Engineering Gels with Time-Evolving Viscoelasticity
Abstract
:1. Introduction
- Elastic—these substrates are described by a constant elastic modulus, E, and represents the classical tool for mechanobiology studies in-vitro;
- Viscoelastic, time-dependent behaviour described by one or more elastic moduli, E, and viscous moduli, ηi, with values that are constant over time;
- Dynamic elastic, described by an elastic modulus, E(t), which change or evolves as a function of time;
- Dynamic viscoelastic, i.e., with viscoelastic properties which evolve over time and are thus described by one or more elastic moduli Ei(t), and viscosities, ηi(t).
2. Materials and Methods
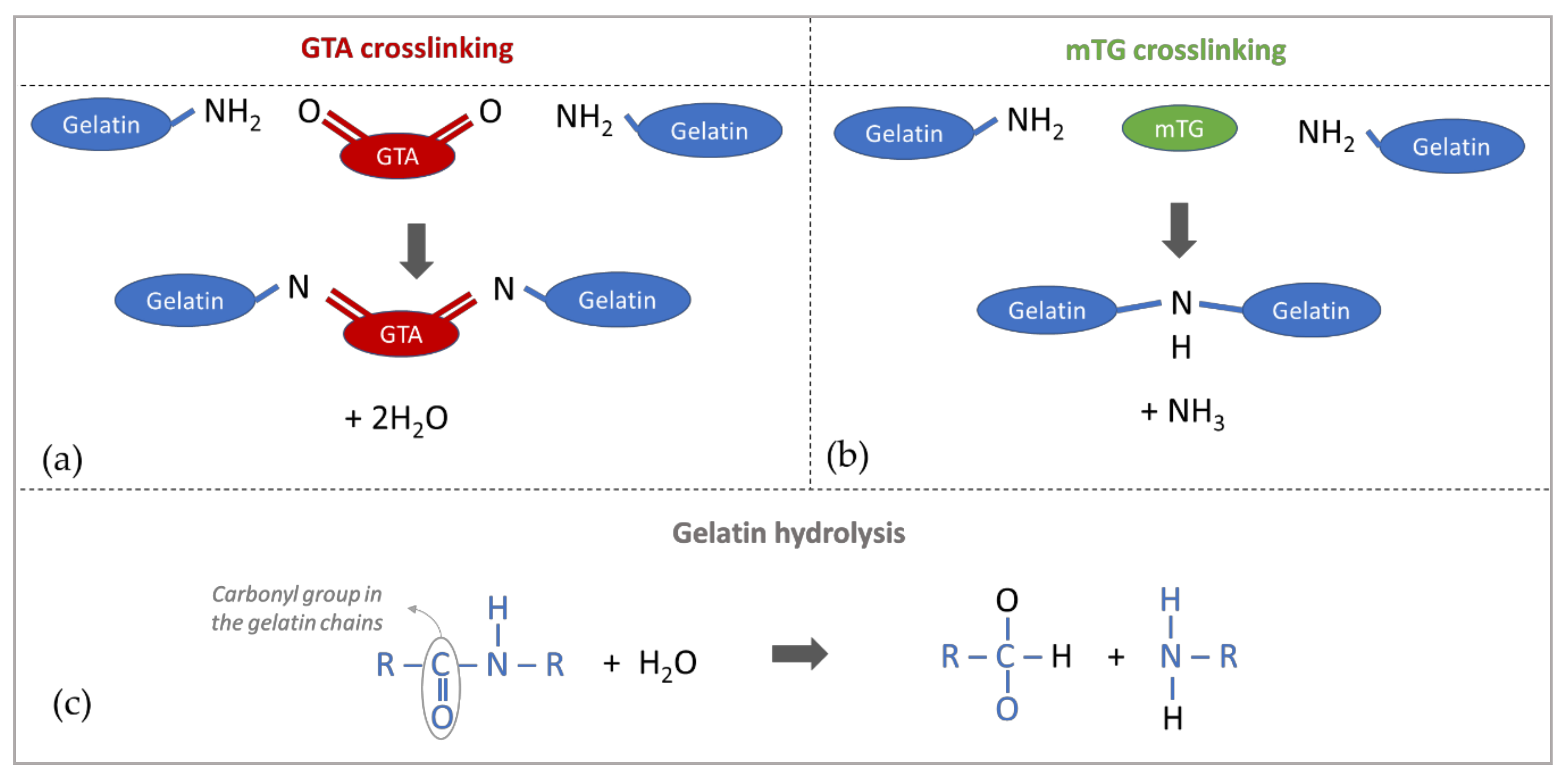
2.1. Sample Preparation and First Step (Chemical) Crosslinking
2.2. Second Step (Enzymatic) Crosslinking
2.3. Viscoelastic Parameter Estimation
2.4. Nano-Indentation Measurements
2.5. Nanoindentation Data Analysis
2.6. Gel Degradation Tests
2.7. Cytocompatibility Tests
2.8. Statistical Analysis
3. Results
3.1. Initial Hydrogel Viscoelastic Properties (Day 0)
3.2. Evolution of Hydrogel Viscoelastic Properties
3.3. Gel Degradation
3.4. Cytocompatibility
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Selfe, J. Fundamentals of Biomechanics. Physiology 2000, 86, 163. [Google Scholar] [CrossRef] [Green Version]
- Dunn, M.G.; Silver, F.H. Viscoelastic behavior of human connective tissues: Relative contribution of viscous and elastic components. Connect. Tissue Res. 1983, 12, 59–70. [Google Scholar] [CrossRef]
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Mih, J.D.; Shea, B.S.; Kho, A.T.; Sharif, A.S.; Tager, A.M.; Tschumperlin, D.J. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 2010, 190, 693–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tse, J.R.; Engler, A.J. Preparation of Hydrogel Substrates with Tunable Mechanical Properties. Curr. Protoc. Cell Biol. 2010, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Roveri, N.; Rubini, K. Stabilization of gelatin films by crosslinking with genipin. Biomaterials 2002, 23, 4827–4832. [Google Scholar] [CrossRef]
- Mattei, G.; Ferretti, C.; Tirella, A.; Ahluwalia, A.; Mattioli-Belmonte, M. Decoupling the role of stiffness from other hydroxyapatite signalling cues in periosteal derived stem cell differentiation. Sci. Rep. 2015, 5, 1–14. [Google Scholar]
