Conjugates of Copper Alginate with Arginine-Glycine-Aspartic Acid (RGD) for Potential Use in Regenerative Medicine
Abstract
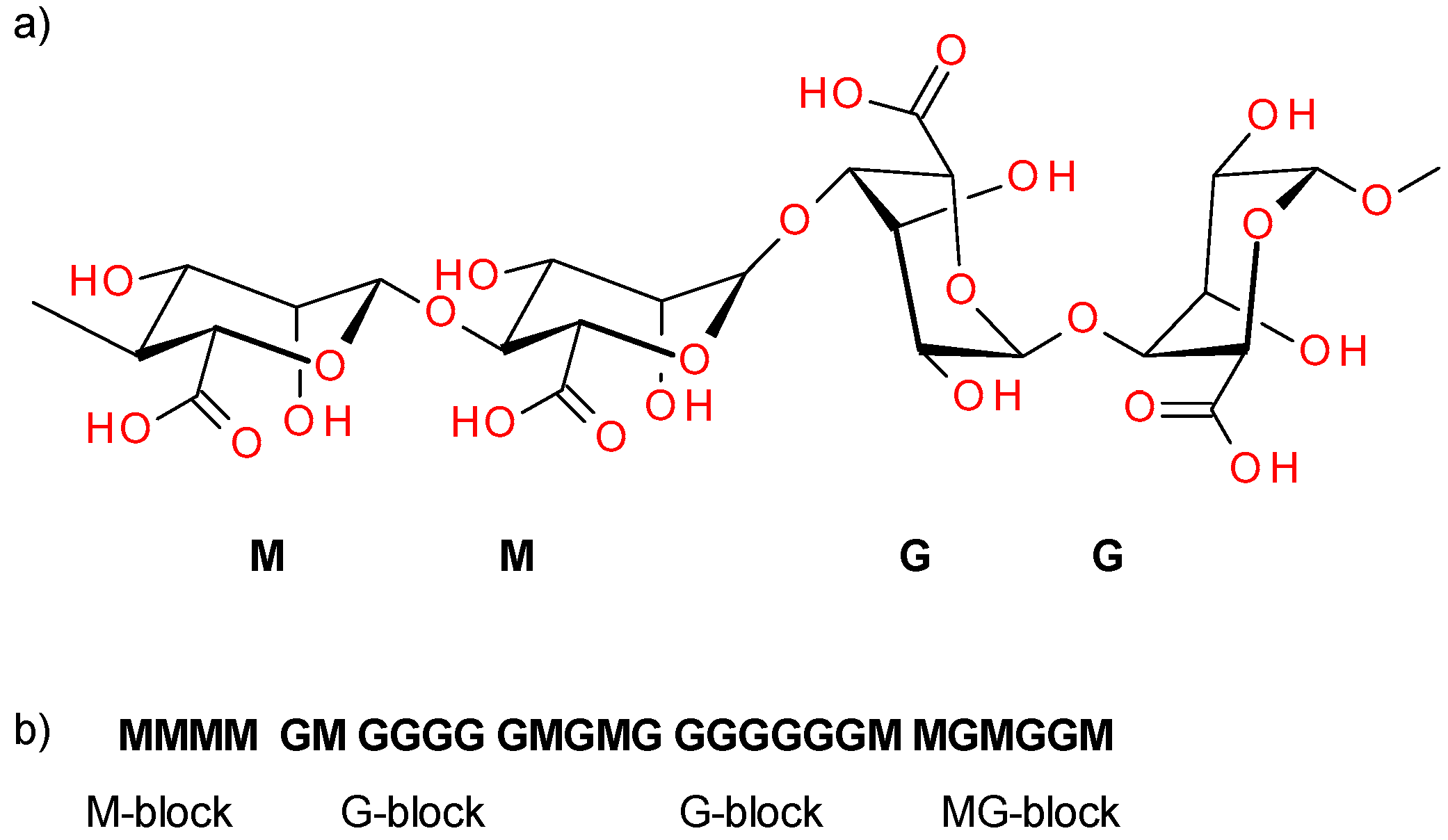
:1. Introduction
2. Materials and Methods
2.1. Formation of Fiber and Nonwovens
2.2. Synthesis of H-RGD-OH and H-RGD-NH2
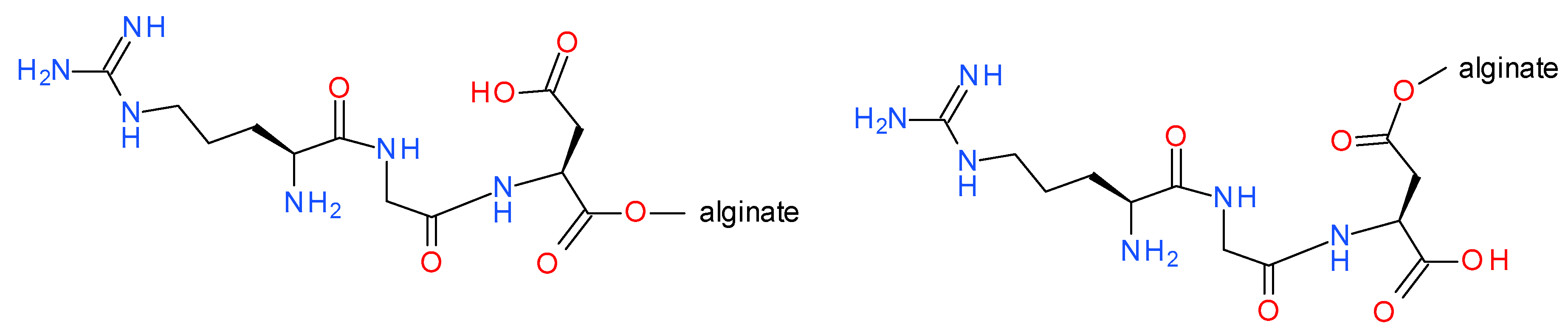
2.3. Preparation of Copper Alginate Nonwovens with Physically Embedded RGD Derivatives
2.4. Preparation of Copper Alginate Nonwovens with Covalently Bound H-RGD-OH (Cu-Alginate-RGD)
2.5. Analysis of Nonwovens Based on Copper Alginate Modified with RGD Peptides
2.6. Testing the Antibacterial Properties of Materials Based on Cu-Alginate-RGD Conjugates
- Staphylococcus aureus American Type Culture Collection (ATCC) 6538,
- Klebsiella pneumoniae ATCC 4352.
