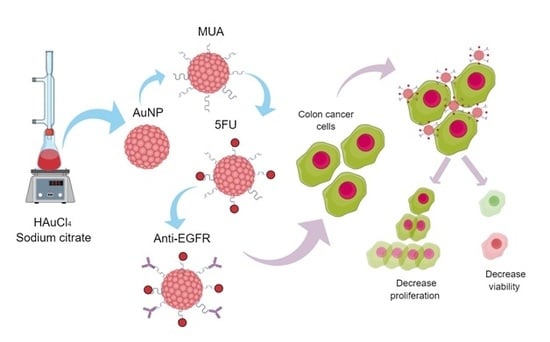
Anti-EGFR-Coated Gold Nanoparticles In Vitro Carry 5-Fluorouracil to Colorectal Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Gold Nanoparticles and Surface Functionalization Based on 11-Mercaptoundecanoic Acid
2.2. Carboxyl Modification by EDC/NHS
2.3. Fluorouracil and Anti-EGFR Incorporation
2.4. Transmission Electron Microscopy
2.5. Zeta Potential (ζ)
2.6. Calculating the Number of Particles
- N = number of particles/mL;
- A450 = absorbance under 450 nm;
- d = particles’ diameter (nm).
2.7. Colorectal Cancer Cell Lines
2.8. Challenging Tumor Cells In Vitro with AuNP Compounds
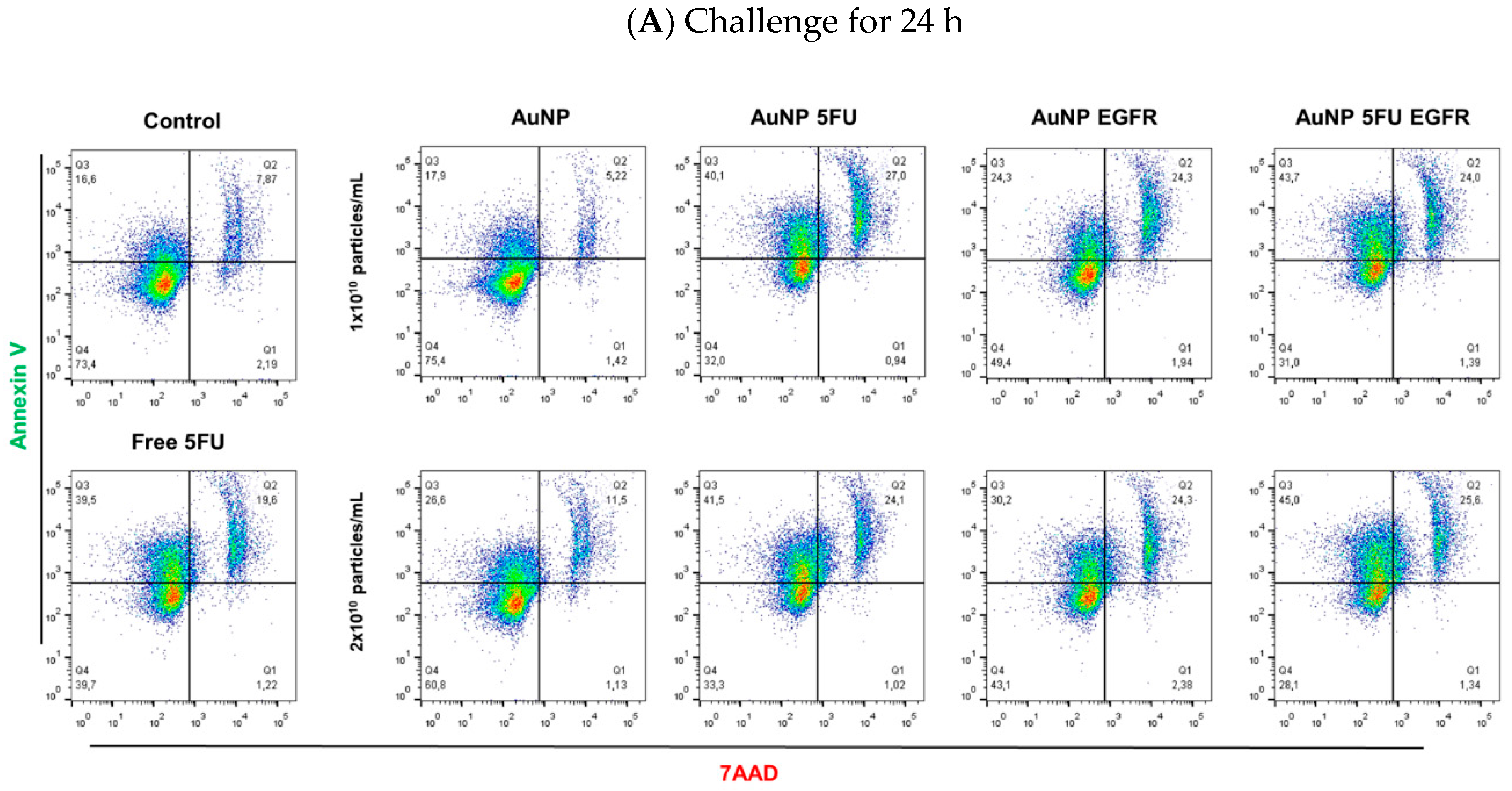
2.9. Tumor Cell Death
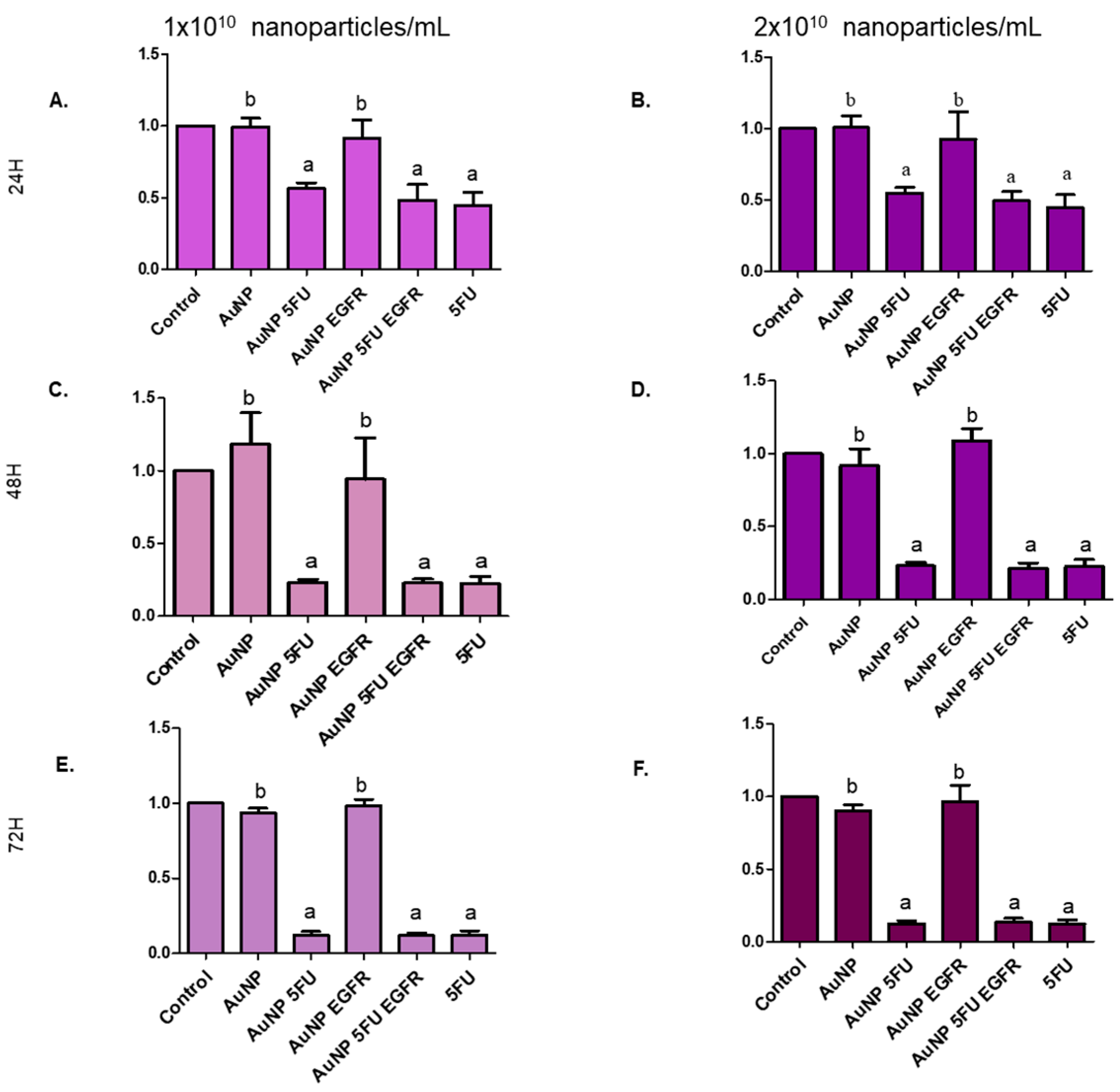
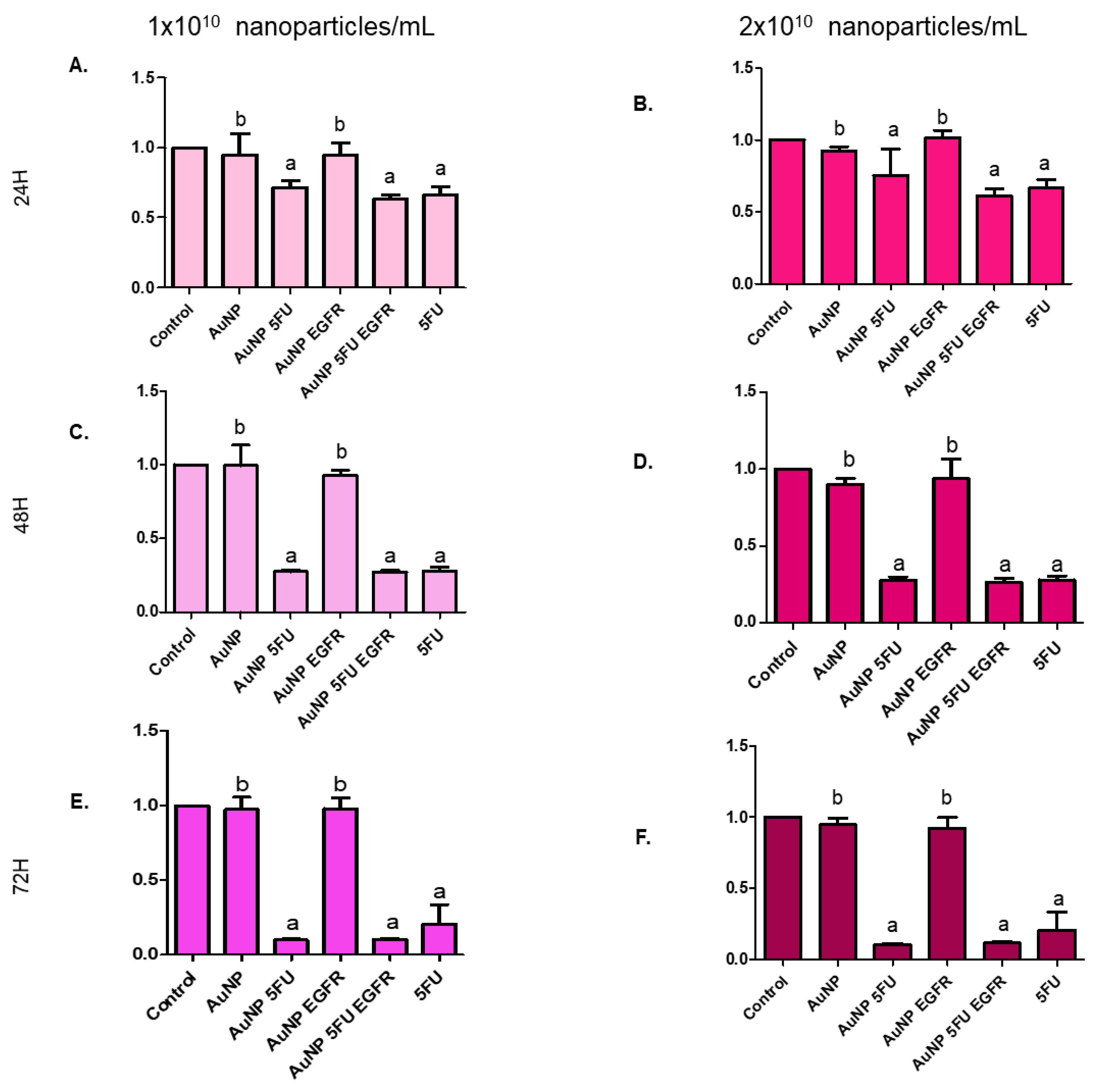
2.10. Cell Proliferation Assay
2.11. Statistical Analysis
3. Results
3.1. Physicochemical Characterization of AuNP
3.2. AuNP 5FU EGFR Induces Apoptosis in Colorectal Tumor Cells
3.3. Nanoparticles Carrying 5FU Have an Antiproliferative Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Arvelo, F.; Sojo, F.; Cotte, C. Biology of colorectal cancer. Ecancermedicalscience 2015, 9, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, N.H.; Cavalcante, L.L.; Lubner, S.J.; Mulkerin, D.L.; LoConte, N.K.; Clipson, L.; Matkowskyj, K.A.; Deming, D.A. Precision medicine in colorectal cancer: The molecular profile alters treatment strategies. Ther. Adv. Med Oncol. 