Micro- and Nanoscale Spectroscopic Investigations of Threonine Influence on the Corrosion Process of the Modified Fe Surface by Cu Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. AFM Measurements
2.3. FT-IR and SEIRA Measurements
2.4. RS and SERS Measurements
2.5. Nano-SEIRA Measurements
2.6. Data Analysis
2.7. Fitting Procedure
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stupnišek-Lisac, E.; Lončarić Božić, A.; Cafuk, I. Low-toxicity copper corrosion inhibitors. Corrosion 1998, 54, 713–720. [Google Scholar] [CrossRef]
- Tang, Z. A review of corrosion inhibitors for rust preventative fluids. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100759–100775. [Google Scholar] [CrossRef]
- Stoyanova, A.E.; Sokolova, E.I.; Raicheva, S.N. The inhibition of mild steel corrosion in 1 M HCL in the presence of linear and cyclic thiocarbamides—Effect of concentration and temperature of the corrosion medium on their protective action. Corros. Sci. 1997, 39, 1595–1604. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Li, Y.; Wang, F.H. Corrosion inhibition and adsorption behavior of methionine on mild steel in sulfuric acid and synergistic effect of iodide ion. J. Colloid Interface Sci. 2007, 310, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Sarkar, T.K.; Purkait, T. Amino acid compounds as eco-friendly corrosion inhibitor for N80 steel in HCl solution: Electrochemical and theoretical approaches. J. Mol. Liq. 2015, 212, 731–738. [Google Scholar] [CrossRef]
- Kesavan, D.; Gopiraman, M.; Sulochana, N. Green inhibitors for corrosion of metals: A review. Chem. Sci. Rev. Lett. 2012, 1, 1–8. Available online: https://chesci.com/wp-content/uploads/2016/12/V1i1_1_CS10204205.pdf (accessed on 20 August 2020).
- Amin, M.A.; Khaled, K.F.; Mohsen, Q.; Arida, H.A. A study of the inhibition of iron corrosion in HCl solutions by some amino acids. Corros. Sci. 2010, 52, 1684–1695. [Google Scholar] [CrossRef]
- Marzorati, S.; Verotta, L.; Trasatti, S.P. Green corrosion inhibitors from natural sources and biomass wastes. Molecules 2019, 24, 48. [Google Scholar] [CrossRef] [Green Version]
- Palaniappan, N.; Cole, I.S.; Kuznetsov, A.E.; Balasubramanian, K.; Thomas, K.R.J. Experimental and computational studies of a graphene oxide barrier layer covalently functionalized with amino acids on Mg AZ13 alloy in salt medium. RSC Adv. 2019, 9, 32441–32447. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.Q.; Cai, Q.R.; Gao, L.X.; Lee, K.Y. Effect of serine, threonine and glutamic acid on the corrosion of copper in aerated hydrochloric acid solution. Corros. Sci. 2008, 50, 3615–3621. [Google Scholar] [CrossRef]