2.7. Cytotoxicity Studies
3. Results and Discussion
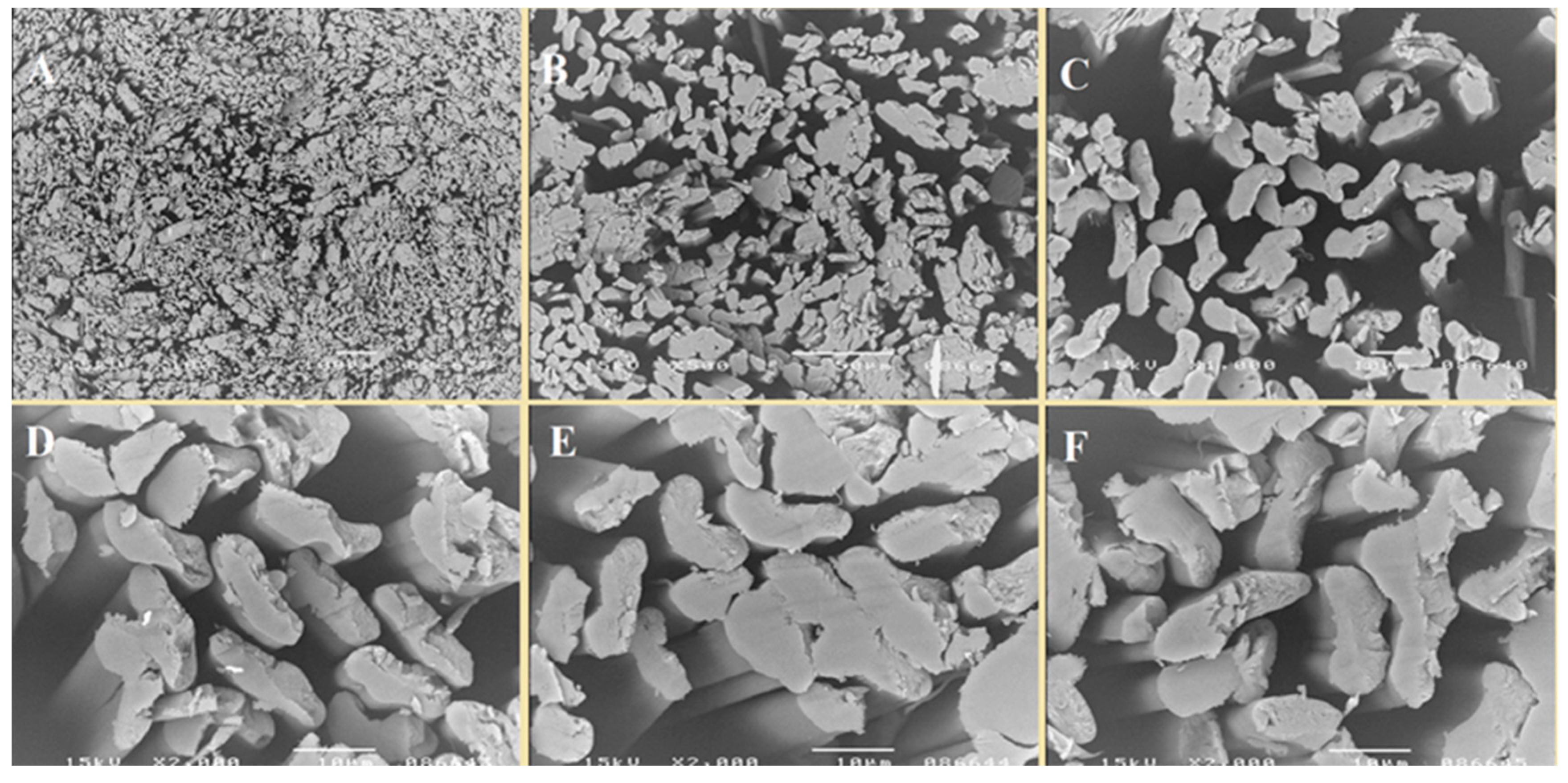
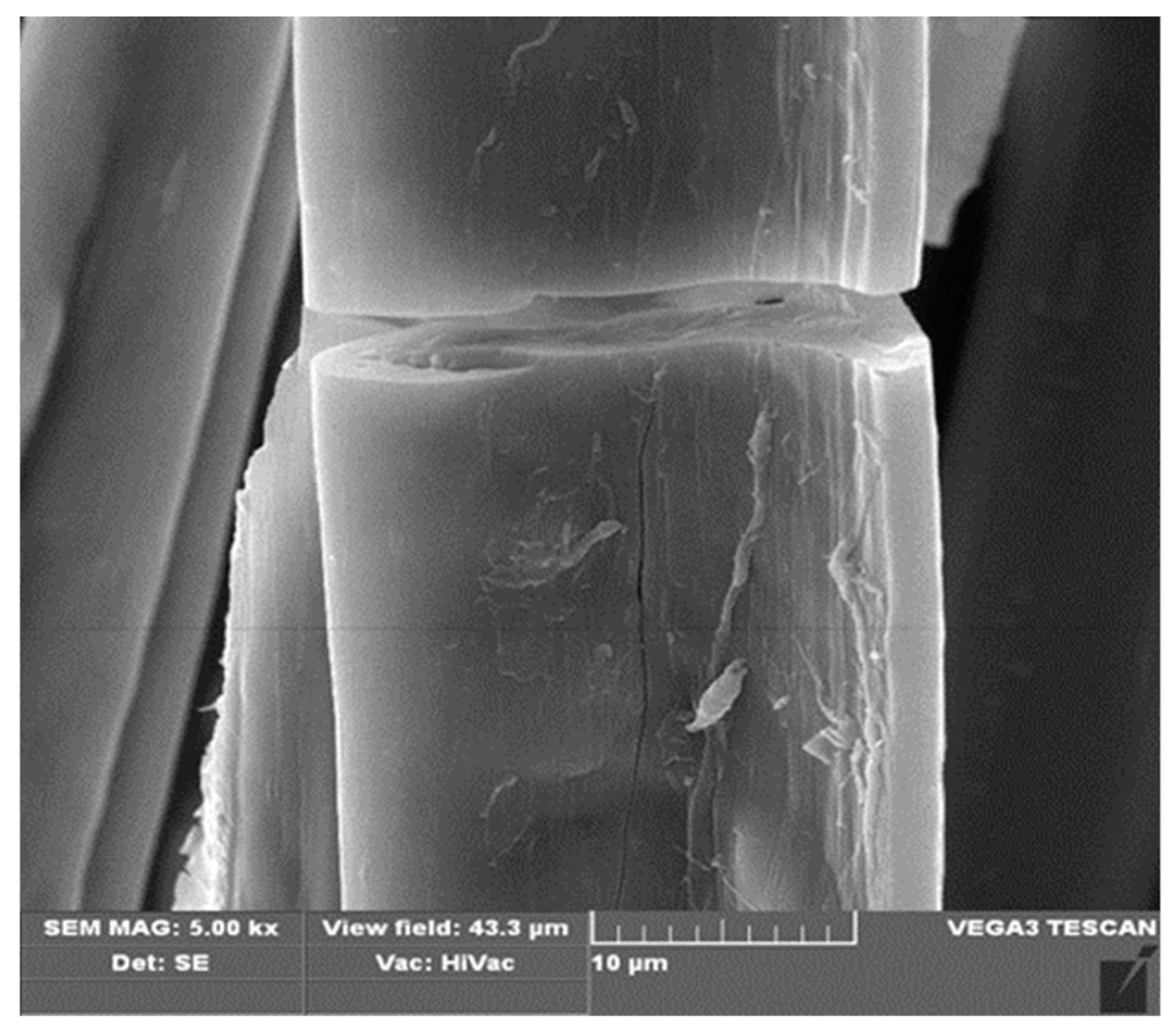
3.1. Preparation of Fibers and Nonwovens Based on Copper Alginate
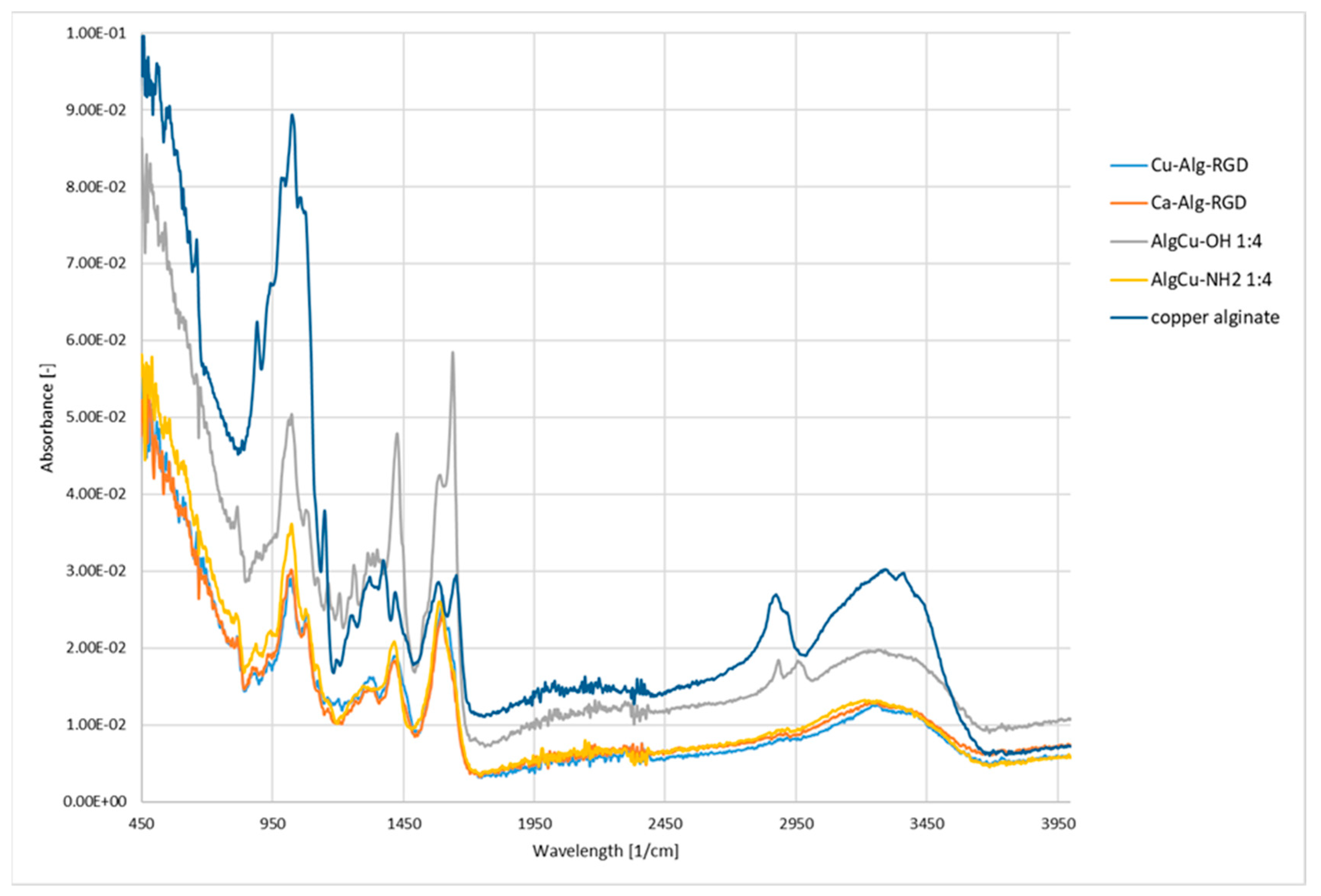
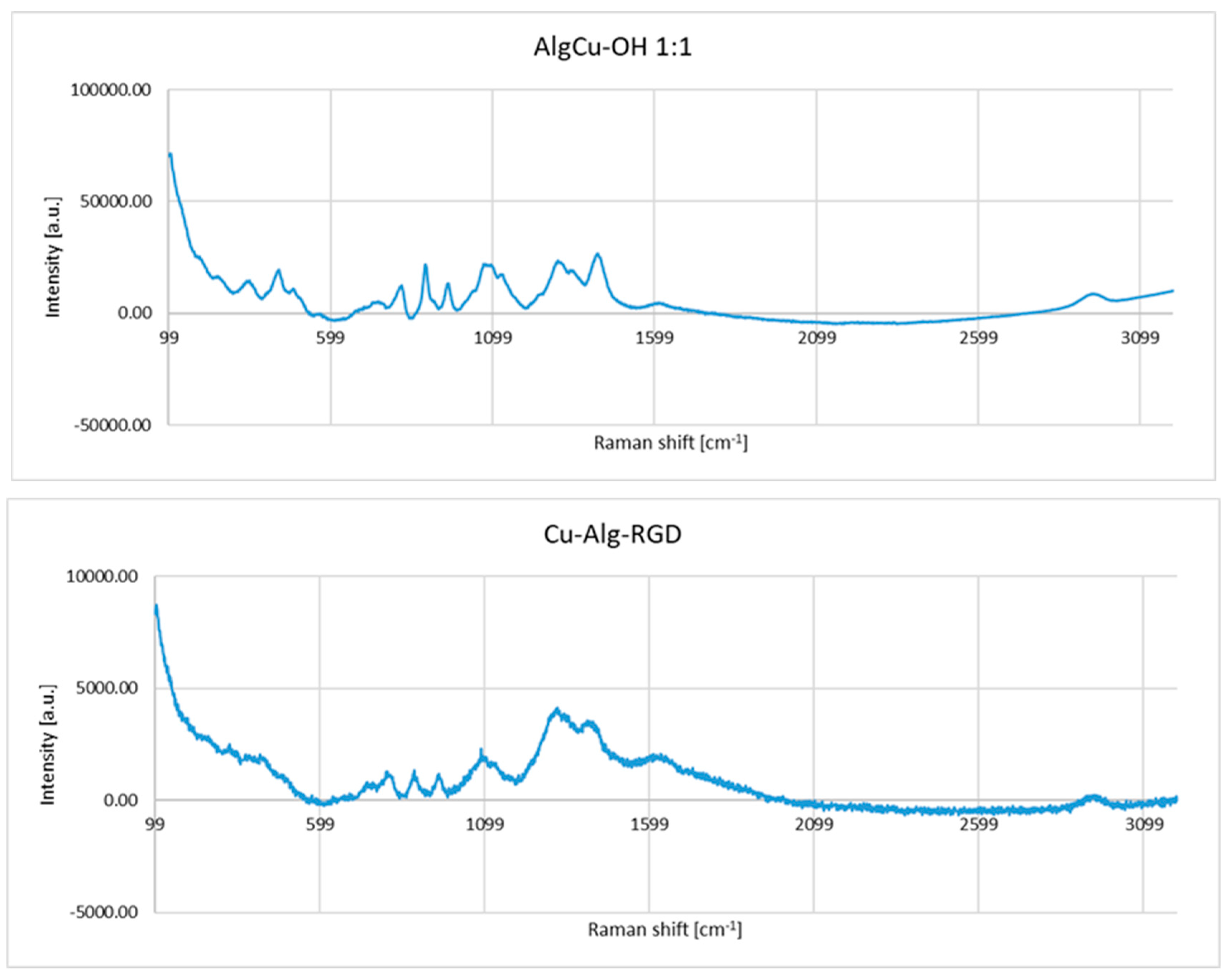
3.2. Functionalization of Copper Alginate Using RGD Derivatives
3.3. Evaluation of Antibacterial Activity
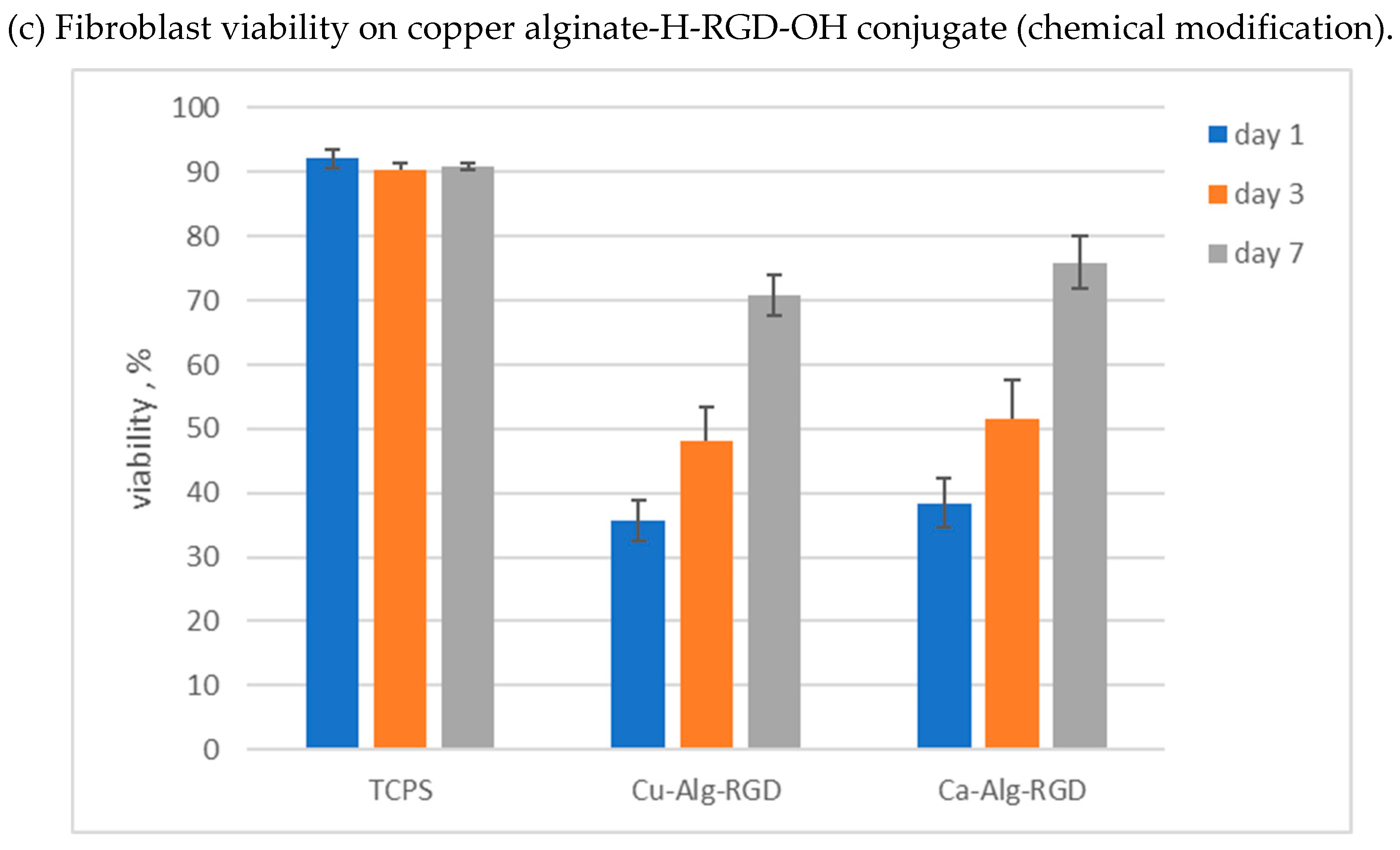
3.4. Cytotoxicity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matsumoto, T.; Kawai, M.; Masuda, T. Influence of concentration and mannuronate/guluronate ratio on steady flow properties of alginate aqueous systems. Biorheology 1992, 29, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Dragenr, K. Alginate. In Hand book of Hydrocolloids, 2nd ed.; Philips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 375–395. [Google Scholar]
- Donati, T.; Holtans, S.; Morsh, Y.; Bergogna, M.; Dentini, M.S.B. New hypothesis on the role of alternating sequences in calcium alginate gels. Biomacromolecules 2005, 16, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Chu, I.M.; Shiao, M.Y.; Hsu, B.R.S.; Fu, S.H. Microencapsulation of islets in PEG-amine modified alginate-poly(lysine)-alginate microcapsules for constructing bioartificial pancreas. J. Ferment. Bioeng. 1998, 86, 185–190. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.B.; Kim, S.J.; Lee, Y.M. Rapid temperature/pH response of porous alginate-g-poly(N-isopropylacrylamide) hydrogels. Polymer 2002, 43, 7549–7558. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Misra, B.N. Grafting: A versatile means to modify polymers: Techniques, factors and applications. Prog. Polym. Sci. 2004, 29, 767–814. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Lan, X.Z.; Tan, Z.C.; Shi, Q.; Yang, C.G. A novel gelling method for stabilization of phase change material Na2HPO4·12H2O with sodium alginate grafted sodium acrylate. Thermochim. Acta 2007, 463, 18–20. [Google Scholar] [CrossRef]
