Graphene Oxide-Assisted Morphology and Structure of Electrodeposited ZnO Nanostructures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. rGO-Assisted Electrodeposition of ZnO Nanostructured Films
2.3. Characterization
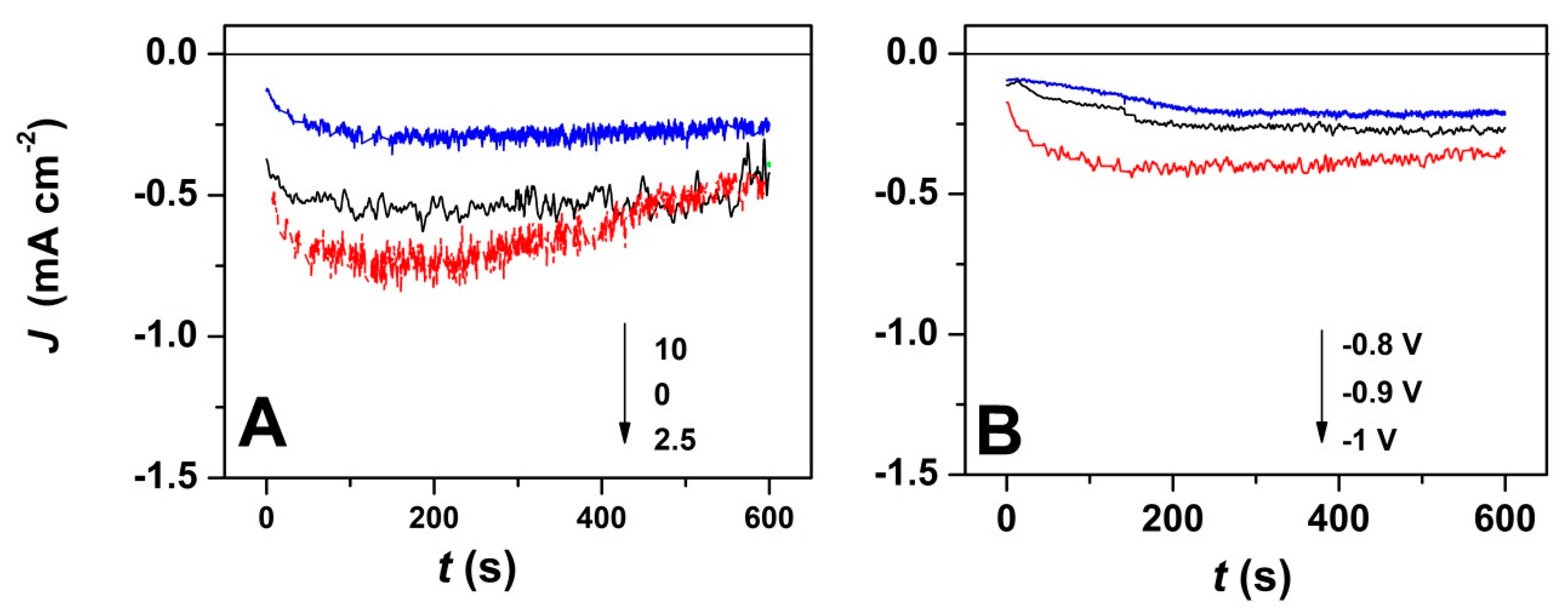
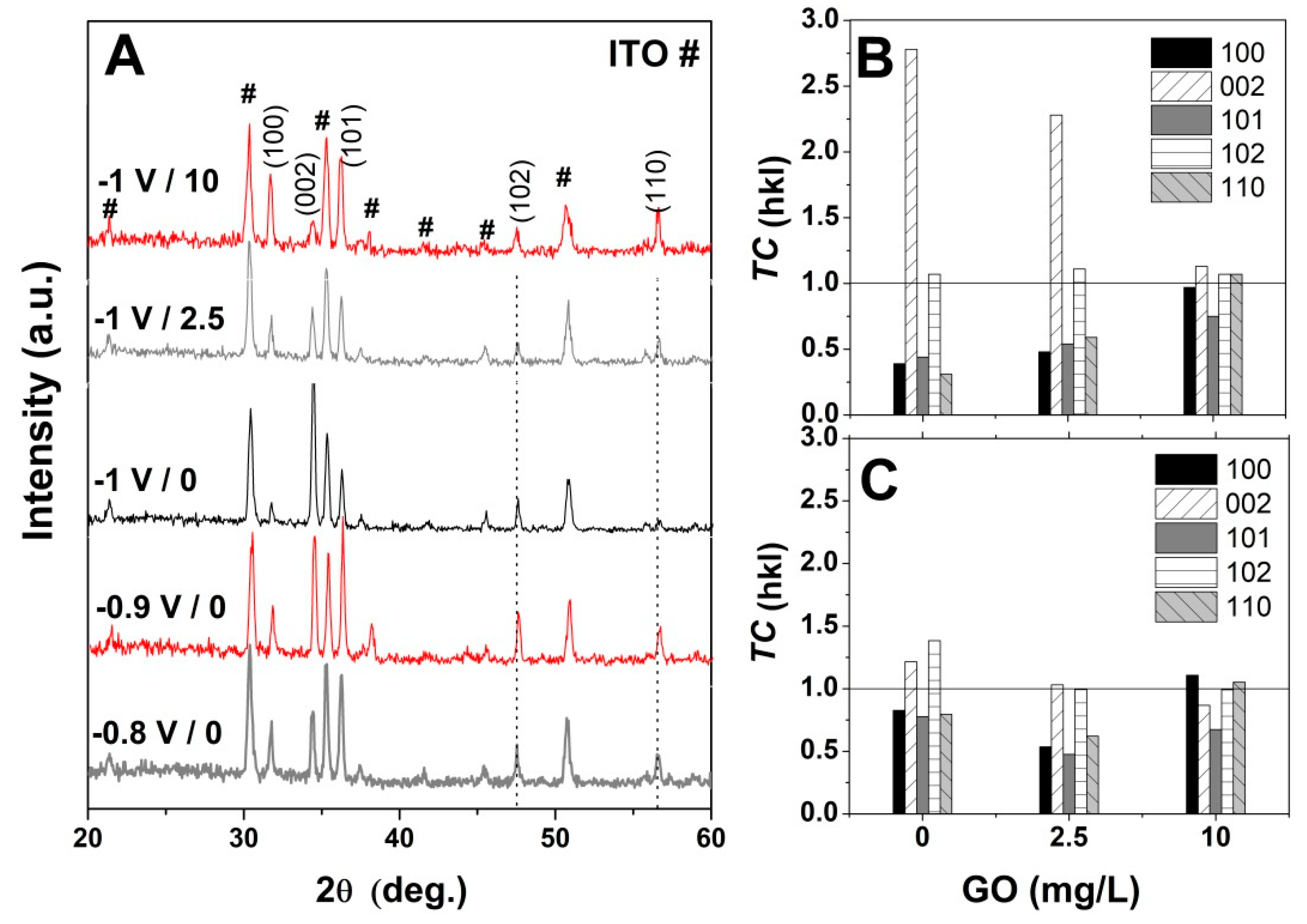
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, T.; Chen, M.; Kong, Q.; Wang, X.; Guo, X.; Li, W.; Jiao, K. Shape-controllable ZnO nanostructures based on synchronously electrochemically reduced graphene oxide and their morphology-dependent electrochemical performance. Electrochim. Acta 2015, 182, 1037–1045. [Google Scholar] [CrossRef]
- Rosas-Laverde, N.M.; Pruna, A.; Cembrero, J.; Orozco-Messana, J.; Manjón, F.J. Performance of graphene oxide-modified electrodeposited ZnO/Cu2O heterojunction solar cells. Boletín Soc. Española Cerámica Y Vidr. 2019, 58, 263–273. [Google Scholar] [CrossRef]
- Rosas-Laverde, N.M.; Pruna, A.; Busquets-Mataix, D.; Marí, B.; Cembrero, J.; Salas Vicente, F.; Orozco-Messana, J. Improving the properties of Cu2O/ZnO heterojunction for photovoltaic application by graphene oxide. Ceram. Int. 2018, 44, 23045–23051. [Google Scholar] [CrossRef]
- Zhang, Z.; Ren, L.; Han, W.; Meng, L.; Wei, X.; Qi, X.; Zhong, J. One-pot electrodeposition synthesis of ZnO/graphene composite and its use as binder-free electrode for supercapacitor. Ceram. Int. 2015, 41, 4374–4380. [Google Scholar] [CrossRef]
- Psychoyios, V.N.; Nikoleli, G.-P.P.; Tzamtzis, N.; Nikolelis, D.P.; Psaroudakis, N.; Danielsson, B.; Israr, M.Q.; Willander, M. Potentiometric Cholesterol Biosensor Based on ZnO Nanowalls and Stabilized Polymerized Lipid Film. Electroanalysis 2013, 25, 367–372. [Google Scholar] [CrossRef]
- Jiang, X.; Lin, Q.; Zhang, M.; He, G.; Sun, Z. Microstructure, optical properties, and catalytic performance of Cu2O-modified ZnO nanorods prepared by electrodeposition. Nanoscale Res. Lett. 2015, 10, 2–7. [Google Scholar] [CrossRef]
- Cui, J. Zinc oxide nanowires. Mater. Charact. 2012, 64, 43–52. [Google Scholar] [CrossRef]
- Da Fonseca, A.F.V.; Siqueira, R.L.; Landers, R.; Ferrari, J.L.; Marana, N.L.; Sambrano, J.R.; de La Porta, F.A.; Schiavon, M.A. A theoretical and experimental investigation of Eu-doped ZnO nanorods and its application on dye sensitized solar cells. J. Alloys Compd. 2018, 739, 939–947. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.-G.; Xi, R.; Zhang, L.; Zhang, S.-H.; Wang, L.-J.; Pan, G.-B. In situ synthesis of flower-like ZnO on GaN using electrodeposition and its application as ethanol gas sensor at room temperature. Sens. Actuators B Chem. 2019, 292, 270–276. [Google Scholar] [CrossRef]