2015, 7, 252–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, W.; Yang, W.; Lou, G.; Jiang, J.; Geng, M.; Xi, W.; Li, H.; Ma, T.; Jin, Y. Phase II trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) as first-line treatment for advanced gastric cancer. Anti Cancer Drugs 2009, 20, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Kanamoto, A.; Miyanaga, S.; Noto, M.; Takeda, T.; Tani, T.; Yagi, M. [The present status of CapeOX as adjuvant chemotherapy for colorectal cancer]. Gan to kagaku ryoho. Cancer Chemother. 2015, 42, 319–322. [Google Scholar]
- Schilsky, R.L. Biochemical and clinical pharmacology of 5-fluorouracil. Oncology 1998, 12, 13–18. [Google Scholar]
- Chen, L.; She, X.; Wang, T.; He, L.; Shigdar, S.; Duan, W.; Kong, L. Overcoming acquired drug resistance in colorectal cancer cells by targeted delivery of 5-FU with EGF grafted hollow mesoporous silica nanoparticles. Nanoscale 2015, 7, 14080–14092. [Google Scholar] [CrossRef]
- Posner, M.R.; Darnowski, J.W.; Weitberg, A.B.; Dudley, M.N.; Corvese, D.; Cummings, F.J.; Clark, J.; Murray, C.; Clendennin, N.; Bigley, J.; et al. High-dose intravenous zidovudine with 5-fluorouracil and leucovorin. A phase I trial. Cancer 1992, 70, 2929–2934. [Google Scholar] [CrossRef]
- Cohen, S.S.; Flaks, J.G.; Barner, H.D.; Loeb, M.R.; Lichtenstein, J. The Mode of Action of 5-Fluorouracil and Its Derivatives. Proc. Natl. Acad. Sci. USA 1958, 44, 1004–1012. [Google Scholar] [CrossRef] [Green Version]
- Bardelli, A.; Janne, P.A. The road to resistance: EGFR mutation and cetuximab. Nat. Med. 2012, 18, 199–200. [Google Scholar] [CrossRef]