- Al-Karawi, A.J.M.; Al-Qaisi, Z.H.J.; Abdullah, H.I.; Al-Mokaram, A.M.A.; Al-Heetimi, D.T.A. Synthesis, characterization of acrylamide grafted chitosan and its use in removal of copper(II) ions from water. Carbohydr. Polym. 2011, 83, 495–500. [Google Scholar] [CrossRef]
- Mishra, D.K.; Arpit, S.; Mithilesh, Y.; Kunj, B. Modification of alginate by grafting of N-vinyl-2-pyrrolidone and studies of physicochemical properties in terms of swelling capacity, metal-ion uptake and flocculation. Carbohydr. Polym. 2010, 80, 1147–1154. [Google Scholar]
- Morsh, Y.; Donati, T.; Strand, B.; Skjak-Braek, G. Effect of Ca2+, Ba2+, Sr2+ on alginate microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.; Cohen, S. Novel alginate sponges for cell culture and transplantation. Biomaterials 1997, 18, 583–590. [Google Scholar] [CrossRef]
- Szekalska, M.; Pucilowska, A.; Szymanska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef] [Green Version]
- Mould, A.P.; Humphries, M.J. Cell biology—Adhesion articulated. Nature 2004, 432, 27–28. [Google Scholar] [CrossRef]
- Marelli, U.K.; Rechenmacher, F.; Ali Sobahi, T.R.; Mas-Moruno, C.; Kessler, H. Tumor targeting via integrin ligands. Front. Oncol. 2013, 3, 222. [Google Scholar] [CrossRef] [Green Version]
- Cox, D.; Brennan, M.; Moran, N. Integrins as therapeutic targets: Lessons and opportunities. Nat. Rev. Drug Discov. 2010, 9, 804–820. [Google Scholar] [CrossRef]
- Ley, K.; Rivera-Nieves, J.; Sandborn, W.J.; Shattil, S. Integrin-based therapeutics: Biological basis, clinical use and new drugs biological basis, clinical use and new drugs. Nat. Rev. Drug Discov. 2016, 15, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Goodman, S.L.; Picard, M. Integrins as therapeutic targets. Trends Pharmacol. Sci. 2012, 33, 405–412. [Google Scholar] [CrossRef]
- Kapp, T.G.; Rechenmacher, F.; Sobahi, T.R.; Kessler, H. Integrin modulators: A patent review. Expert Opin. Thr. Pat. 2013, 23, 1273–1295. [Google Scholar] [CrossRef]
- Degrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, A.M.; Aidoudi-Ahmed, S.; Sharma, S.; Kotamraju, V.R.; Foster, P.J.; Sugahara, K.N.; Ruoslahti, E.; Rutt, B.K. Nanoparticles coated with the tumor-penetrating peptide iRGD reduce experimental breast cancer metastasis in the brain. J. Mol. Med. 2015, 93, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Schottelius, M.; Laufer, B.; Kessler, H.; Wester, H.-J. Ligands for mapping αvβ3-integrin expression in vivo. Acc. Chem. Res. 2009, 42, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, S.V.; Pasapera, A.M.; Sabass, B.; Waterman, C.M. Force flctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell 2012, 151, 1513–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mas-Moruno, C.; Fraioli, R.; Rechenmacher, F.; Neubauer, S.; Kapp, T.G.; Kessler, H. αv β3- or α5 β1-Integrin-selective peptidomimetics for surface coating. Angew. Chem. Int. Ed. 2016, 55, 7048–7067. [Google Scholar] [CrossRef]
- Formo, K.; Cho, C.H.; Vallier, L.; Stran, B.L. Culture of hESC-derived pancreatic progenitors in alginate-based scaffolds. J. Biomed. Mater. Res. Part A 2015, 103, 3717–3726. [Google Scholar] [CrossRef] [Green Version]
- Rowley, J.A.; Mooney, D.J. Alginate type and RGD density control myoblast phenotype. J. Biomed. Mater. Res. 2002, 60, 217–223. [Google Scholar] [CrossRef]
- Sandvig, I.; Karstensen, K.; Rokstad, A.M.; Aachmann, F.L.; Formo, K.; Sandvig, A.; Skjåk-Bræk, G.; Strand, B.L. RGD-peptide modified alginate by a chemoenzymatic strategy for tissue engineering applications. J. Biomed. Mater. Res. Part A 2015, 103, 896–906. [Google Scholar] [CrossRef]
- Fonseca, K.B.; Bidarra, S.J.; Oliveira, M.J.; Granja, P.L.; Barrias, C.C. Molecularly designed alginate hydrogels susceptible to local proteolysis as three-dimensional cellular microenvironments. Acta Biomater. 2011, 7, 1674–1682. [Google Scholar] [CrossRef]
- Bidarra, S.J.; Barrias, C.C.; Fonseca, K.B.; Barbosa, M.A.; Soares, R.A.; Granja, P.L. Injectable in situ crosslinkable RGD-modified alginate matrix for endothelial cells delivery. Biomaterials 2011, 32, 7897–7904. [Google Scholar] [CrossRef]
- Dhoot, N.O.; Tobias, C.A.; Fischer, I.; Wheatley, M.A. Peptide-modified alginate surfaces as a growth permissive substrate for neurite outgrowth. J. Biomed. Mater. Res. A 2004, 71, 191–200. [Google Scholar] [CrossRef]
