, S.; Sato, T.; Wang, Y. Visible light-driven photocatalytic activity of oleic acid-coated TiO2 nanoparticles synthesized from absolute ethanol solution. Nanoscale Res. Lett. 2015, 10, 415. [Google Scholar] [CrossRef]
- Maeda, K.; Kuriki, R.; Ishitani, O. Photocatalytic activity of carbon nitride modified with a Ruthenium(II) complex having carboxylic- or phosphonic acid anchoring groups for visible-light CO2 reduction. Chem. Lett. 2016, 45, 182–184. [Google Scholar] [CrossRef]
- Ma, S.; Gu, J.; Han, Y.; Gao, Y.; Zong, Y.; Ye, Z.; Xue, J. Facile fabrication of C-TiO2 nanocomposites with enhanced photocatalytic activity for degradation of tetracycline. ACS Omega 2019, 4, 21063–21071. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xiao, K.; He, Y.; Zhang, T.; Dong, F.; Du, X.; Zhang, Y. In situ assembly of BiOI@Bi12O17Cl2 P-n junction: Charge induced unique front-lateral surfaces coupling heterostructure with high exposure of BiOI {001} active facets for robust and nonselective photocatalysis. Appl. Catal. B Environ. 2016, 199, 75–86. [Google Scholar] [CrossRef]
- Huang, H.; Li, X.; Wang, J.; Dong, F.; Chu, P.K.; Zhang, T.; Zhang, Y. Anionic group self-doping as a promising strategy: Band-gap engineering and multi-functional applications of high-performance CO32−-Doped Bi2O2CO3. ACS Catal. 2015, 5, 4094–4103. [Google Scholar] [CrossRef]
- Górska, P.; Zaleska, A.; Kowalska, E.; Klimczuk, T.; Sobczak, J.; Skwarek, E.; Janusz, W.; Hupka, J. TiO2 photoactivity in Vis and UV light: The influence of calcination temperature and surface properties. Appl. Catal. B 2008, 84, 440–447. [Google Scholar] [CrossRef]
- International Organization for Standarization. Water Quality—Determination of Selected Plant Treatment Agents—Method Using High Performance Liquid Chromatography with UV Detection After Solid-Liquid Extraction; ISO 11369; International Organization for Standarization: Geneva, Switzerland, 1997. [Google Scholar]
- Lee, J.Y.; Jo, W.-K. Application of ultrasound-aided method for the synthesis of CdS-Incorporated three-dimensional TiO2 photocatalysts with enhanced performance. Ultrason. Sonochem. 2017, 35, 440–448. [Google Scholar] [CrossRef]
- Elhalil, A.; Elmoubarki, R.; Farnane, M.; Machrouhi, A.; Sadiq, M.; Mahjoubi, F.Z.; Qourzal, S.; Barka, N. Photocatalytic degradation of caffeine as a model pharmaceutical pollutant on Mg doped ZnO-Al2O3 heterostructure. Environ. Nanotechnol. Monit. Manag. 2018, 10, 63–72. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous Photocatalysis: State of the art and present applications in honor of Pr. R.L. Burwell Jr. (1912–2003), Former Head of Ipatieff Laboratories, Northwestern University, Evanston (Ill). Top. Catal. 2005, 34, 49–65. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Garg, A.; Singhania, T.; Singh, A.; Sharma, S.; Rani, S.; Neogy, A.; Yadav, S.R.; Sangal, V.K.; Garg, N. Photocatalytic degradation of bisphenol-A using N, Co Codoped TiO2 catalyst under solar light. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Kumar, K.V.; Porkodi, K.; Rocha, F. Langmuir–hinshelwood kinetics—A theoretical study. Catal. Commun. 2008, 9, 82–84. [Google Scholar] [CrossRef]
- Asenjo, N.G.; Santamaría, R.; Blanco, C.; Granda, M.; Álvarez, P.; Menéndez, R. Correct Use of the Langmuir–Hinshelwood equation for proving the absence of a synergy effect in the photocatalytic degradation of phenol on a suspended mixture of Titania and activated carbon. Carbon 2013, 55, 62–69. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.K. Preparation and Study of TiO2/C photocatalysts for Water and Wastewater Treatment. 2012. Available online: https://zbc.ksiaznica.szczecin.pl/dlibra/publication/30931/edition/29344/content (accessed on 5 November 2019).