- Makarenko, N.V.; Kharchenko, U.V.; Zemnukhova, L.A. Effect of amino acids on corrosion of copper and steel in acid medium. Russ. J. Appl. Chem. 2011, 84, 1362–1365. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, M.; Hu, J.; Wang, C.; Xu, S.; Han, C.C. Preparation and optimization of silver nanoparticles embedded electrospun membrane for implant associated infections prevention. ACS Appl. Mater. Interfaces 2013, 5, 11014–11021. [Google Scholar] [CrossRef] [PubMed]
- Kusior, A.; Kollbek, K.; Kowalski, K.; Borysiewicz, M.; Wojciechowski, T.; Adamczyk, A.; Trenczek-Zajac, A.; Radecka, M.; Zakrzewska, K. Sn and Cu oxide nanoparticles deposited on TiO2 nanoflower 3D substrates by inert gas condensation technique. Appl. Surf. Sci. 2016, 380, 193–202. [Google Scholar] [CrossRef]
- Abbasi Kesbi, F.; Rashid, A.M.; Astinchap, B. Preparation of ultrafine grained copper nanoparticles via immersion deposit method. Appl. Nanosci. 2018, 8, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Ustunol, I.B.; Gonzalez-Pech, N.I.; Grassian, V.H. pH-dependent adsorption of α-amino acids, lysine, glutamic acid, serine and glycine, on TiO2 nanoparticle surfaces. J. Colloid Interface Sci. 2019, 554, 362–375. [Google Scholar] [CrossRef]
- van Hengel, I.A.J.; Tierolf, M.W.A.M.; Valerio, V.P.M.; Minneboo, M.; Fluit, A.C.; Fratila-Apachitei, L.E.; Apachiteia, I.; Zadpoor, A.A. Self-defending additively manufactured bone implants bearing silver and copper nanoparticles. J. Mater. Chem. B 2020, 8, 1589–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starowicz, M. Electrochemical synthesis of copper oxide particles with controlled oxidation state, shape and size. Mater. Res. Express. 2019, 6, 0850a3. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Kim, K.; Shin, I.-S.; Shin, K.S. Antioxidative metallic copper nanoparticles prepared by modified polyol method and their catalytic activities. J. Nanopart. Res. 2020, 22, 8. [Google Scholar] [CrossRef]
- Magdassi, S.; Grouchko, M.; Kamyshny, A. Copper nanoparticles for printed electronics: Routes towards achieving oxidation stability. Materials 2010, 3, 4626–4638. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef]
- Bogdanović, U.; Lazić, V.; Vodnik, V.; Budimir, M.; Marković, Z.; Dimitrijević, S. Copper nanoparticles with high antimicrobial activity. Mater. Lett. 2014, 128, 75–78. [Google Scholar] [CrossRef]