- Andersen, T.; Markussen, C.; Dornish, M.; Heier-Baardson, H.; Melvik, J.E.; Alsberg, E.; Christensen, B.E. In situ gelation for cell immobilization and culture in alginate foam scaffolds. Tissue Eng. Part A 2014, 20, 600–610. [Google Scholar] [PubMed] [Green Version]
- Rokstad, A.M.; Donati, I.; Borgogna, M.; Oberholzer, J.; Strand, B.L.; Espevik, T.; Skjåk-Braek, G. Cell-compatible covalently reinforced beads obtained from a chemoenzymatically engineered alginate. Biomaterials 2006, 27, 4726–4737. [Google Scholar] [CrossRef] [PubMed]
- Ertesvåg, H. Alginate-modifying enzymes: Biological roles and biotechnological uses. Front. Microbiol. 2015, 6, 523. [Google Scholar] [PubMed] [Green Version]
- Bush, K.; Courvalin, P.; Dantas, G.; Davies, J.; Eisenstein, B.; Huovinen, P.; Jacoby, G.; Kishony, R.; Kreiswirth, B.; Kutter, E.; et al. Tackling antibiotic resistance. Nat. Rev. Microbiol. 2011, 9, 894–896. [Google Scholar]
- Spellberg, B.; Shlaes, D. Prioritized current unmet needs for antibacterial therapies. Clin. Pharmacol. Ther. 2014, 96, 151–153. [Google Scholar] [CrossRef]
- Prokhorov, E.; España-Sánchez, B.L.; Luna-Bárcenas, G.; Padilla-Vaca, F.; Cruz-Soto, M.-E.; OVázquez-Lepe, M.; Kovalenko, Y.; Elizalde-Peña, E.A. Chitosan/copper nanocomposites: Correlation between electrical and antibacterial properties. Colloids Surf. B 2019, 180, 186–192. [Google Scholar] [CrossRef]
- Pereira, I.C.; Duarte, A.S.; Neto, A.S.; Ferreira, J.M.F. Chitosan and polyethylene glycol based membranes with antibacterial properties for tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 606–615. [Google Scholar] [CrossRef]
- Safaei, M.; Taran, M. Optimized synthesis, characterization, and antibacterial activity of an alginate–cupric oxide bionanocomposite. J. Appl. Polym. Sci. 2018, 135, 45682. [Google Scholar] [CrossRef]
- Lee, I.C.; Ko, J.W.; Park, S.-H.; Lim, J.O.; Shin, I.S.; Moon, C.; Kim, S.H.; Heo, J.D.; Kim, J.C. Comparative toxicity and biodistribution of copper nanoparticles and cupric ions in rats. Int. J. Nanomed. 2016, 11, 2883–2900. [Google Scholar]