- Marimuthu, T.; Anandhan, N.; Thangamuthu, R.; Surya, S.; Panneerselvam, R.; Ganesan, K.P. Effect of Deposition Potential and Bath Temperature on One-Step Electrochemical Synthesis of One and Two Dimensional Nanostructured ZnO Thin Films on Fluorine Doped Tin Oxide Substrates. J. Nanosci. Nanotechnol. 2019, 19, 7014–7025. [Google Scholar] [CrossRef]
- Rosas-Laverde, N.M.; Pruna, A. Electrodeposition of ZnO Nanostructured Films for Photovoltaics and Photoelectrochemical Sensing. In ZnO Thin Films Properties, Performance and Applications; Mele, P., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2019; ISBN 978-1-53616-086-4. [Google Scholar]
- Al-Nassar, S.I.; Hussein, F.I.; Adel, K.M. The effect of laser pulse energy on ZnO nanoparticles formation by liquid phase pulsed laser ablation. J. Mater. Res. Technol. 2019, 8, 4026–4031. [Google Scholar] [CrossRef]
- Ozerov, I.; Bulgakov, A.V.; Nelson, D.K.; Castell, R.; Marine, W. Production of gas phase zinc oxide nanoclusters by pulsed laser ablation. Appl. Surf. Sci. 2005, 247, 1–7. [Google Scholar] [CrossRef][Green Version]
- Shao, Q.; Chen, S.Q.Y.; Yeung, O.L.; Foo, Y.S.; Ng, S.M.; Zapien, J.A.; Leung, C.W.; Ruotolo, A. Magnetism as a tool for band-gap narrowing of zinc oxide films prepared by sol–gel method. J. Sol Gel Sci. Technol. 2016, 77, 240–243. [Google Scholar] [CrossRef]
- Wang, X.L.; Luan, C.Y.; Shao, Q.; Pruna, A.; Leung, C.W.; Lortz, R.; Zapien, J.A.; Ruotolo, A. Effect of the magnetic order on the room-temperature band-gap of Mn-doped ZnO thin films. Appl. Phys. Lett. 2013, 102, 102112. [Google Scholar] [CrossRef]
- Haga, K.; Kamidaira, M.; Kashiwaba, Y.; Sekiguchi, T.; Watanabe, H. ZnO thin films prepared by remote plasma-enhanced CVD method. J. Cryst. Growth 2000, 214–215, 77–80. [Google Scholar] [CrossRef]
- Abd Samad, N.A.; Lai, C.W.; Abd Hamid, S.B. Easy Formation of Nanodisk-Dendritic ZnO Film via Controlled Electrodeposition Process. J. Nanomater. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Künze, S.; Schlettwein, D. Electrochemical and electroless deposition of porous zinc oxide on aluminium. Electrochim. Acta 2014, 128, 360–367. [Google Scholar] [CrossRef]
- Neuthe, K.; Bittner, F.; Stiemke, F.; Ziem, B.; Du, J.; Zellner, M.; Wark, M.; Schubert, T.; Haag, R. Phosphonic acid anchored ruthenium complexes for ZnO-based dye-sensitized solar cells. Dyes Pigments 2014, 104, 24–33. [Google Scholar] [CrossRef]