- Fan, C.W.; Fan, H.A.; Hsu, S.H.; Chan, C.C.; Chen, S.Y.; Hsu, Y.H.; Chan, E.C. An in vitro short time-high dose drug exposure assay for predicting 5FU-resistance of colorectal cancer. Cancer Lett. 2004, 214, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Austin, L.A.; Mackey, M.A.; El-Sayed, M.A. Size matters: Gold nanoparticles in targeted cancer drug delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leite, P.E.C.; Pereira, M.R.; Harris, G.; Pamies, D.; Dos Santos, L.M.G.; Granjeiro, J.M.; Hogberg, H.T.; Hartung, T.; Smirnova, L. Suitability of 3D human brain spheroid models to distinguish toxic effects of gold and poly-lactic acid nanoparticles to assess biocompatibility for brain drug delivery. Part. Fibre Toxicol. 2019, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Jabir, M.S.; Taha, A.A.; Sahib, U.I.; Taqi, Z.J.; Al-Shammari, A.M.; Salman, A.S. Novel of nano delivery system for Linalool loaded on gold nanoparticles conjugated with CALNN peptide for application in drug uptake and induction of cell death on breast cancer cell line. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 949–964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, B.; Du, J.; Wang, Y. Regulation the morphology of cationized gold nanoparticles for effective gene delivery. Colloids Surf. B Biointerfaces 2017, 157, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Moynihan, K.D.; Bekdemir, A.; Dichwalkar, T.M.; Noh, M.M.; Watson, N.; Melo, M.; Ingram, J.; Suh, H.; Ploegh, H.; et al. Targeting small molecule drugs to T cells with antibody-directed cell-penetrating gold nanoparticles. Biomater. Sci. 2018, 7, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prisner, L.; Bohn, N.; Hahn, U.; Mews, A. Size dependent targeted delivery of gold nanoparticles modified with the IL-6R-specific aptamer AIR-3A to IL-6R-carrying cells. Nanoscale 2017, 9, 14486–14498. [Google Scholar] [CrossRef]