- Mabry, K.M.; Lawrence, R.L.; Anseth, K.S. Dynamic stiffening of poly(ethylene glycol)-based hydrogels to direct valvular interstitial cell phenotype in a three-dimensional environment. Biomaterials 2015, 49, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, J.L.; Tuler, J.; Braden, R.; Schüp-Magoffin, P.; Schaefer, J.; Kretchmer, K.; Christman, K.L.; Engler, A.J. In vivo response to dynamic hyaluronic acid hydrogels. Acta Biomater. 2013, 9, 7151–7157. [Google Scholar] [CrossRef] [Green Version]
- Stowers, R.S.; Allen, S.C.; Suggs, L.J. Dynamic phototuning of 3D hydrogel stiffness. Proc. Natl. Acad. Sci. USA 2015, 112, 1953–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- McKinnon, D.D.; Domaille, D.W.; Cha, J.N.; Anseth, K.S. Biophysically defined and cytocompatible covalently adaptable networks as viscoelastic 3d cell culture systems. Adv. Mater. 2014, 26, 865–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yung, C.W.; Wu, L.Q.; Tullman, J.A.; Payne, G.F.; Bentley, W.E.; Barbari, T.A. Transglutaminase crosslinked gelatin as a tissue engineering scaffold. J. Biomed. Mater. Res. A 2007, 83, 1039–1046. [Google Scholar] [CrossRef]
- Young, J.L.; Engler, A.J. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials 2011, 32, 1002–1009. [Google Scholar] [CrossRef] [Green Version]
- Shirke, P.U.; Goswami, H.; Kumar, V.; Shah, D.; Das, S.; Bellare, J.; Venkatesh, K.V.; Seth, J.R.; Majumder, A. “Viscotaxis”—Directed Migration of Mesenchymal Stem Cells in Response to Loss Modulus Gradient. bioRxiv 2019, 804492. [Google Scholar]
- Chaudhuri, O. Viscoelastic hydrogels for 3D cell culture. Biomater. Sci. 2017, 5, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Dey, K.; Agnelli, S.; Sartore, L. Dynamic freedom: Substrate stress relaxation stimulates cell responses. Biomater. Sci. 2019, 7, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.R.; Frith, J.E.; Cooper-White, J.J. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials 2011, 32, 5979–5993. [Google Scholar] [CrossRef] [PubMed]
- Charrier, E.E.; Pogoda, K.; Wells, R.G.; Janmey, P.A. Control of cell morphology and differentiation by substrates with independently tunable elasticity and viscous dissipation. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhuri, O.; Gu, L.; Darnell, M.; Klumpers, D.; Sidi, A.; Weaver, J.C.; Huebsch, N.; Mooney, D.J.; Francisco, S. Substrate stress relaxation regulates cell spreading. Nat. Commun. 2015, 6, 6364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacopardo, L.; Guazzelli, N.; Nossa, R.; Mattei, G.; Ahluwalia, A. Engineering hydrogel viscoelasticity. J. Mech. Behav. Biomed. Mater. 