- Ibrahim, N.A.; Abo-Shosha, M.H.; Gaffar, M.A.; Elshafei, A.M.; Abdel-Fatah, O.M. Antibacterial properties of ester-cross-linked cellulose-containing fabrics post-treated with metal salts. Polym. Plast. Technol. 2006, 45, 719–727. [Google Scholar] [CrossRef]
- Taran, M.; Etemadi, S.; Safaei, M. Microbial levan biopolymer production and its use for the synthesis of an antibacterial iron (II, III) oxide-levan nanocomposite. J. Appl. Polym. Sci. 2017, 134, 44613. [Google Scholar] [CrossRef]
- Bogun, M.; Mikołajczyk, T. Sorption and tensile strength properties of selected fibers of cupric alginate. Fibres Text. East. Eur. 2008, 16, 39–42. [Google Scholar]
- Wimonwan, K.; Pitt, S. Novel copper (II) alginate hydrogels and their potential for use as anti-bacterial wound dressings. Biomed. Mater. 2014, 9, 45008. [Google Scholar]
- Willenberg, B.J.; Zheng, T.; Meng, F.W.; Meneses, J.C.; Rossignol, C.; Batich, C.D.; Terada, N.; Steindler, D.A.; Weiss, M.D. Gelatinized copper–capillary alginate gel functions as an injectable tissue scaffolding system for stem cell transplants. J. Biomat. Sci. 2011, 22, 1621–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolesinska, B.; Rozniakowski, K.K.; Fraczyk, J.; Relich, I.; Papini, A.M.; Kaminski, Z.J. The effect of counterion and tertiary amine on the efficiency of N-triazinylammonium sulfonates in solution and solid-phase peptide synthesis. Eur. J. Org. Chem. 2015, 2015, 401–408. [Google Scholar] [CrossRef]
- Cipriani, F.; Bernhagen, D.; García-Arévalo, C.; de Torre, I.G.; Timmerman, P.; Rodríguez-Cabello, J.C. Bicyclic RGD peptides with high integrin α v β 3 and α 5 β 1 affinity promote cell adhesion on elastin-like recombinamers. Biomed. Mater. 2019, 14, 35009. [Google Scholar] [CrossRef]
- Fraczyk, J.; Kaminski, Z.J.; Katarzynska, J.; Kolesinska, B. 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium toluene-4-sulfonate (DMT/NMM/TsO−) universal coupling reagent for synthesis in solution. Helv. Chim. Acta 2018, 101, e1700187. [Google Scholar] [CrossRef] [Green Version]
