Influence of Micro-Arc Oxidation Coatings on Stress Corrosion of AlMg6 Alloy
Abstract
:1. Introduction
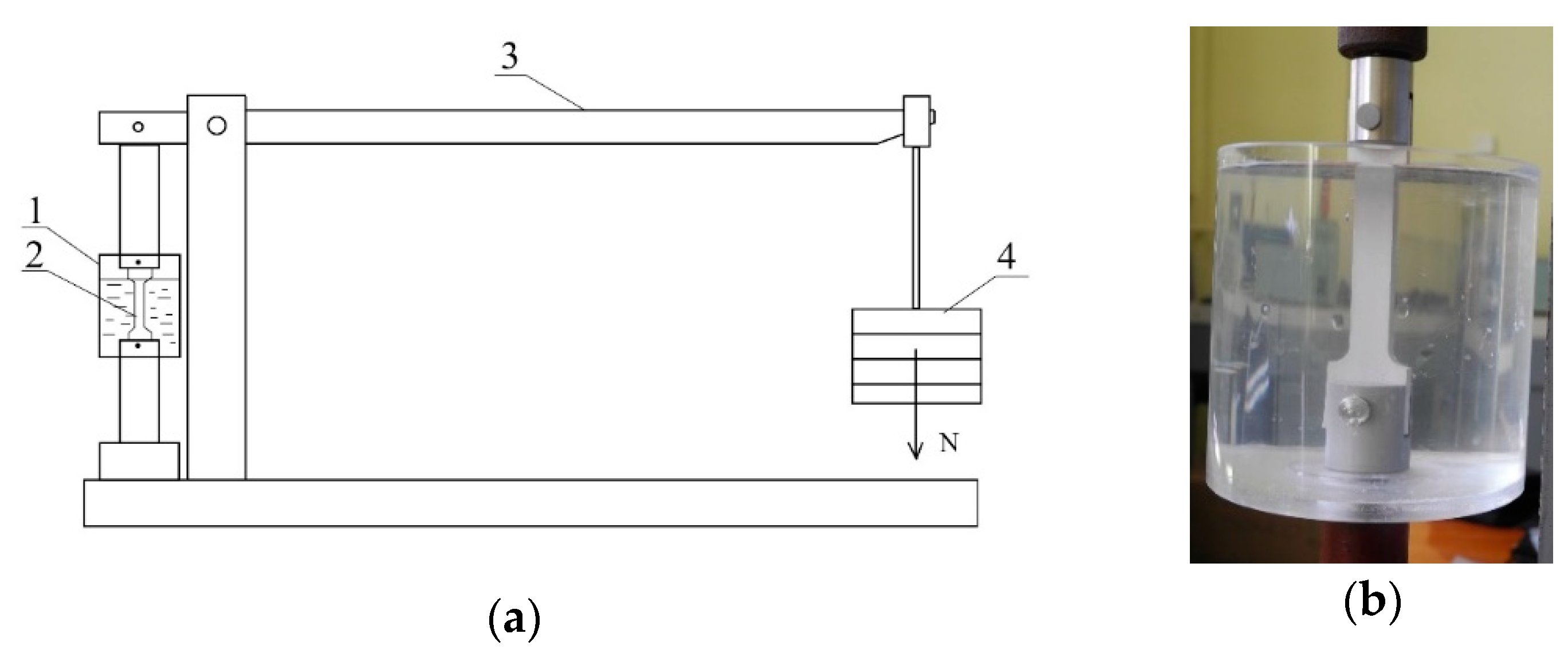
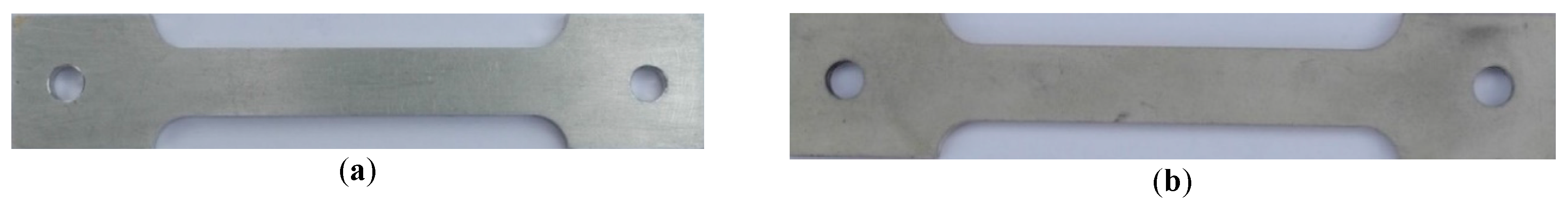
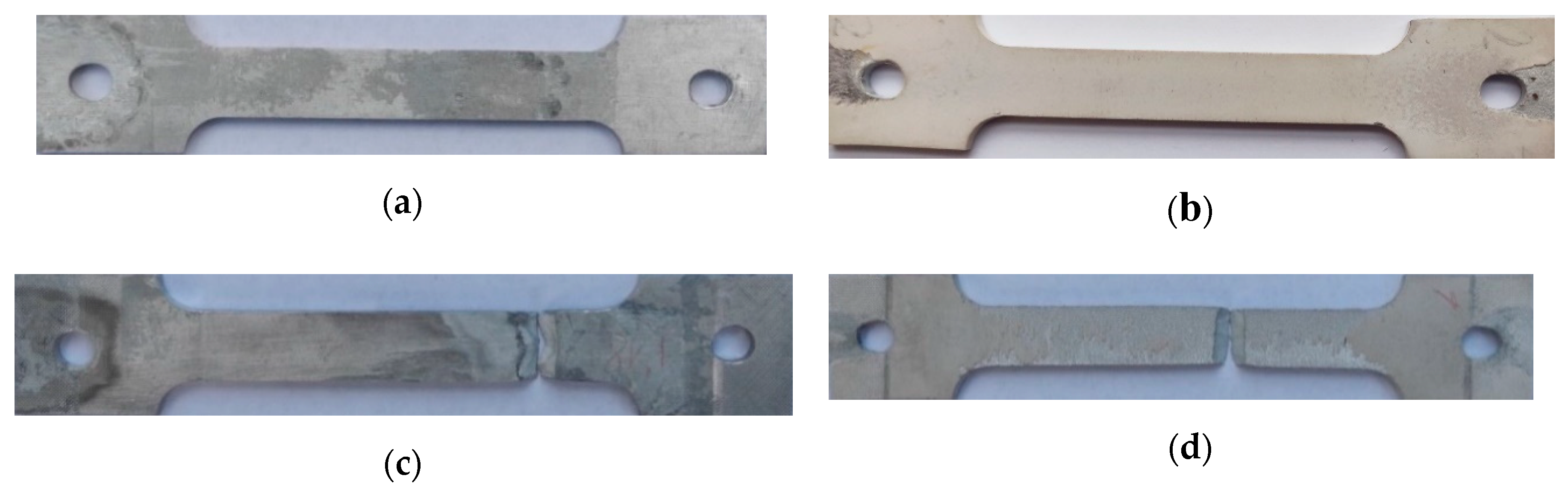
2. Materials and Methods
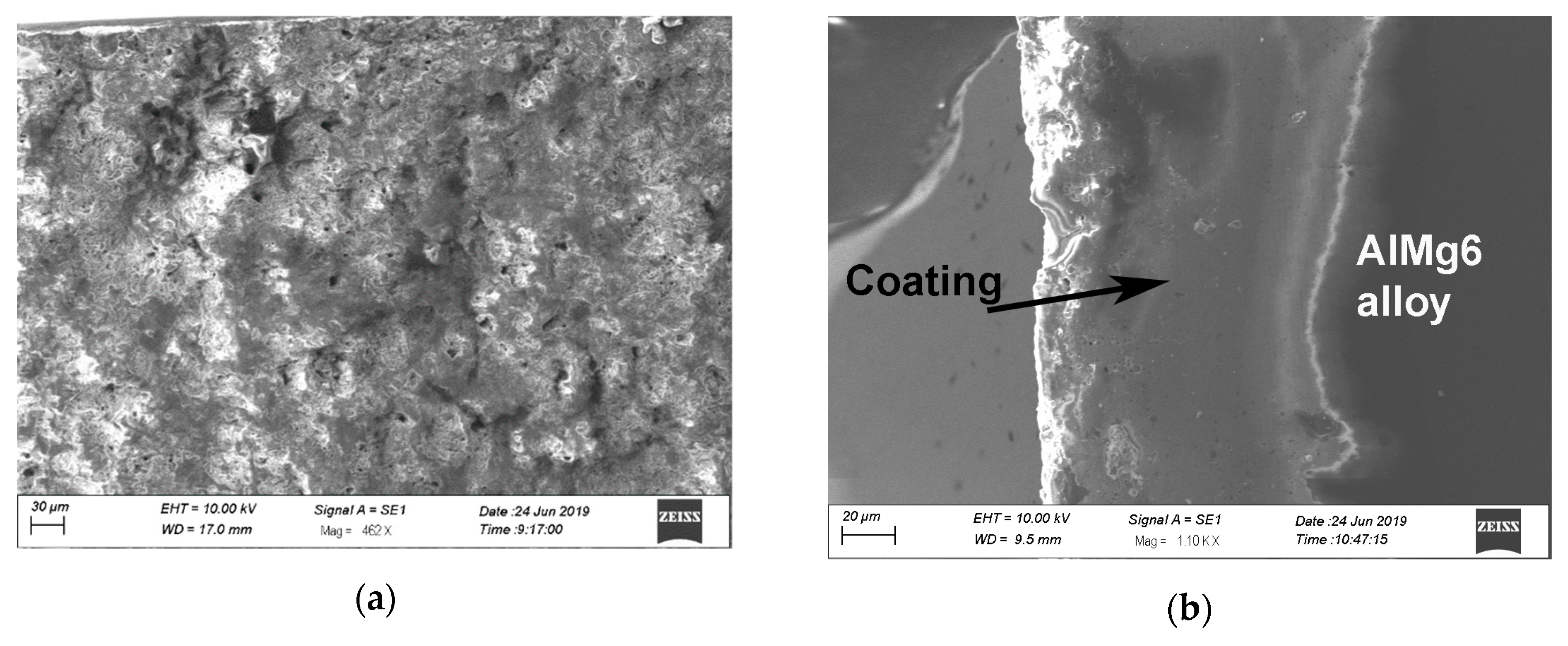
3. Research Results and Their Analysis
- -
- The samples without coating exhibited a decline of R0.2 by 15%, Rm by 6%, and A5 by over 20%;
- -
- The samples with coating did not exhibit any deterioration of mechanical properties. The protective coating served its purpose well, protecting the material against corrosion.
- -
- The samples without coating exhibited a decline of R0.2 by 20%, Rm by 8%, and A5 by over 36%;
- -
- The samples with coating exhibited an insignificant decline of R0.2 by 5%, no change, and A5 by 7%.
- -
- the samples without coating exhibited a decline of R0.2 by 25%, Rm by 18%, and A5 by over 50%;
- -
- the samples with coatings exhibited a decline of R0.2 by 3%, Rm by 6%, and A5 by 14%.
- -
- The samples without coating exhibited a decline of R0.2 by over 30%, Rm by 23%, and A5 by nearly 60%;
- -
- The samples with coatings exhibited a decline of R0.2 by 4%, Rm by 8%, and A5 by 18%.