- Liu, R.; Memarzadeh, K.; Chang, B.; Zhang, Y.; Ma, Z.; Allaker, R.P.; Ren, L.; Yang, K. Antibacterial effect of copper bearing titanium alloy (Ti-Cu) against Streptococcus mutans and Porphyromonas gingivalis. Sci. Rep. 2016, 6, 29985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Yang, C.; Ren, G.; Ren, L. Study on behaviour and mechanism of Cu2+ ion release from Cu bearing antibacterial stainless steel. Mater. Technol. 2015, 30, B126–B132. [Google Scholar] [CrossRef]
- Hameed, H.A.; Ariffin, A.; Luddin, N.; Husein, A. Evaluation of antibacterial properties of copper nanoparticles surface coating on titanium dental implant. J. Pharm. Sci. Res. 2018, 10, 1157–1160. Available online: https://www.jpsr.pharmainfo.in/Documents/Volumes/vol10Issue05/jpsr10051840.pdf (accessed on 15 August 2020).
- Li, D.; Chen, S.; Zhao, S.; Ma, H. The corrosion inhibition of the self- assembled Au, and Ag nanoparticles films on the surface of copper. Colloids Surf. A 2006, 273, 16–23. [Google Scholar] [CrossRef]
- El-Ansary, A.; Faddah, L.M. Nanoparticles as biochemical sensors. Nanotechnol. Sci. Appl. 2010, 3, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Bozzini, B.; De Gaudenzi, G.P.; Mele, C. An in-situ FT-IR investigation of the anodic behaviour of WC-Co hardmetal. Mater. Corros. 2003, 54, 694–696. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, K.; Dong, C.; Wu, J.; Li, X.; Huang, Y. In situ Raman spectroscopy study of corrosion products on the surface of carbon steel in solution containing Cl− and SO42−. Eng. Faul. Anal. 2011, 18, 1981–1989. [Google Scholar] [CrossRef]
- Piergies, N.; Pięta, E.; Paluszkiewicz, C.; Domin, H.; Kwiatek, W.M. Polarization effect in tip-enhanced infrared nanospectroscopy studies of the selective Y5 receptor antagonist Lu AA33810. Nano Res. 2018, 11, 4401–4411. [Google Scholar] [CrossRef] [Green Version]
- Pięta, E.; Paluszkiewicz, C.; Kwiatek, W.M. Multianalytical approach to surface- and tip-enhanced infrared spectroscopy study of molecule-metal conjugate: Deducing the adsorption geometry. Phys. Chem. Chem. Phys. 2018, 20, 27992–28000. [Google Scholar] [CrossRef]
- Pięta, E.; Petibois, C.; Paluszkiewicz, C.; Kwiatek, W.M. Physico-chemical analysis of molecular binding to the colloidal metal nanostructure: Multiple micro- and nanospectroscopy study. Appl. Surf. Sci. 2020, 499, 143975. [Google Scholar] [CrossRef]
- Doering, W.E.; Nie, S. Single-molecule and single-nanoparticle SERS: Examining the roles of surface active sites and chemical enhancement. J. Phys. Chem. B 2002, 106, 311–317. [Google Scholar] [CrossRef]
- Aroca, R.F. Surface-Enhanced Vibrational Spectroscopy; John Wiley & Sons Ltd.: Chichester, UK, 2006. [Google Scholar]