- Ichinose, K.; Mizuno, T.; Schuette White, M.; Yoshida, T. Control of Nanostructure and Crystallographic Orientation in Electrodeposited ZnO Thin Films via Structure Directing Agents. J. Electrochem. Soc. 2014, 161, D195–D201. [Google Scholar] [CrossRef]
- Nguyen, T.H.Q.; Ruess, R.; Schlettwein, D. Adjusting Porosity and Pore Radius of Electrodeposited ZnO Photoanodes. J. Electrochem. Soc. 2019, 166, B3040–B3046. [Google Scholar] [CrossRef]
- Zou, J.-P.; Ma, J.; Huang, Q.; Luo, S.-L.; Yu, J.; Luo, X.-B.; Dai, W.-L.; Sun, J.; Guo, G.-C.; Au, C.-T.; et al. Graphene oxide as structure-directing and morphology-controlling agent for the syntheses of heterostructured graphene-Bi2MoO6/Bi3.64Mo0.36O6.55 composites with high photocatalytic activity. Appl. Catal. B Environ. 2014, 156, 447–455. [Google Scholar] [CrossRef]
- Wei, A.; Xiong, L.; Sun, L.; Liu, Y.; Li, W.; Lai, W.; Liu, X.; Wang, L.; Huang, W.; Dong, X. One-step electrochemical synthesis of a graphene–ZnO hybrid for improved photocatalytic activity. Mater. Res. Bull. 2013, 48, 2855–2860. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T. Graphene oxide as a structure-directing agent for the two-dimensional interface engineering of sandwich-like graphene-G-C3 N4 hybrid nanostructures with enhanced visible-light photoreduction of CO2 to methane. Chem. Commun. 2015, 51, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1957, 208, 1937. [Google Scholar] [CrossRef]
- Du, Y.; Yao, H.; Zhao, L.; Yang, H.; Wang, M.; Yuan, L.; Xu, Y.; Li, J. Graphene Oxide Induced High Crystallinity of SAPO-11 Molecular Sieves for Improved Alkane Isomerization Performance. ChemNanoMat 2019, 5, 1225–1232. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Xiong, T.; Xu, A.; Pan, B.; Tang, K. Graphene Oxide Reinforced Alginate/PVA Double Network Hydrogels for Efficient Dye Removal. Polymer 2018, 10, 835. [Google Scholar] [CrossRef]
- Muthu Prabhu, S.; Park, C.M.; Shahzad, A.; Lee, D.S. Designed synthesis of sulfide-rich bimetallic-assembled graphene oxide sheets as flexible materials and self-tuning adsorption cum oxidation mechanisms of arsenic from water. J. Mater. Chem. A 2019, 7, 12253–12265. [Google Scholar] [CrossRef]
- Yang, J.; Hao, J.; Xu, S.; Dai, J.; Wang, Y.; Pang, X. Visible-light-driven photocatalytic degradation of 4-CP and the synergistic reduction of Cr(VI) on one-pot synthesized amorphous Nb2O5 nanorods/graphene heterostructured composites. Chem. Eng. J. 2018, 353, 100–114. [Google Scholar] [CrossRef]