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Kyzioł, L. Wpływ obróbki cieplnej na odporność na korozje naprężeniową i wytrzymałość zmęczeniowo−korozyjną stopów AlMg6 i AlZn5Mg2CrZr przeznaczonych na kadłuby okrętów. Ph.D. Thesis, Akademia Marynarki Wojennej, Gdynia, Poland, August 1990. [Google Scholar]
- Kyzioł, L.; Zganiacz, F. Wstępne badania korozji naprężeniowej stopów AlMg6 i AlMg4.5Mn. Zeszyty Naukowe AMW, Gdynia 1988, 2, 85–104. [Google Scholar]
- Cudny, K.; Jasiński, R.; Kyzioł, L. Badanie stopów AlMg6 i AlZn5Mg2CrZr w celu ich zastosowania w budownictwie okrętowym. Budownictwo Okrętowe i Gospodarka Morska, Gdańsk 1991, 9, 10–14. [Google Scholar]
- Sinavskij, V.S.; Volov, V.D.; Kalinin, V.D. Korrozja i zaŝĉita aluminievych splavov; Izdatielstvo Metallurgija: Moskva, Russia, 1986. [Google Scholar]
- Jones, R.H.; Vetrano, J.S.; Windisch, C., Jr. Stress corrosion cracking of Al-Mg and Mg-Al alloys. Corrosion 2004, 60, 1144–1154. [Google Scholar] [CrossRef]
- Torkan, A.; Rabiei, B.A.; Khakpour, I. Corrosion Behavior of AA5038 Nanostructured Aluminum Alloy Produced by Accumulative Roll-Bonding. Nanosci. Nanometr. 2018, 4, 34–40. [Google Scholar] [CrossRef]
- Gao, J.; David, J.Q. Enhancement of the Stress Corrosion Sensitivity of AA5083 by Heat Treatment. Metall. Mater. Trans. A 2011, 42A, 356–364. [Google Scholar] [CrossRef]
- Goswami, G.; Spanos, P.S.; Paoa, R.; Holtz, L. Precipitation behavior of the phase in Al-5083. Mater. Sci. Eng. 2010, 527, 1089–1095. [Google Scholar] [CrossRef]
- Choi, D.-H.; Ahn, B.-W.; Quesnel, D.J.; Seung, B.J. Behavior of β-phase (Al3Mg2) in AA 5083 during friction stir welding. Intermetallics 2013, 35, 120–127. [Google Scholar] [CrossRef]
- Radović, L.; Bučko, M.; Miladinov, M. Corrosion Behavior of TIG Welded AlMg6Mn Alloy. Sci. Tech. Rev. 2016, 66, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Romhanji, E.; Popović, M.; Radmilović, V. Room temperature deformation behaviour of AlMg6.5 alloy sheet. Z. Metallkunde 1999, 90, 305–310. [Google Scholar]
- Timoshenko, Y.B. On the relation between the Luders deformation and grain boundary structure in aluminium alloy. Revue Phys. Appl. 1990, 25, 1001–1004. [Google Scholar] [CrossRef]
- Radović, L.J.; Nikačević, M.; Jordović, B. Deformation behaviour and microstructure evolution of AlMg6Mn alloy during shear spinning . Trans. Nonferrous Met. Soc. China 2012, 22, 991–1000. [Google Scholar] [CrossRef]
- Radović, L.J.; Nikačević, M. Microstructure and properties of cold rolled and annealed Al-Mg alloys. Sci. Tech. Rev. 2008, 58, 14–20. [Google Scholar]
- Zhu, Y. Characterization of Beta Phase Growth and Experimental Validation of Long Term Thermal Exposure Sensitization of AA5xxx Alloys. Master’ Thesis, The University of Utah, Salt Lake City, UT, USA, 2013. [Google Scholar]
- Jones, R.H.; Baer, D.R.; Danielson, M.J.; Vetrano, J.S. Role of Mg in the Stress Corrosion Cracking of an Al-Mg Alloy. Metall. Mater. Trans. A 2001, 32A, 1699–1711. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Voevodin, A.A.; Lyubimov, V.V.; Zabinski, J.; Donley, M. Plasma Electrolytic Fabrication of Oxide Ceramic Surface Layers for Tribotechnical Purposes on Aluminium Alloys. Surf. Coat. Technol. 1998, 110, 140–146. [Google Scholar] [CrossRef]