- Blaber, M.G.; Arnold, M.D.; Harris, N.; Ford, M.J.; Cortie, M.B. Plasmon absorption in nanosphers: A comparision of sodium, potassium, aluminium, silver and gold. Physica B Cond. Mat. 2007, 394, 184–187. [Google Scholar] [CrossRef] [Green Version]
- Hartstein, A.; Kirtley, J.R.; Tsang, J.C. Enhancement of the infrared absorption from molecular monolayers with thin metal overlayers. Phys. Rev. Lett. 1980, 15, 201–204. [Google Scholar] [CrossRef]
- Osawa, M.M.; Ikeda, M. Surface-enhanced infrared absorption of p-nitrobenzoic acid deposited on silver island films: Contribution of electromagnetic and chemical mechanisms. J. Phys. Chem. 1991, 95, 9914–9919. [Google Scholar] [CrossRef]
- Dazzi, A.; Prazeres, R.; Glotin, F.; Ortega, J.M. Subwavelength infrared spectromicroscopy using an AFM as a local absorption sensor. Infrared Phys. Technol. 2006, 49, 113–121. [Google Scholar] [CrossRef]
- Dazzi, A.; Prater, C.B. AFM-IR: Technology and applications in nanoscale infrared spectroscopy and chemical imaging. Chem. Rev. 2017, 117, 5146–5173. [Google Scholar] [CrossRef]
- Ruggeri, F.S.; Habchi, J.; Cerreta, A.; Dietler, G. AFM-based single molecule techniques: Unraveling the amyloid Pathogenic Species. Curr. Pharm. Des. 2016, 22, 3950–3970. [Google Scholar] [CrossRef] [Green Version]
- Dazzi, A.; Prater, C.B.; Hu, Q.C.; Chase, D.B.; Rabolt, J.F.; Marcott, C. AFM-IR: Combining atomic force microscopy and infrared spectroscopy for nanoscale chemical characterization. Appl. Spectrosc. 2012, 66, 1365–1384. [Google Scholar] [CrossRef] [Green Version]
- Święch, D.; Ozaki, Y.; Kim, Y.; Proniewicz, E. Surface- and tip-enhanced Raman scattering of bradykinin onto the colloidal suspended Ag surface. Phys. Chem. Chem. Phys. 2015, 17, 17140–17149. [Google Scholar] [CrossRef]
- Piergies, N.; Dazzi, A.; Deniset-Beseau, A.; Mathurin, J.; Oćwieja, M.; Paluszkiewicz, C.; Kwiatek, W.M. Nanoscale image of the drug/metal mono-layer interaction: Tapping AFM-IR investigations. Nano Res. 2020, 13, 1020–1028. [Google Scholar] [CrossRef] [Green Version]
- Chisanga, M.; Muhamadali, H.; Ellis, D.I.; Goodacre, R. Enhancing disease diagnosis: Biomedical applications of surface-enhanced Raman scattering. Appl. Sci. 2019, 9, 1163. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Schlücker, S. Surface-enhanced Raman spectroscopic detection of molecular chemo- and plasmo-catalysis on noble metal nanoparticles. Chem. Commun. 2018, 54, 2326–2336. [Google Scholar] [CrossRef]
- Vendrell, M.; Maiti, K.K.; Dhaliwal, K.; Chang, Y.T. Surface-enhanced Raman scattering in cancer detection and imaging. Trends Biotechnol. 2013, 31, 249–257. [Google Scholar] [CrossRef]