- Mashtalir, O.; Kurtoglu, M.; Pogulay, S.; Gogotsi, A.; Naguib, M.; Gogotsi, Y. Photocatalytic WO3 and TiO2 films on brass. Int. J. Appl. Ceram. Technol. 2013, 10, 26–32. [Google Scholar] [CrossRef]
- Guo, H.; Lin, K.; Zheng, Z.; Xiao, F.; Li, S. Sulfanilic acid-modified P25 TiO2 nanoparticles with improved photocatalytic degradation on congo red under visible light. Dye. Pigment. 2012, 92, 1278–1284. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C.; Yu, J.C. Photocatalyst TiO2 supported on glass fiber for indoor air purification: Effect of NO on the photodegradation of CO and NO2. J. Photochem. Photobiol. A Chem. 2003, 156, 171–177. [Google Scholar] [CrossRef]
- Maira, A.J.; Yeung, K.L.; Soria, J.; Coronado, J.M.; Belever, C.; Lee, C.Y.; Augugkario, V. Gas-phase photo-oxidation of toluene using nanometer-size TiO2 catalysts. Appl. Catal. B Environ. 2001, 29, 327–336. [Google Scholar] [CrossRef]
- Hsieh, M.-S.; Su, H.-J.; Hsieh, P.-L.; Chiang, Y.-W.; Huang, M.H. Synthesis of Ag3PO4 crystals with tunable shapes for facet-dependent optical property, photocatalytic activity, and electrical conductivity examinations. ACS Appl. Mater. Interfaces 2017, 9, 39086–39093. [Google Scholar] [CrossRef]
- Lin, H.; Ye, H.; Xu, B.; Cao, J.; Chen, S. Ag3PO4 quantum dot sensitized BiPO4: A novel p–n junction Ag3PO4/BiPO4 with enhanced visible-light photocatalytic activity. Catal. Commun. 2013, 37, 55–59. [Google Scholar] [CrossRef]
- Inamdar, J.; Singh, S.K. Photocatalytic detoxification method for zero effluent discharge in dairy industry: Effect of operational parameters. World Acad. Sci. Eng. Technol. J. Civ. Environ. Eng. 2008, 2, 10–14. [Google Scholar] [CrossRef]
- Kudlek, E. The Sequential System Photocatalysis—Membrane Pressure Process in Depth Treatment of Effluents Containing Pharmaceutical Active Compounds from Municipal Wastewater Treatment Plants. 2016. Available online: http://delibra.bg.polsl.pl/dlibra/doccontent?id=37846 (accessed on 5 November 2019).