- Vityaz’, P.A.; Komarov, A.I.; Komarova, V.I. Triboengineering properties of ceramic oxide coatings under boundary friction against steel. J Frict. Wear. 2008, 29, 325–329. [Google Scholar] [CrossRef]
- Nie, X.; Leyland, A.; Song, H.W.; Yerokhin, A.L.; Dowey, S.J.; Matthews, A. Thickness Effects on the Mechanical Properties of Micro-arc Discharge Oxide Coatings on Aluminium alloys. Surf. Coat. Technol. 1999, 116–119, 1055–1060. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Khrisanfova, O.A.; Zavidnaya, A.G.; Sinebrukhov, S.L.; Kovryanov, A.N.; Scorobogatova, T.M.; Gordienko, P.S. Production of Hard and Heat-Resistant Coatings on Aluminium Using a Plasma Micro-discharge. Surf. Coat. Technol. 2000, 123, 24–28. [Google Scholar] [CrossRef]
- Wang, Y.K.; Sheng, L.; Xiong, R.Z.; Li, B.S. Study of Ceramic Coatings Formed by Microarc Oxidation on Al Matrix Composite Surface. Surf. Eng. 1999, 15, 112–114. [Google Scholar] [CrossRef]
- Butyagin, P.I.; Khokhryakov, Y.V.; Mamaev, A.I. Microplasma Systems for Creating Coatings on Aluminium Alloys. Mater. Lett. 2003, 57, 1748–1751. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, C.; Wang, F.; Lou, H.; Cao, C. Study on the Environmentally Friendly Anodizing of AZ91D Magnesium Alloy. Surf. Coat. Technol. 2002, 161, 36–43. [Google Scholar] [CrossRef]
- Rama Krishna, L.; Somaraju, K.R.C.; Sundararajan, G. The Tribological Performance of Ultra-hard Ceramic Composite Coatings Obtained Through Microarc Oxidation. Surf. Coat. Technol. 2003, 163–164, 484–490. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Yerokhin, A.L.; Lyubimov, V.V.; Donley, M.S.; Zabinski, J.S. Characterization of Wear Protective Al-Si-O Coatings Formed on Al-based Alloys by Micro-arc Discharge Treatment. Surf. Coat. Technol. 1996, 86–87, 516–521. [Google Scholar] [CrossRef]
- Tian, J.; Luo, Z.; Qi, S.; Sun, X. Structure and Antiwear Behavior of Micro-arc Oxidized Coatings on Aluminum Alloy. Surf. Coat. Technol. 2002, 154, 1–7. [Google Scholar] [CrossRef]
- Kuhn, A.T. Plasma anodized aluminum-A 2000/2000 Ceramic Coating. Met. Fnish. 2002, 100, 44. [Google Scholar] [CrossRef]
- Nie, X.; Meletis, E.I.; Jiang, J.C.; Leyland, A.; Yerokhin, A.L.; Matthews, A. Abrasive Wear/Corrosion Properties and TEM Analysis of Al2O3 Coatings Fabricated Using Plasma Electrolysis. Surf. Coat. Technol. 2002, 149, 245–251. [Google Scholar] [CrossRef]
- Barik, R.C.; Wharton, J.A.; Wood, R.J.K.; Stokes, K.R.; Jones, R.L. Corrosion, Erosion and Erosion-Corrosion Performance of Plasma Electrolytic Oxidation (PEO) Deposited Al2O3 Coatings. Surf. Coat. Technol. 2005, 199, 158–167. [Google Scholar] [CrossRef]
- Nitin Wasekar, P.; Jyothirmayi, A.; Rama Krishna, L.; Sundararajan, G. Effect of Micro Arc Oxidation Coatings on Corrosion Resistance of 6061-Al Alloy 2008. J. Mater. Eng. Perform. 2008, 17, 708–713. [Google Scholar] [CrossRef]
- Sundararajan, G.; Rama Krishna, L. Mechanisms Underlying the Formation of Thick Alumina Coatings Through the MAO Coating Technology. Surf. Coat. Technol. 2003, 167, 269–277. [Google Scholar] [CrossRef]
- Guangliang, Y.; Xianyi, L.; Yizhen, B.; Haifeng, C.; Zengsun, J. The Effects of Current Density on the Phase Composition and Microstructure Properties of Micro-arc Oxidation Coating. J. Alloy Compd. 2002, 345, 196–200. [Google Scholar] [CrossRef]