- Pabla, B.; Bissonnette, M.; Konda, V.J. Colon cancer and the epidermal growth factor receptor: Current treatment paradigms, the importance of diet, and the role of chemoprevention. World J. Clin. Oncol. 2015, 6, 133–141. [Google Scholar] [CrossRef]
- Basso, C.R.; Tozato, C.C.; Junior, J.P.A.; Pedrosa, V.A. A fast and highly sensitive method for the detection of canine distemper virus by the naked eye. Anal. Methods 2015, 7, 2264–2267. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Bhadra, D.; Bhadra, S.; Jain, S.; Jain, N.K. A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int. J. Pharm. 2003, 257, 111–124. [Google Scholar] [CrossRef]
- Deka, J.; Mech, R.; Ianeselli, L.; Amenitsch, H.; Cacho-Nerin, F.; Parisse, P.; Casalis, L. Surface Passivation Improves the Synthesis of Highly Stable and Specific DNA-Functionalized Gold Nanoparticles with Variable DNA Density. ACS Appl. Mater. Interfaces 2015, 7, 7033–7040. [Google Scholar] [CrossRef] [PubMed]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV-Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Kong, L.J.; Li, L.; Li, N.E.; Yan, P. Antitumor Activity of Doxorubicin-Loaded Carbon Nanotubes Incorporated Poly(Lactic-Co-Glycolic Acid) Electrospun Composite Nanofibers. Nanoscale Res. Lett. 2015, 10, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safwat, M.A.; Soliman, G.M.; Sayed, D.; Attia, M.A. Gold nanoparticles enhance 5-fluorouracil anticancer efficacy against colorectal cancer cells. Int. J. Pharm. 