- Rauf, M.A.; Ashraf, S.S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 2009, 151, 10–18. [Google Scholar] [CrossRef]
- Nsib, M.F.; Maayoufi, A.; Moussa, N.; Tarhouni, N.; Massouri, A.; Houas, A.; Chevalier, Y. TiO2 modified by salicylic acid as a photocatalyst for the degradation of monochlorobenzene via pickering emulsion way. J. Photochem. Photobiol. A Chem. 2013, 251, 10–17. [Google Scholar] [CrossRef]
- Ni, W.; Wu, S.; Ren, Q. Preparation and Characterization of silanized TiO2 nanoparticles and their application in toner. Ind. Eng. Chem. Res. 2012, 51, 13157–13163. [Google Scholar] [CrossRef]
- Afrin, U.; Mian, M.R.; Breedlove, B.K.; Hossain, M.M. Enhanced photocatalytic activity of an acid-modified TiO2 surface for degradation of the azo dye remazol red. ChemistrySelect 2017, 2, 10371–10374. [Google Scholar] [CrossRef]
- Kosmulski, M. The PH-dependent surface charging and points of zero charge: V. update. J. Colloid Interface Sci. 2011, 353, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; An, T.; Li, G.; Song, W.; Cooper, W.J.; Luo, H.; Guo, X. Photocatalytic degradation kinetics and mechanism of environmental pharmaceuticals in aqueous suspension of TiO2: A Case of β-blockers. J. Hazard. Mater. 2010, 179, 834–839. [Google Scholar] [CrossRef]
- Udom, I.; Myers, P.D.; Ram, M.K.; Hepp, A.F.; Archibong, E.; Stefanakos, E.K.; Goswami, D.Y. Optimization of photocatalytic degradation of phenol using simple photocatalytic reactor. Am. J. Anal. Chem. 2014, 5, 743–750. [Google Scholar] [CrossRef]
- Alkaim, A.F.; Aljeboree, A.M.; Alrazaq, N.A.; Baqir, S.J.; Hussein, F.H.; Lilo, A.J. Effect of pH on adsorption and photocatalytic degradation efficiency of different catalysts on removal of methylene blue. Asian J. Chem. 2014, 26, 8445–8448. [Google Scholar] [CrossRef]
- Fernández-Domene, R.M.; Roselló-Márquez, G.; Sánchez-Tovar, R.; Lucas-Granados, B.; García-Antón, J. Photoelectrochemical removal of chlorfenvinphos by using WO3 nanorods: Influence of annealing temperature and operation PH. Sep. Purif. Technol. 2019, 212, 458–464. [Google Scholar] [CrossRef]
- Liu, S.X.; Chen, X.Y.; Chen, X. A TiO2/AC Composite photocatalyst with high activity and easy separation prepared by a hydrothermal method. J. Hazard. Mater. 2007, 143, 257–263. [Google Scholar] [CrossRef]
- Li Puma, G.; Bono, A.; Krishnaiah, D.; Collin, J.G. Preparation of titanium dioxide photocatalyst loaded onto activated carbon support using chemical vapor deposition: A review paper. J. Hazard. Mater. 2008, 157, 209–219. [Google Scholar] [CrossRef]
- Nadia, E.M.; Moustapha, B.; Yahia, A.I. UV/TiO2 photocatalytic oxidation of commercial pesticide in aqueous solution. Am. J. Innov. Res. Appl. Sci. 2018, 5, 36–43. [Google Scholar]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 Photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Tseng, C.-S.; Wu, T.; Lin, Y.-W. Facile synthesis and characterization of Ag3PO4 microparticles for degradation of organic dyestuffs under white-light light-emitting-diode irradiation. Materials 2018, 11, 708. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zu, L.; Jiang, Y.; Shi, D.; Cai, X.; Ni, Y.; Lin, S.; Qin, Y. A titanium-based photo-fenton bifunctional catalyst of Mp-MXene/TiO2−x nanodots for dramatic enhancement of catalytic efficiency in advanced oxidation processes. Chem. Commun. 2018, 54, 11622–11625. [Google Scholar] [CrossRef] [PubMed]