- Kharitonov, D.S.; Sommertune, J.; Örnek, C.; Ryl, J.; Kurilo, I.I.; Claessona, P.M.; Pan, J. Corrosion inhibition of aluminium alloy AA6063-T5 by vanadates: Local surface chemical events elucidated by confocal Raman micro-spectroscopy. Corros. Sci. 2019, 148, 237–250. [Google Scholar] [CrossRef]
- Johnson, C.M.; Böhmler, M. Nano-FTIR microscopy and spectroscopy studies of atmospheric corrosion with a spatial resolution of 20 nm. Corros. Sci. 2016, 108, 60–65. [Google Scholar] [CrossRef]
- Ruggeri, F.S.; Longo, G.; Faggiano, S.; Lipiec, E.; Pastore, A.; Dietler, G. Infrared nanospectroscopy characterization of oligomeric and fibrillar aggregates during amyloid formation. Nat. Commun. 2015, 6, 7831. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.P.; Moon, A.P.; Sengupta, S.; Deo, G.; Sangal, S.; Mondal, K. Corrosion behavior of IF steel in various media and its comparison with mild steel. J. Mater. Eng. Perform. 2015, 24, 1961–1974. [Google Scholar] [CrossRef]
- Veneranda, M.; Aramendia, J.; Bellot-Gurlet, L.; Colomban, P.; Castro, K.; Madariaga, J.M. FTIR spectroscopic semi-quantification of iron phases: A new method to evaluate the protection ability index (PAI) of archaeological artefacts corrosion systems. Corros. Sci. 2018, 133, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Kwon, S.-K.; Suzuki, S.; Saito, M.; Waseda, Y. Atomic-scale structure and morphology of ferric oxyhydroxides formed by corrosion of an iron–silicon alloy. Mater. Trans. 2006, 47, 243–246. [Google Scholar] [CrossRef] [Green Version]
- Johnson, G.E.; Colby, R.; Laskin, J. Soft landing of bare nanoparticles with controlled size, composition, and morphology. Nanoscale 2015, 7, 3491–3503. [Google Scholar] [CrossRef]
- Musić, S.; Gotić, M.; Popović, S. X-ray diffraction and Fourier transform-infrared analysis of the rust formed by corrosion of steel in aqueous solutions. J. Mater. Sci. 1993, 28, 5744–5752. [Google Scholar] [CrossRef]
- Hedberga, J.; Karlssonb, H.L.; Hedberga, Y.; Blomberga, E.; Wallinder, I.O. The importance of extracellular speciation and corrosion of copper nanoparticles on lung cell membrane integrity. Colloid Surf. B 2016, 141, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Debbichi, L.; Marco de Lucas, M.C.; Pierson, J.F.; Krüger, P. Vibrational properties of CuO and Cu4O3 from first-principles calculations, and Raman and infrared Spectroscopy. J. Phys. Chem. C 2012, 116, 10232–10237. [Google Scholar] [CrossRef]
- Rémazeilles, C.; Refait, P. On the formation of b-FeOOH (akaganéite) in chloride-containing environments. Corros. Sci. 2007, 49, 844–857. [Google Scholar] [CrossRef]