2019, 89, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, A.A.; Lee, J.; Ashwin Bharadwaj, N.; Ewoldt, R.H.; Kilian, K.A.; Abdeen, A.A.; Lee, J.; Kilian, K.A.; Bharadwaj, N.A.; Ewoldt, R.H. Temporal Modulation of Stem Cell Activity Using Magnetoactive Hydrogels. Adv. Healthc. Mater. 2016, 5, 2536–2544. [Google Scholar] [CrossRef] [Green Version]
- Rodell, C.B.; MacArthur, J.W.; Dorsey, S.M.; Wade, R.J.; Wang, L.L.; Woo, Y.J.; Burdick, J.A. Shear-thinning supramolecular hydrogels with secondary autonomous covalent crosslinking to modulate viscoelastic properties in vivo. Adv. Funct. Mater. 2015, 25, 636–644. [Google Scholar] [CrossRef]
- Guvendiren, M.; Burdick, J.A. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat. Commun. 2012, 3, 792–799. [Google Scholar] [CrossRef] [Green Version]
- Rosales, A.M.; Vega, S.L.; DelRio, F.W.; Burdick, J.A.; Anseth, K.S. Hydrogels with Reversible Mechanics to Probe Dynamic Cell Microenvironments. Angew. Chem. Int. Ed. 2017, 56, 12132–12136. [Google Scholar] [CrossRef]
- Mattei, G.; Cacopardo, L.; Ahluwalia, A. Micro-Mechanical Viscoelastic Properties of Crosslinked Hydrogels Using the Nano-Epsilon Dot Method. Materials 2017, 10, 889. [Google Scholar] [CrossRef] [Green Version]
- Broderick, E.P.; Halloran, D.M.O.; Rochev, Y.A.; Griffin, M.; Collighan, R.J.; Pandit, A.S. Enzymatic Stabilization of Gelatin-Based Scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 72, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Chau, D.Y.S.; Collighan, R.J.; Verderio, E.A.M.; Addy, V.L.; Griffin, M. The cellular response to transglutaminase-cross-linked collagen. Biomaterials 2005, 26, 6518–6529. [Google Scholar] [CrossRef] [PubMed]
- Mcdermott, M.K.; Chen, T.; Williams, C.M.; Markley, K.M.; Payne, G.F. Mechanical Properties of Biomimetic Tissue Adhesive Based on the Microbial Transglutaminase-Catalyzed Crosslinking of Gelatin. Biomacromolecules 2004, 5, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Paguirigan, A.L.; Beebe, D.J. Protocol for the fabrication of enzymatically crosslinked gelatin microchannels for microfluidic cell culture. Nat. Protoc. 2007, 2, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Wangtueai, S.; Noomhorm, A.; Regenstein, J.M. Effect of Microbial Transglutaminase on Gel Properties and Film Characteristics of Gelatin from Lizardfish (Saurida spp.) scales. J. Food Sci. 2010, 75, 731–739. [Google Scholar] [CrossRef]