- Fraczyk, J.; Kolesinska, B.; Kaminski, Z.J. N-lipidated oligopeptides immobilized on cellulose as new type of organocatalysts. Comb. Chem. High Throughput Screen. 2013, 16, 562–571. [Google Scholar] [CrossRef]
- Fraczyk, J.; Walczak, M.; Kaminski, Z.J. New methodology for automated SPOT synthesis of peptides on cllulose using 1,3,5-triazine derivatives as linkers and as coupling reagents. J. Pep. Sci. 2018, 24, e3063. [Google Scholar] [CrossRef]
- Bak, A.; Kozik, V.; Walczak, M.; Fraczyk, J.; Kaminski, Z.J.; Kolesinska, B.; Smolinski, A.; Jampilek, J. Towards intelligent drug design system: Application of artificial peptide receptor library in QSAR-oriented studies. Molecules 2018, 23, 1964. [Google Scholar] [CrossRef] [Green Version]
- Fraczyk, J.; Kaminski, Z.J. N-Lipidated amino acids and peptides immobilized on cellulose able to split amide bonds. Materials 2019, 12, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Zhu, J.; Chen, X.; Dong, H.; Li, Q.; Zeng, L.; Cao, X. Alginate based antimicrobial hydrogels formed by integrating Diels–Alder “click chemistry” and the thiol –ene reaction. RSC Adv. 2018, 8, 11036–11042. [Google Scholar] [CrossRef] [Green Version]
- Labre, F.; Mathieu, S.; Chaud, P.; Morvan, P.Y.; Vallée, R.; Helbert, W.; Fort, S. DMTMM-mediated amidation of alginate oligosaccharides aimed at modulating their interaction with proteins. Carbohydr. Polym. 2018, 184, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Preussmann, R.; Schneider, H.; Epple, F. Identification of alkylating agents. II. Identification of different classes of alkylating agents by a modification of the color reaction with 4-(4-nitrobenzyl)-pyridine (NBP). Arzneimittelforschung 1969, 19, 1059–1073. [Google Scholar] [PubMed]
- Lall, N.; Henley-Smith, C.J.; De Canha, M.N.; Oosthuizen, C.B.; Berrington, D. Viability Reagent, PrestoBlue, in comparison with other available reagents, utilized in cytotoxicity and antimicrobial assays. Int. J. Microbiol. 2013, 2013, 420601. [Google Scholar] [CrossRef] [Green Version]