- Pawlukojc´, A.; Leciejewicz, J.; Tomkinson, J.; Parker, S.F. Neutron scattering, infrared, Raman spectroscopy and ab initio study of L-threonine. Spectrochim. Acta A 2001, 57, 2513–2523. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, X.; Fan, Q.; Wan, X. Raman spectra of amino acids and their aqueous solutions. Spectrochim. Acta A 2011, 78, 1187–1195. [Google Scholar] [CrossRef]
- Hernández, B.; Pflüger, F.; Adenier, A.; Nsangou, M.; Kruglik, S.G.; Ghomi, M. Energy maps, side chain conformational flexibility, and vibrational features of polar amino acids L-serine and L-threonine in aqueous environment. J. Phys. Chem. 2011, 135, 08B601. [Google Scholar] [CrossRef]
- Wolpert, M.; Hellwig, P. Infrared spectra and molar absorption coefficients of the 20 alpha amino acids in aqueous solutions in the spectral range from 1800 to 500 cm−1. Spectrochim. Acta A 2006, 64, 987–1001. [Google Scholar] [CrossRef]
- Stewart, S.; Fredericks, P.M. Surface-enhanced Raman spectroscopy of amino acids adsorbed on an electrochemically prepared silver surface. Spectrochim. Acta A 1999, 55, 1641–1660. [Google Scholar] [CrossRef]
- Negri, P.; Schultz, Z.D. Online SERS detection of the 20 proteinogenic L-amino acids separated by capillary zone electrophoresis. Analyst 2014, 139, 5989–5998. [Google Scholar] [CrossRef] [Green Version]
- Guzzetti, K.A.; Brizuela, A.B.; Romano, E.; Brandán, S.A. Structural and vibrational study on zwitterions of L-threonine in aqueous phase using the FT-Raman and SCRF calculations. J. Mol. Struct. 2013, 1045, 171–179. [Google Scholar] [CrossRef]
- Quesada-Moreno, M.M.; Márquez-García, A.A.; Avilés-Moreno, J.R.; López-González, J.J. Conformational landscape of L-threonine in neutral, acid and basic solutions from vibrational circular dichroism spectroscopy and quantum chemical calculations. Tetrahedron 2013, 24, 1537–1547. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Meyer, R.A., Ed.; John Wiley & Sons Ltd: Chichester, UK, 2000. [Google Scholar]
- Costa, D.; Savio, L.; Pradier, C.-M. Adsorption of amino acids and peptides on metal and oxide surfaces in water environment: A synthetic and prospective review. J. Phys. Chem. B 2016, 120, 7039–7052. [Google Scholar] [CrossRef]
- Talley, C.E.; Jusinski, L.; Hollars, C.W.; Lane, S.M.; Huser, T. Intracellular pH sensors based on surface-enhanced Raman scattering. Anal. Chem. 2004, 76, 7064–7068. [Google Scholar] [CrossRef]
- Moskovits, M. Surface selection rules. J. Chem. Phys. 1982, 77, 4408–4416. [Google Scholar] [CrossRef]