2016, 513, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, M.H.; Amani, H.; Pourfatollah, A.A.; Pazoki-Toroudi, H.; Sedighimoghaddam, B. Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens. Bio-Sens. Res. 2016, 9, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Al-Thawabeia, R.A.; Hodali, H.A. Use of Zeolite ZSM-5 for Loading and Release of 5-Fluorouracil. J. Chem. 2015, 2015, 403597. [Google Scholar] [CrossRef] [Green Version]
- Comenge, J.; Sotelo, C.; Romero, F.; Gallego, O.; Barnadas, A.; Parada, T.G.C.; Dominguez, F.; Puntes, V.F. Detoxifying Antitumoral Drugs via Nanoconjugation: The Case of Gold Nanoparticles and Cisplatin. PLoS ONE 2012, 7, e47562. [Google Scholar] [CrossRef] [Green Version]
- Chegel, V.; Rachkov, O.; Lopatynskyi, A.; Ishihara, S.; Yanchuk, I.; Nemoto, Y.; Hill, J.P.; Ariga, K. Gold Nanoparticles Aggregation: Drastic Effect of Cooperative Functionalities in a Single Molecular Conjugate. J. Phys. Chem. C 2012, 116, 2683–2690. [Google Scholar] [CrossRef]
- Wang, G.; Sun, W.F. Optical limiting of gold nanoparticle aggregates induced by electrolytes. J. Phys. Chem. B 2006, 110, 20901–20905. [Google Scholar] [CrossRef]
- Mohamed, M.B.; Adbel-Ghani, N.T.; El-Borady, O.M.; El-Sayed, M.A. 5-Fluorouracil Induces Plasmonic Coupling in Gold Nanospheres: New Generation of Chemotherapeutic Agents. J. Nanomed. Nanotechol. 2012, 3, 146–153. [Google Scholar] [CrossRef]