- Li, F.; Xie, L.; Sun, G.; Kong, Q.; Su, F.; Lei, H.; Guo, X.; Zhang, B.; Chen, C. Regulating pore structure of carbon aerogels by graphene oxide as ‘shape-directing’ agent. Microporous Mesoporous Mater. 2017, 240, 145–148. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, Q.; Qu, N.; Yang, H.; Wang, M.; Liu, A.; Yang, J. Ultrathin 2D nitrogen-doped carbon nanosheets for high performance supercapacitors: Insight into the effects of graphene oxides. Nanoscale 2019, 11, 8588–8596. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, M.; Liu, Q.; Wang, X.; Lv, T.; Jia, L. Graphene oxide mediated cellulose-derived carbon as a highly selective catalyst for the hydrolysis of cellulose to glucose. Appl. Catal. A Gen. 2017, 545, 167. [Google Scholar] [CrossRef]
- Cai, J.; Lu, J.-Y.; Chen, Q.-Y.; Qu, L.-L.; Lu, Y.-Q.; Gao, G.-F. Eu-Based MOF/graphene oxide composite: A novel photocatalyst for the oxidation of benzyl alcohol using water as oxygen source. New J. Chem. 2017, 41, 3882–3886. [Google Scholar] [CrossRef]
- Zhang, L.; He, W.; Ling, M.; Shen, K.; Liu, Y.; Guo, S. Self-standing MgMoO4/Reduced Graphene Oxide Nanosheet Arrays for Lithium and Sodium Ion Storage. Electrochim. Acta 2017, 252, 322–330. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, Y.; Chen, W. Graphene nanosheet-tailored PtPd concave nanocubes with enhanced electrocatalytic activity and durability for methanol oxidation. Nanoscale 2014, 6, 3309–3315. [Google Scholar] [CrossRef] [PubMed]
- Teh, S.J.; Yeoh, S.L.; Lee, K.M.; Lai, C.W.; Abdul Hamid, S.B.; Thong, K.L. Effect of reduced graphene oxide-hybridized ZnO thin films on the photoinactivation of Staphylococcus aureus and Salmonella enterica serovar Typhi. J. Photochem. Photobiol. B Biol. 2016, 161, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Pruna, A.; Wu, Z.; Zapien, J.A.A.; Li, Y.Y.Y.; Ruotolo, A. Enhanced photocatalytic performance of ZnO nanostructures by electrochemical hybridization with graphene oxide. Appl. Surf. Sci. 2018, 441, 936–944. [Google Scholar] [CrossRef]
- Bu, Y.; Chen, Z.; Li, W.; Hou, B. Highly Efficient Photocatalytic Performance of Graphene–ZnO Quasi-Shell–Core Composite Material. ACS Appl. Mater. Interfaces 2013, 5, 12361–12368. [Google Scholar] [CrossRef]
- Pruna, A.; Cembrero, J.; Pullini, D.; Mocioiu, A.M.; Busquets-Mataix, D. Effect of reduced graphene oxide on photocatalytic properties of electrodeposited ZnO. Appl. Phys. A 2017, 123, 792. [Google Scholar] [CrossRef]
- Maiti, S.; Pal, S.; Chattopadhyay, K.K. Recent advances in low temperature, solution processed morphology tailored ZnO nanoarchitectures for electron emission and photocatalysis applications. CrystEngComm 2015, 17, 9264–9295. [Google Scholar] [CrossRef]