- Creighton, J.A. Surface Raman electromagnetic enhancement factors for molecules at the surface of small isolated metal spheres: The determination of adsorbate orientation from SERS relative intensities. Surf. Sci. 1983, 124, 209–219. [Google Scholar] [CrossRef]
- Suh, J.S.; Moskovits, M. Surface-enhanced Raman spectroscopy of amino acids and nucleotide bases adsorbed on silver. J. Am. Chem. Soc. 1986, 108, 4711–4718. [Google Scholar] [CrossRef]
- Suh, J.S.; Kim, J. Three distinct geometries of surface-adsorbed carboxylate groups. J. Raman Spectrosc. 1998, 29, 143–148. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. A unified approach to surface-enhanced Raman spectroscopy. J. Phys. Chem. C 2008, 112, 5605–5617. [Google Scholar] [CrossRef]
- Dou, X.; Jung, Y.M.; Yamamoto, H.; Doi, S.; Ozaki, Y. Near-Infrared excited surface-enhanced Raman scattering of biological molecules on gold colloid I: Effects of pH of the solutions of amino acids and of their polymerization. Appl. Spectrosc. 1999, 53, 133–138. [Google Scholar] [CrossRef]
- Morad, M.S. Effect of amino acids containing sulfur on the corrosion of mild steel in phosphoric acid solutions containing Cl−, F− and Fe3+ ions: Behavior under polarization conditions. J. Appl. Electrochem. 2005, 35, 889–895. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; García, P.F.; Merck, G.K.; Bodensteiner, F.A.; Heissler, S.; Günther, S.; Berensmeier, S. On the nature of interactions of amino acids with bare magnetite nanoparticles. J. Phys. Chem. C 2015, 119, 23032–23041. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Grassian, V.H. Histidine adsorption on TiO2 nanoparticles: An integrated spectroscopic, thermodynamic, and molecular-based approach toward understanding nano-bio interactions. Langmuir 2014, 30, 8751–8760. [Google Scholar] [CrossRef]
- Begonja, S.; Rodenas, L.G.; Borghi, E.B.; Morando, P.J. Adsorption of cysteine on TiO2 at different pH values: Surface complexes characterization by FTIR-ATR and Langmuir isotherms analysis. Colloids Surf. A 2012, 403, 114–120. [Google Scholar] [CrossRef]

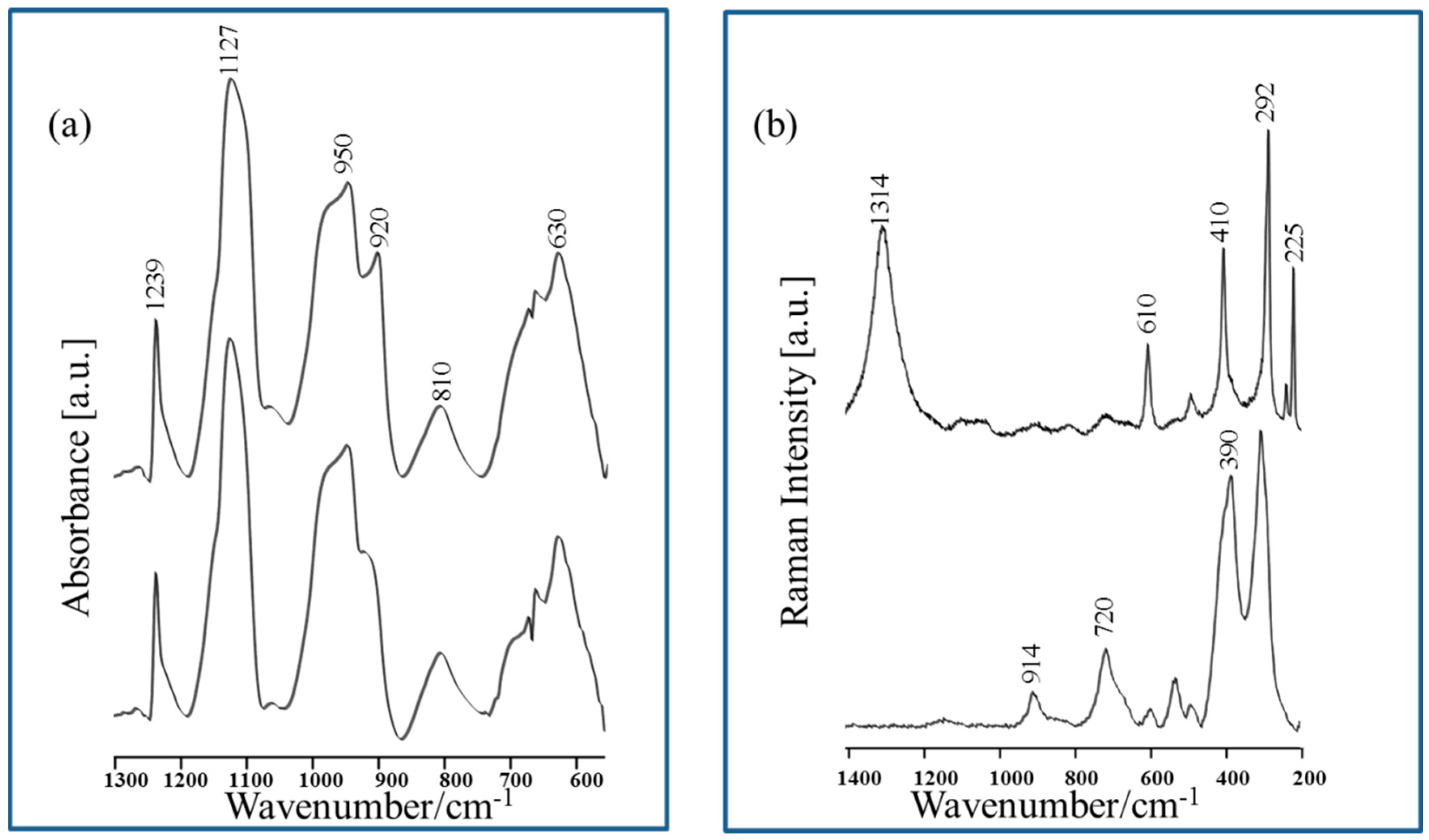


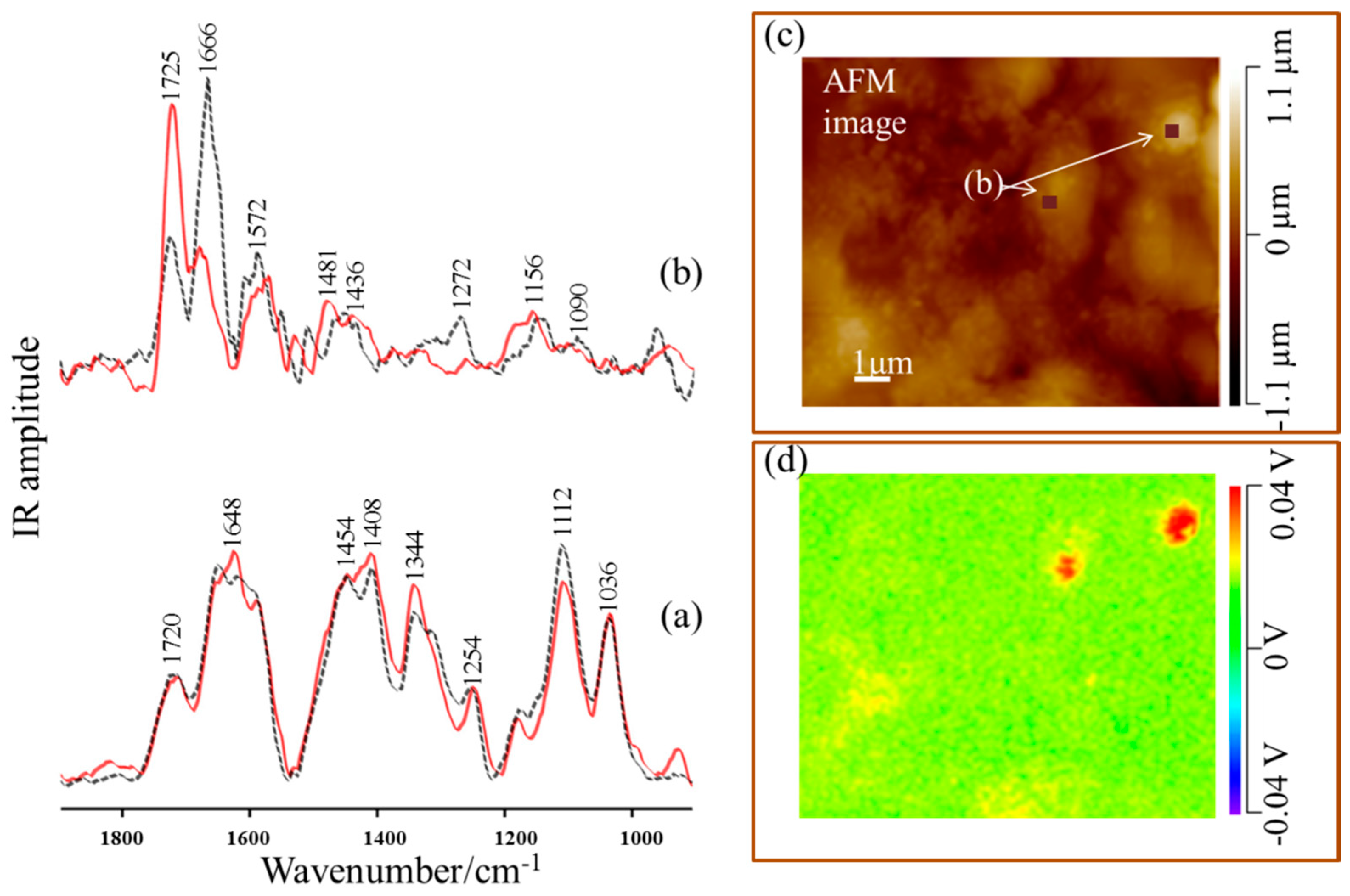
| Band Assignments | RS | SERS | FT-IR | SEIRA | Nano-SEIRA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ν (cm−1) | FWHM (cm−1) | ν (cm−1) | FWHM (cm−1) | ν (cm−1) | FWHM (cm−1) | ν (cm−1) | FWHM (cm−1) | s-pol | p-pol | |
| ν(C=O) | − | − | − | − | 1723 vw | 30 | 1744 m | 45 | 1725 vs | 1724 m |
| δas(NH3+) | 1641 vw | 15 | 1631 sh | 23 | 1624 vs | 39 | 1620 sh | 54 | 1666 vs | 1672 m |
| δas(NH3+)/ νas(COO) | 1599 vw | 16 | 1597 vs/ 1576 sh | 28 20 | 1598 sh | 27 | 1579 vs | 68 | 1572 m | 1572 m |