- Labus, K.; Drozd, A.; Trusek-Holownia, A. Preparation and characterisation of gelatine hydrogels predisposed to use as matrices for effective immobilisation of biocatalystst. Chem. Pap. 2016, 70, 523–530. [Google Scholar]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X.; Zhang, J. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018, 8, 1616. [Google Scholar] [CrossRef]
- Mattei, G.; Gruca, G.; Rijnveld, N.; Ahluwalia, A. The nano-epsilon dot method for strain rate viscoelastic characterisation of soft biomaterials by spherical nano-indentation. J. Mech. Behav. Biomed. Mater. 2015, 50, 150–159. [Google Scholar] [CrossRef]
- Clayton, E.H.; Okamoto, R.J.; Wilson, K.S.; Namani, R.; Bayly, P.V. Comparison of Dynamic Mechanical Testing and MR Elastography of Biomaterials. In Application of Imaging Techniques to Mechanics of Materials and Structures; Springer: New York, NY, USA, 2013; Volume 4, pp. 143–150. [Google Scholar]
- Pulieri, E.; Chiono, V.; Ciardelli, G.; Vozzi, G.; Ahluwalia, A.; Domenici, C.; Vozzi, F.; Giusti, P. Chitosan/gelatin blends for biomedical applications. J. Biomed. Mater. Res. Part A 2008, 86, 311–322. [Google Scholar] [CrossRef]
- Tirella, A.; Mattei, G.; Ahluwalia, A. Strain rate viscoelastic analysis of soft and highly hydrated biomaterials. J. Biomed. Mater. Res. Part A 2014, 102, 3352–3360. [Google Scholar] [CrossRef] [Green Version]
- Roylance, D. Engineering Viscoelasticity; Department of Materials Science and Engineering—Massachusetts Institute of Technology: Cambridge, MA, USA, 2001; Volume 2139. [Google Scholar]
- Cacopardo, L.; Mattei, G.; Ahluwalia, A. A new load-controlled testing method for viscoelastic characterisation through stress-rate measurements. Materialia 2020, 9, 100552. [Google Scholar] [CrossRef]
- Bartolini, L.; Iannuzzi, D.; Mattei, G. Comparison of frequency and strain-rate domain mechanical characterization. Sci. Rep. 2018, 8, 13697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qiang, B.; Greenleaf, J. Comparison of the surface wave method and the indentation method for measuring the elasticity of gelatin phantoms of different concentrations. Ultrasonics 2011, 51, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Czerner, M.; Fellay, L.S.; Suárez, M.P.; Frontini, P.M.; Fasce, L.A. Determination of Elastic Modulus of Gelatin Gels by Indentation Experiments. Procedia Mater. Sci. 2015, 8, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Mattei, G.; Tirella, A.; Gallone, G.; Ahluwalia, A. Viscoelastic characterisation of pig liver in unconfined compression. J. Biomech. 2014, 47, 2641–2646. [Google Scholar] [CrossRef] [PubMed]