- Kapp, T.G.; Rechenmacher, F.; Neubauer, S.; Maltsev, O.V.; Cavalcanti-Adam, E.A.; Zarka, R.; Reuning, U.; Notni, J.; Wester, H.J.; Mas-Moruno, C.; et al. A comprehensive evaluation of the activity and selectivity profile of ligands for RGD-binding integrins. Sci. Rep. 2017, 7, 39805. [Google Scholar] [CrossRef] [Green Version]






| The Value of Antibacterial Activity for the Seeding Method | ||
|---|---|---|
| Bacteria | Staphylococcus aureus ATCC 6538 | Klebsiella pneumonie ATCC 4352 |
| Inoculum concentration (colony-forming unit (CFU)/mL) | 2.6 × 105 | 1.0 × 105 |
| Value of growth F (F = log CT−log C0) | 1.75 | 2.75 |
| Value of growth G (G = log TT−log T0) | −1.15 | −1.81 |
| Value of antibacterial activity A (A = F−G) | 2.90 | 4.56 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraczyk, J.; Wasko, J.; Walczak, M.; Kaminski, Z.J.; Puchowicz, D.; Kaminska, I.; Bogun, M.; Kolasa, M.; Stodolak-Zych, E.; Scislowska-Czarnecka, A.; et al. Conjugates of Copper Alginate with Arginine-Glycine-Aspartic Acid (RGD) for Potential Use in Regenerative Medicine. Materials 2020, 13, 337. https://doi.org/10.3390/ma13020337
Fraczyk J, Wasko J, Walczak M, Kaminski ZJ, Puchowicz D, Kaminska I, Bogun M, Kolasa M, Stodolak-Zych E, Scislowska-Czarnecka A, et al. Conjugates of Copper Alginate with Arginine-Glycine-Aspartic Acid (RGD) for Potential Use in Regenerative Medicine. Materials. 2020; 13(2):337. https://doi.org/10.3390/ma13020337
Chicago/Turabian StyleFraczyk, Justyna, Joanna Wasko, Malgorzata Walczak, Zbigniew J. Kaminski, Dorota Puchowicz, Irena Kaminska, Maciej Bogun, Marcin Kolasa, Ewa Stodolak-Zych, Anna Scislowska-Czarnecka, and et al. 2020. "Conjugates of Copper Alginate with Arginine-Glycine-Aspartic Acid (RGD) for Potential Use in Regenerative Medicine" Materials 13, no. 2: 337. https://doi.org/10.3390/ma13020337
APA StyleFraczyk, J., Wasko, J., Walczak, M., Kaminski, Z. J., Puchowicz, D., Kaminska, I., Bogun, M., Kolasa, M., Stodolak-Zych, E., Scislowska-Czarnecka, A., & Kolesinska, B. (2020). Conjugates of Copper Alginate with Arginine-Glycine-Aspartic Acid (RGD) for Potential Use in Regenerative Medicine. Materials, 13(2), 337. https://doi.org/10.3390/ma13020337