- Nakarada, Ð.; Petković, M. Mechanistic insights on how hydroquinone disarms OH and OOH radicals. Int. J. Quantum Chem. 2018, 118, e25496. [Google Scholar] [CrossRef]
- Azarpira, H.; Sadani, M.; Abtahi, M.; Vaezi, N.; Rezaei, S.; Atafar, Z.; Mohseni, S.M.; Sarkhosh, M.; Ghaderpoori, M.; Keramati, H.; et al. Photo-catalytic degradation of triclosan with UV/Iodide/ZnO process: Performance, kinetic, degradation pathway, energy consumption and toxicology. J. Photochem. Photobiol. A Chem. 2019, 371, 423–432. [Google Scholar] [CrossRef]
- Pelaez, M.; Falaras, P.; Likodimos, V.; O’Shea, K.; de la Cruz, A.A.; Dunlop, P.S.M.; Byrne, J.A.; Dionysiou, D.D. Use of selected scavengers for the determination of NF-TiO2 reactive oxygen species during the degradation of microcystin-lr under visible light irradiation. J. Mol. Catal. A Chem. 2016, 425, 183–189. [Google Scholar] [CrossRef]
- Abdullah, H.; Khan, M.R.; Pudukudy, M.; Yaakob, Z.; Ismail, N.A. CeO2-TiO2 as a visible light active catalyst for the photoreduction of CO2 to methanol. J. Rare Earths 2015, 33, 1155–1161. [Google Scholar] [CrossRef]
- Huang, H.; Tu, S.; Zeng, C.; Zhang, T.; Reshak, A.H.; Zhang, Y. Macroscopic polarization enhancement promoting photo- and piezoelectric-induced charge separation and molecular oxygen activation. Angew. Chem. 2017, 56, 11860–11864. [Google Scholar] [CrossRef]
- Chen, F.; Huang, H.; Guo, L.; Zhang, Y.; Ma, T. The role of polarization in photocatalysis. Angew. Chem. 2019, 58, 10061–10073. [Google Scholar] [CrossRef]











| Chemical Structure | Physico-Chemical Properties | |
|---|---|---|
 | Molecular formula | C12H14Cl3O4P |
| Molecular weight (g/mol) | 359.57 | |
| CAS number | 470-90-6 | |
| Water solubility at 20 °C (mg/L) | 124.0 | |
| Density (g/L) | 1.36 | |
| Vapor pressure (25 °C) (mmHg) | 7.5 × 10−6 | |
| logKOW (−) | 3.81 | |
| Chemical Structure | Physico-Chemical Properties | |
|---|---|---|
 | Symbol, origin | P-25, Sigma-Aldrich (Poznań, Poland) |
| Crystal structure | Anatase:rutile = 80:20 | |
| Surface area (m2/g) | 35.0–65.0 | |
| Particle size (nm) | 21.0 | |
| Density (g/cm3) | 4.26 | |
| Symbol | Explanation |
|---|---|
| TiO2 | Pure Titanium(IV) Oxide |
| TiO2/PA/99:1 | TiO2 modified with pyruvic acid in a ratio of 99:1 (w/w) |
| TiO2/PA/90:10 | TiO2 modified with pyruvic acid in a ratio of 90:10 (w/w) |
| TiO2/PA/80:20 | TiO2 modified with pyruvic acid in a ratio of 80:20 (w/w) |
| TiO2/PA/50:50 | TiO2 modified with pyruvic acid in a ratio of 50:50 (w/w) |
| TiO2/PA/20:80 | TiO2 modified with pyruvic acid in a ratio of 20:80 (w/w) |
| TiO2/SA/99:1 | TiO2 modified with succinic acid in a ratio of 99:1 (w/w) |
| TiO2/SA/90:10 | TiO2 modified with succinic acid in a ratio of 90:10 (w/w) |
| TiO2/SA/80:20 | TiO2 modified with succinic acid in a ratio of 80:20 (w/w) |