- Pruna, A.; Pullini, D.; Busquets, D. Structure and Properties of Chemically-reduced Functionalized Graphene Oxide Platelets. J. Mater. Sci. Technol. 2015, 31, 458–462. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, Y.; Zhai, Y.; Zhai, J.; Ren, W.; Wang, F.; Dong, S. Controlled Synthesis of Large-Area and Patterned Electrochemically Reduced Graphene Oxide Films. Chem. A Eur. J. 2009, 15, 6116–6120. [Google Scholar] [CrossRef] [PubMed]
- Septina, W.; Ikeda, S.; Khan, M.A.; Hirai, T.; Harada, T.; Matsumura, M.; Peter, L.M. Potentiostatic electrodeposition of cuprous oxide thin films for photovoltaic applications. Electrochim. Acta 2011, 56, 4882–4888. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, X.; Li, J.; Dong, S. The large-area preparation and photoelectrochemical properties of graphene/ZnO nanorod composite film. RSC Adv. 2017, 7, 55673–55679. [Google Scholar] [CrossRef]
- Sánchez Zeferino, R.; Barboza Flores, M.; Pal, U. Photoluminescence and raman scattering in ag-doped zno nanoparticles. J. Appl. Phys. 2011, 109, 014208. [Google Scholar]
- Wu, S.; Yin, Z.; He, Q.; Huang, X.; Zhou, X.; Zhang, H. Electrochemical deposition of semiconductor oxides on reduced graphene oxide-based flexible, transparent, and conductive electrodes. J. Phys. Chem. C 2010, 114, 11816–11821. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; He, J. Controlled fabrication and photocatalytic properties of a three-dimensional ZnO nanowire/reduced graphene oxide/CdS heterostructure on carbon cloth. Nanoscale 2013, 5, 11291–11297. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.Q.; Xu, Y.J. Morphology control, defect engineering and photoactivity tuning of ZnO crystals by graphene oxide—A unique 2D macromolecular surfactant. Phys. Chem. Chem. Phys. 2014, 16, 5589–5599. [Google Scholar] [CrossRef]
- Skompska, M.; Zarȩbska, K. Electrodeposition of ZnO nanorod arrays on transparent conducting substrates—A review. Electrochim. Acta 2014, 127, 467–488. [Google Scholar] [CrossRef]
- Arslan, A.; Hür, E.; Ilican, S.; Caglar, Y.; Caglar, M. Controlled growth of c-axis oriented ZnO nanorod array films by electrodeposition method and characterization. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 716–723. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, D.; Yang, L.; Li, C.; Liu, Y.; Lu, J.; Wang, Y. Preparation of 3D urchin-like RGO/ZnO and its photocatalytic activity. J. Mater. Sci. Mater. Electron. 2017, 28, 7935–7942. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Li, W.; He, Y. Photoelectric conversion properties of electrochemically codeposited graphene oxide–ZnO nanocomposite films. J. Alloys Compd. 2015, 648, 942–950. [Google Scholar] [CrossRef]