- Mattei, G.; Ahluwalia, A. Sample, testing and analysis variables affecting liver mechanical properties: A review. Acta Biomater. 2016, 45, 60–71. [Google Scholar] [CrossRef]
- Wang, Z.; Golob, M.J.; Chesler, N.C. Viscoelastic Properties of Cardiovascular Tissues. In Viscoelastic and Viscoplastic Materials; InTech: London, UK, 2016. [Google Scholar]
- Mijailovic, A.S.; Qing, B.; Fortunato, D.; Van Vliet, K.J. Characterizing viscoelastic mechanical properties of highly compliant polymers and biological tissues using impact indentation. Acta Biomater. 2018, 71, 388–397. [Google Scholar] [CrossRef]
- Chen, P.R.; Kang, P.L.; Su, W.Y.; Lin, F.H.; Chen, M.H. The evaluation of thermal properties and in vitro test of carbodiimide or glutaraldehyde cross-linked gelatin for PC 12 cells culture. Biomed. Eng. Appl. Basis Commun. 2005, 17, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Rachel, N.M.; Quaglia, D.; Lévesque, É.; Charette, A.B.; Pelletier, J.N. Engineered, highly reactive substrates of microbial transglutaminase enable protein labeling within various secondary structure elements. Protein Sci. 2017, 26, 2268–2279. [Google Scholar] [CrossRef]
- Englert, C.; Blunk, T.; Müller, R.; von Glasser, S.S.; Baumer, J.; Fierlbeck, J.; Heid, I.M.; Nerlich, M.; Hammer, J. Bonding of articular cartilage using a combination of biochemical degradation and surface cross-linking. Arthritis Res. Ther. 2007, 9, R47. [Google Scholar] [CrossRef] [Green Version]
- Correia, D.M.; Padrão, J.; Rodrigues, L.R.; Dourado, F.; Lanceros-méndez, S. Thermal and hydrolytic degradation of electrospun fi sh gelatin membranes. Polym. Test. 2013, 32, 995–1000. [Google Scholar] [CrossRef] [Green Version]
- Kiewiet, M.B.G.; Faas, M.M.; de Vos, P. Immunomodulatory protein hydrolysates and their application. Nutrients 2018, 10, 904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karathanasopoulos, N.; Ganghoffer, J. Investigating the Effect of Aging on the Viscosity of Tendon Fascicles and Fibers. Front. Bioeng. Biotechnol. 2019, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Escoffier, C.; de Rigal, J.; Rochefort, A.; Vassalet, R.; Lèvêque, J.-L.; Agache, P.G. Age-Related Mechanical An In Vivo Study. J. Investig. Dermatol. 1989, 93, 353–357. [Google Scholar] [CrossRef]