| TiO2/SA/50:50 | TiO2 modified with succinic acid in a ratio of 50:50 (w/w) |
| TiO2/SA/20:80 | TiO2 modified with succinic acid in a ratio of 20:80 (w/w) |
| TiO2/PA | TiO2/SA | ||||||
|---|---|---|---|---|---|---|---|
| Symbol | k, 1/min | R2 | t/2, min | Symbol | k, 1/min | R2 | t/2, min |
| TiO2 | 0.0027 | 0.86 | 257.0 | TiO2 | 0.0027 | 0.86 | 257.0 |
| 99:1 | 0.0046 | 0.87 | 151.0 | 99:1 | 0.0034 | 0.94 | 204.0 |
| 90:10 | 0.0110 | 0.93 | 63.0 | 90:10 | 0.0064 | 0.93 | 108.0 |
| 80:20 | 0.0063 | 0.93 | 110.0 | 80:20 | 0.0039 | 0.91 | 178.0 |
| 50:50 | 0.0036 | 0.81 | 193.0 | 50:50 | 0.0031 | 0.86 | 224.0 |
| 20:80 | 0.0022 | 0.91 | 315.0 | 20:80 | 0.0031 | 0.80 | 224.0 |
| pH | Catalyst | k, 1/min | R2 | t/2, min |
|---|---|---|---|---|
| 3 | TiO2 | 0.0019 | 0.85 | 365.0 |
| TiO2/PA/90:10 | 0.0104 | 0.97 | 67.0 | |
| TiO2/SA/90:10 | 0.0063 | 0.95 | 110.0 | |
| 6 | TiO2 | 0.0031 | 0.94 | 224.0 |
| TiO2/PA/90:10 | 0.0084 | 0.91 | 83.0 | |
| TiO2/SA/90:10 | 0.0082 | 0.98 | 85.0 | |
| 9 | TiO2 | 0.0027 | 0.82 | 257.0 |
| TiO2/PA/90:10 | 0.0057 | 0.82 | 122.0 | |
| TiO2/SA/90:10 | 0.0078 | 0.86 | 89.0 |
| Without Aeration | With Aeration | ||||||
|---|---|---|---|---|---|---|---|
| Symbol | k, 1/min | R2 | t/2, min | Symbol | k, 1/min | R2 | t/2, min |
| TiO2 | 0.0031 | 0.94 | 224.0 | TiO2 | 0.0071 | 0.92 | 98.0 |
| TiO2/PA/90:10 | 0.0084 | 0.91 | 83.0 | TiO2/PA/90:10 | 0.0255 | 0.97 | 27.0 |
| TiO2/SA/90:10 | 0.0082 | 0.98 | 85.0 | TiO2/SA/90:10 | 0.0178 | 0.93 | 39.0 |
| Catalyst | Scavenger | k, 1/min | R2 | t/2, min |
|---|---|---|---|---|
| TiO2 | Control | 0.0027 * | 0.86 | 257.0 |
| MeOH | 0.0023 | 0.87 | 301.0 | |
| Hydroquinone | 0.0027 | 0.94 | 257.0 | |
| EDTA | 0.0032 | 0.95 | 217.0 | |
| TiO2/PA/90:10 | Control | 0.0046 | 0.87 | 151.0 |
| MeOH | 0.0049 | 0.90 | 141.0 | |
| Hydroquinone | 0.0024 | 0.92 | 289.0 | |
| EDTA | 0.0030 | 0.92 | 231.0 | |
| TiO2/SA/90:10 | Control | 0.0039 | 0.91 | 178.0 |
| MeOH | 0.0037 | 0.91 | 187.0 | |
| Hydroquinone | 0.0015 | 0.90 | 462.0 | |
| EDTA | 0.0026 | 0.88 | 267.0 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawadzki, P. TiO2 Modified with Organic Acids for the Decomposition of Chlorfenvinphos under the Influence of Visible Light: Activity, Performance, Adsorption, and Kinetics. Materials 2020, 13, 289. https://doi.org/10.3390/ma13020289
Zawadzki P. TiO2 Modified with Organic Acids for the Decomposition of Chlorfenvinphos under the Influence of Visible Light: Activity, Performance, Adsorption, and Kinetics. Materials. 2020; 13(2):289. https://doi.org/10.3390/ma13020289
Chicago/Turabian StyleZawadzki, Piotr. 2020. "TiO2 Modified with Organic Acids for the Decomposition of Chlorfenvinphos under the Influence of Visible Light: Activity, Performance, Adsorption, and Kinetics" Materials 13, no. 2: 289. https://doi.org/10.3390/ma13020289
APA StyleZawadzki, P. (2020). TiO2 Modified with Organic Acids for the Decomposition of Chlorfenvinphos under the Influence of Visible Light: Activity, Performance, Adsorption, and Kinetics. Materials, 13(2), 289. https://doi.org/10.3390/ma13020289