| δs(NH3+) | 1547 vw | 13 | 1545 sh | 26 | − | − | 1523 sh | 23 | − | − |
| δ(CH3)/ δ(NH3+) | 1451 m | 9 | 1440 s | 25 | 1454 vs/ 1420 sh | 40 44 | 1450 sh | 60 | 1481 m | 1468 m |
| νs (COO)/ δ(CH) | 1419 m | 16 | 1396 sh | 27 | 1385 sh | 22 | 1399 s | 28 | 1436 sh | 1438 sh |
| δ(CH3)/ δ(CH) | 1342 s | 15 | 1334 m | 7 | 1345 m | 46 | 1340 m | 63 | − | − |
| δ(CH) | 1302 sh | 27 | 1315 m | 20 | 1320 sh | 29 | − | − | − | − |
| δ(NH3+)/ ν(C-OH) | 1246 vw | 11 | 1233 m | 13 | 1242 w | 49 | 1270 sh | 34 | 1272 m | 1260 w |
| ρr(CH),ν(C-C), δ(OH) | 1194 m | 11 | 1168 w | 8 | 1180 vw | 47 | 1200 w | 36 | 1156 m | 1140 w |
| δ(C-O) ν(CC), δ(NH) | 1114 sh | 22 | − | − | 1115 m | 43 | 1117 sh | 45 | − | − |
| ν(C-O) | 1097 s | 16 | − | − | 1086 sh | 19 | − | − | 1090 w | 1089 w |
| ν(C-OH), ν(C-N) | 1030 m | 9 | 1021 m | 27 | 1039 m | 26 | 1043 m | 35 | − | − |
| ν (CC) | 929 s | 9 | − | − | 929 w | 53 | 945 w | 34 | − | − |
| ν(C-N), ν(C-C) | 902 w | 11 | − | − | 893 w | 35 | 899 w | 28 | − | − |
| ν(CCN)/ δip(COO) | 870 s | 8 | 865 m | 20 | − | − | − | − | − | − |
| δ(COO) | − | − | 823 m | 18 | − | − | − | − | − | − |
| δoop(COO) | 678 m | 35 | ||||||||
| ν(Cu-O), ρw(COO) | − | − | 622 m | 23 | − | − | − | − | − | − |
| ρr(COO) | 563 s | 12 | − | − | − | − | − | − | − | − |
| ν(Cu-O)/ Cu-Thr | − | − | 282 s | 45 | − | − | − | − | − | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Święch, D.; Paluszkiewicz, C.; Piergies, N.; Pięta, E.; Kollbek, K.; Kwiatek, W.M. Micro- and Nanoscale Spectroscopic Investigations of Threonine Influence on the Corrosion Process of the Modified Fe Surface by Cu Nanoparticles. Materials 2020, 13, 4482. https://doi.org/10.3390/ma13204482
Święch D, Paluszkiewicz C, Piergies N, Pięta E, Kollbek K, Kwiatek WM. Micro- and Nanoscale Spectroscopic Investigations of Threonine Influence on the Corrosion Process of the Modified Fe Surface by Cu Nanoparticles. Materials. 2020; 13(20):4482. https://doi.org/10.3390/ma13204482
Chicago/Turabian StyleŚwięch, Dominika, Czesława Paluszkiewicz, Natalia Piergies, Ewa Pięta, Kamila Kollbek, and Wojciech M. Kwiatek. 2020. "Micro- and Nanoscale Spectroscopic Investigations of Threonine Influence on the Corrosion Process of the Modified Fe Surface by Cu Nanoparticles" Materials 13, no. 20: 4482. https://doi.org/10.3390/ma13204482
APA StyleŚwięch, D., Paluszkiewicz, C., Piergies, N., Pięta, E., Kollbek, K., & Kwiatek, W. M. (2020). Micro- and Nanoscale Spectroscopic Investigations of Threonine Influence on the Corrosion Process of the Modified Fe Surface by Cu Nanoparticles. Materials, 13(20), 4482. https://doi.org/10.3390/ma13204482