- Ambrosi, A.; Pumera, M. Precise tuning of surface composition and electron-transfer properties of graphene oxide films through electroreduction. Chem. A Eur. J. 2013, 19, 4748–4753. [Google Scholar] [CrossRef] [PubMed]
- Gutić, S.J.; Kozlica, D.K.; Korać, F.; Bajuk-Bogdanović, D.; Mitrić, M.; Mirsky, V.M.; Mentus, S.V.; Pašti, I.A. Electrochemical tuning of capacitive response of graphene oxide. Phys. Chem. Chem. Phys. 2018, 20, 22698–22709. [Google Scholar] [CrossRef] [PubMed]
- Viinikanoja, A.; Wang, Z.; Kauppila, J.; Kvarnström, C. Electrochemical reduction of graphene oxide and its in situ spectroelectrochemical characterization. Phys. Chem. Chem. Phys. 2012, 14, 14003–14009. [Google Scholar] [CrossRef] [PubMed]
- Moshgi Asl, S.; Afshar, A.; Yaghoubinezhad, Y. An Electrochemical Synthesis of Reduced Graphene Oxide/Zinc Nanocomposite Coating through Pulse-Potential Electrodeposition Technique and the Consequent Corrosion Resistance. Int. J. Corros. 2018, 2018, 3028693. [Google Scholar] [CrossRef]
- Henni, A.; Harfouche, N.; Karar, A.; Zerrouki, D.; Perrin, F.X.; Rosei, F. Synthesis of graphene–ZnO nanocomposites by a one-step electrochemical deposition for efficient photocatalytic degradation of organic pollutant. Solid State Sci. 2019, 98, 106039. [Google Scholar] [CrossRef]
- Oekermann, T.; Yoshida, T.; Schlettwein, D.; Sugiura, T.; Minoura, H. Photoelectrochemical properties of ZnO/tetrasulfophthalocyanine hybrid thin films prepared by electrochemical self assembly. Phys. Chem. Chem. Phys. 2001, 3, 3387–3392. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosas-Laverde, N.M.; Pruna, A.; Busquets-Mataix, D.; Pullini, D. Graphene Oxide-Assisted Morphology and Structure of Electrodeposited ZnO Nanostructures. Materials 2020, 13, 365. https://doi.org/10.3390/ma13020365
Rosas-Laverde NM, Pruna A, Busquets-Mataix D, Pullini D. Graphene Oxide-Assisted Morphology and Structure of Electrodeposited ZnO Nanostructures. Materials. 2020; 13(2):365. https://doi.org/10.3390/ma13020365
Chicago/Turabian StyleRosas-Laverde, N. Ma., A. Pruna, D. Busquets-Mataix, and D. Pullini. 2020. "Graphene Oxide-Assisted Morphology and Structure of Electrodeposited ZnO Nanostructures" Materials 13, no. 2: 365. https://doi.org/10.3390/ma13020365
APA StyleRosas-Laverde, N. M., Pruna, A., Busquets-Mataix, D., & Pullini, D. (2020). Graphene Oxide-Assisted Morphology and Structure of Electrodeposited ZnO Nanostructures. Materials, 13(2), 365. https://doi.org/10.3390/ma13020365