- Pernodet, N.; Fang, X.H.; Sun, Y.; Bakhtina, A.; Ramakrishnan, A.; Sokolov, J.; Ulman, A.; Rafailovich, M. Adverse effects of citrate/gold nanoparticles on human dermal fibroblasts. Small 2006, 2, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.J.; Wang, S.W.; Liu, M.; Ma, H.; Zeng, X.; Zhang, M.; Xu, L.L.; Cui, Y.D.; Xu, H.X.; Tang, Y.; et al. Norcantharidin Inhibits cell growth by suppressing the expression and phosphorylation of both EGFR and c-Met in human colon cancer cells. BMC Cancer 2017, 17, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Formulation * | Diameter (nm) | Zeta Potential (mV) | Radius (nm) | A450 | N° of Particles (×1012) mL−1 |
|---|---|---|---|---|---|
| AuNP | 11.5 ± 1.06 | −46.5 ± 1.10 | 5.75 | 1.556 | 2.95 |
| AuNP 5FU | 12.8 ± 1.5 | −44.4 ± 2.90 | 6.4 | 1.150 | 1.73 |
| AuNP EGFR | 14.9 ± 1.23 | −7.45 ± 0.131 | 7.45 | 0.71T6 | 0.843 |
| AuNP 5FU EGFR | 18.83 ± 1.52 | −33.1 ± 3.78 | 9.415 | 1.316 | 1.186 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liszbinski, R.B.; Romagnoli, G.G.; Gorgulho, C.M.; Basso, C.R.; Pedrosa, V.A.; Kaneno, R. Anti-EGFR-Coated Gold Nanoparticles In Vitro Carry 5-Fluorouracil to Colorectal Cancer Cells. Materials 2020, 13, 375. https://doi.org/10.3390/ma13020375
Liszbinski RB, Romagnoli GG, Gorgulho CM, Basso CR, Pedrosa VA, Kaneno R. Anti-EGFR-Coated Gold Nanoparticles In Vitro Carry 5-Fluorouracil to Colorectal Cancer Cells. Materials. 2020; 13(2):375. https://doi.org/10.3390/ma13020375
Chicago/Turabian StyleLiszbinski, Raquel B., Graziela G. Romagnoli, Carolina M. Gorgulho, Caroline R. Basso, Valber A. Pedrosa, and Ramon Kaneno. 2020. "Anti-EGFR-Coated Gold Nanoparticles In Vitro Carry 5-Fluorouracil to Colorectal Cancer Cells" Materials 13, no. 2: 375. https://doi.org/10.3390/ma13020375