- Boyer, G.; Laquièze, L.; Le Bot, A.; Laquièze, S.; Zahouani, H. Dynamic indentation on human skin in vivo: Ageing effects. Ski. Res. Technol. 2009, 15, 55–67. [Google Scholar] [CrossRef]
- Sack, I.; Beierbach, B.; Wuerfel, J.; Klatt, D.; Hamhaber, U.; Papazoglou, S.; Martus, P.; Braun, J. The impact of aging and gender on brain viscoelasticity. Neuroimage 2009, 46, 652–657. [Google Scholar] [CrossRef]
- Snedeker, J.G.; Gautieri, A. The role of collagen crosslinks in ageing and diabetes—The good, the bad, and the ugly. Muscles Ligaments Tendons J. 2014, 4, 303–308. [Google Scholar] [CrossRef]
- Vogel, H.G. Influence of maturation and aging on mechanical and biochemical properties of connective tissue in rats. Mech. Ageing Dev. 1980, 14, 283–292. [Google Scholar] [CrossRef]
- Ebihara, T.; Venkatesan, N.; Tanaka, R.; Ludwig, M.S. Changes in Extracellular Matrix and Tissue Viscoelasticity in Bleomycin-induced Lung Fibrosis Temporal Aspects. Am. J. Respir. Crit. Care Med. 2000, 162, 1569–1576. [Google Scholar] [CrossRef]
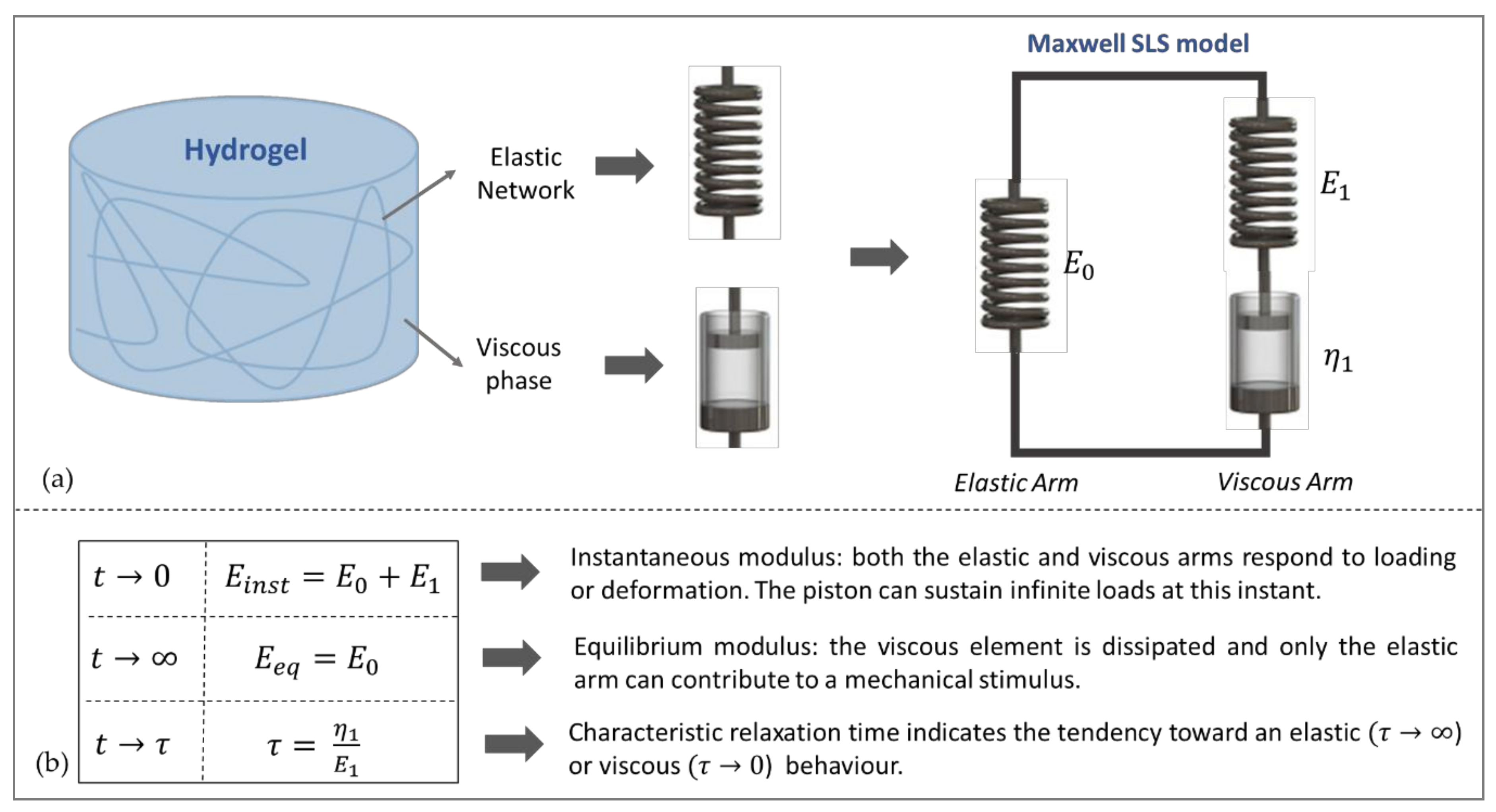



| Change in Properties with Time | Viscoelasticity | Parameter(s): | Examples of Typical Substrates | Refs | |
|---|---|---|---|---|---|
| Elastic | no | no | E | GTA or genepin crosslinked gelatin, Ha/Gel, PAAm | [8,9,10,11] |
| Viscoelastic | no | yes | Ei, ηi | PAAm, alginate, agarose | [22,23,24,25] |
| Dynamic/time-evolving Elastic | yes | no | E (t) | PEG-thiol, HA/PEGDA, Ca2+-liposome loaded alginate | [12,13,14,18] |
| Dynamic/time-evolving Viscoelastic | yes | yes | Ei (t), ηi (t) | Magneto active PAAm, HA-based gels | [26,27,28,29] |
| Day 1 | Day 7 | |
|---|---|---|
| GTA crosslinked gels (controls) | 8.3% ± 0.7% a | 10.3% ± 0.6% c |
| mTG-GTA crosslinked gels | 2.1% ± 0.3% b | 6.2% ± 0.2% d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattei, G.; Cacopardo, L.; Ahluwalia, A. Engineering Gels with Time-Evolving Viscoelasticity. Materials 2020, 13, 438. https://doi.org/10.3390/ma13020438
Mattei G, Cacopardo L, Ahluwalia A. Engineering Gels with Time-Evolving Viscoelasticity. Materials. 2020; 13(2):438. https://doi.org/10.3390/ma13020438
Chicago/Turabian StyleMattei, Giorgio, Ludovica Cacopardo, and Arti Ahluwalia. 2020. "Engineering Gels with Time-Evolving Viscoelasticity" Materials 13, no. 2: 438. https://doi.org/10.3390/ma13020438
APA StyleMattei, G., Cacopardo, L., & Ahluwalia, A. (2020). Engineering Gels with Time-Evolving Viscoelasticity. Materials, 13(2), 438. https://doi.org/10.3390/ma13020438