- Sobolev, A.; Kossenko, A.; Zinigrad, M.; Borodianskiy, K. Comparison of plasma electrolytic oxidation coatings on Al alloy created in aqueous solution and molten salt electrolytes. Surf. Coat. Technol. 2018, 344, 590–595. [Google Scholar] [CrossRef]
- Hua, S.; Song, R.G.; Zong, Y.; Cai, S.W.; Wang, C. Effect of solution pH on stress corrosion and electrochemical behavior of aluminum alloy with micro-arc oxidation coating. Mater. Res. Express 2019, 6, 096441. [Google Scholar] [CrossRef]
- Lianxi, C.; Yinying, S.; Hanyu, Z.; Zhibin, L.; Xiaojian, W.; Wei, L. Influence of a MAO + PLGA coating on biocorrosion and stress corrosion cracking behavior of a magnesium alloy in a physiological environment. Corros. Sci. 2019, 148, 134–143. [Google Scholar]






| Material | Sheet Thickness, mm | Parameters of Production Technology | Chemical Composition, % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mg | Mn | Ti | Zn | Si | Fe | Cu | Al | |||
| AlMg6 | 6 | annealing at temp. 319 °C/10 h | 6.15 | 0.61 | 0.05 | 0.05 | 0.16 | 0.27 | 0.05 | rest |
| State of the Samples. | Yield Strength R0.2 | Tensile Strength Rm | Relative Elongation A5 |
|---|---|---|---|
| without coatings | 211 | 373 | 13.25 |
| 215 | 372 | 13.45 | |
| 210 | 371 | 13.20 | |
| average | 212 | 372 | 13.30 |
| with coatings | 215 | 369 | 13.65 |
| 218 | 371 | 13.45 | |
| 218 | 370 | 13.40 | |
| average | 217 | 370 | 13.50 |
| State of the Samples | Stress Level | |||
|---|---|---|---|---|
| Without coatings | 185 | 352 | 10.25 | |
| 182 | 351 | 10.32 | ||
| 178 | 347 | 10.33 | ||
| Average | 180 | 350 | 10.30 | |
| With coatings | 212 | 365 | 12.85 | |
| 209 | 374 | 13.18 | ||
| 209 | 371 | 12.97 | ||
| Average | 210 | 370 | 13.00 | |
| Without coatings | 167 | 338 | 8.45 | |
| 171 | 343 | 8.65 | ||
| 172 | 339 | 8.40 | ||
| Average | 170 | 340 | 8.50 | |
| With coatings | 208 | 368 | 12.40 | |
| 210 | 361 | 12.65 | ||
| 206 | 366 | 12.45 | ||
| Average | 208 | 365 | 12.50 |
| State of the Samples | Stress Level | |||
|---|---|---|---|---|
| Without coatings | 155 | 303 | 6.48 | |
| 162 | 307 | 6.54 | ||
| 163 | 305 | 6.48 | ||
| Average | 160 | 305 | 6.50 | |
| With coatings | 213 | 354 | 11.62 | |
| 209 | 342 | 11.48 | ||
| 208 | 348 | 11.40 | ||
| Average | 210 | 348 | 11.50 | |
| Without coatings | 140 | 288 | 5.48 | |
| 148 | 280 | 5.53 | ||
| 147 | 287 | 5.55 | ||
| average | 145 | 285 | 5.52 | |
| With coatings | 208 | 341 | 10.98 | |
| 205 | 336 | 11.12 | ||
| 202 | 341 | 10.90 | ||
| Average | 205 | 340 | 11.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyzioł, L.; Komarov, A. Influence of Micro-Arc Oxidation Coatings on Stress Corrosion of AlMg6 Alloy. Materials 2020, 13, 356. https://doi.org/10.3390/ma13020356
Kyzioł L, Komarov A. Influence of Micro-Arc Oxidation Coatings on Stress Corrosion of AlMg6 Alloy. Materials. 2020; 13(2):356. https://doi.org/10.3390/ma13020356
Chicago/Turabian StyleKyzioł, Lesław, and Aleksandr Komarov. 2020. "Influence of Micro-Arc Oxidation Coatings on Stress Corrosion of AlMg6 Alloy" Materials 13, no. 2: 356. https://doi.org/10.3390/ma13020356
APA StyleKyzioł, L., & Komarov, A. (2020). Influence of Micro-Arc Oxidation Coatings on Stress Corrosion of AlMg6 Alloy. Materials, 13(2), 356. https://doi.org/10.3390/ma13020356





