Novel Nanocomposites Based on Bacterial Polyester/LDH-SDS Clay for Stem Cells Delivery in Modern Wound Healing Management
Abstract
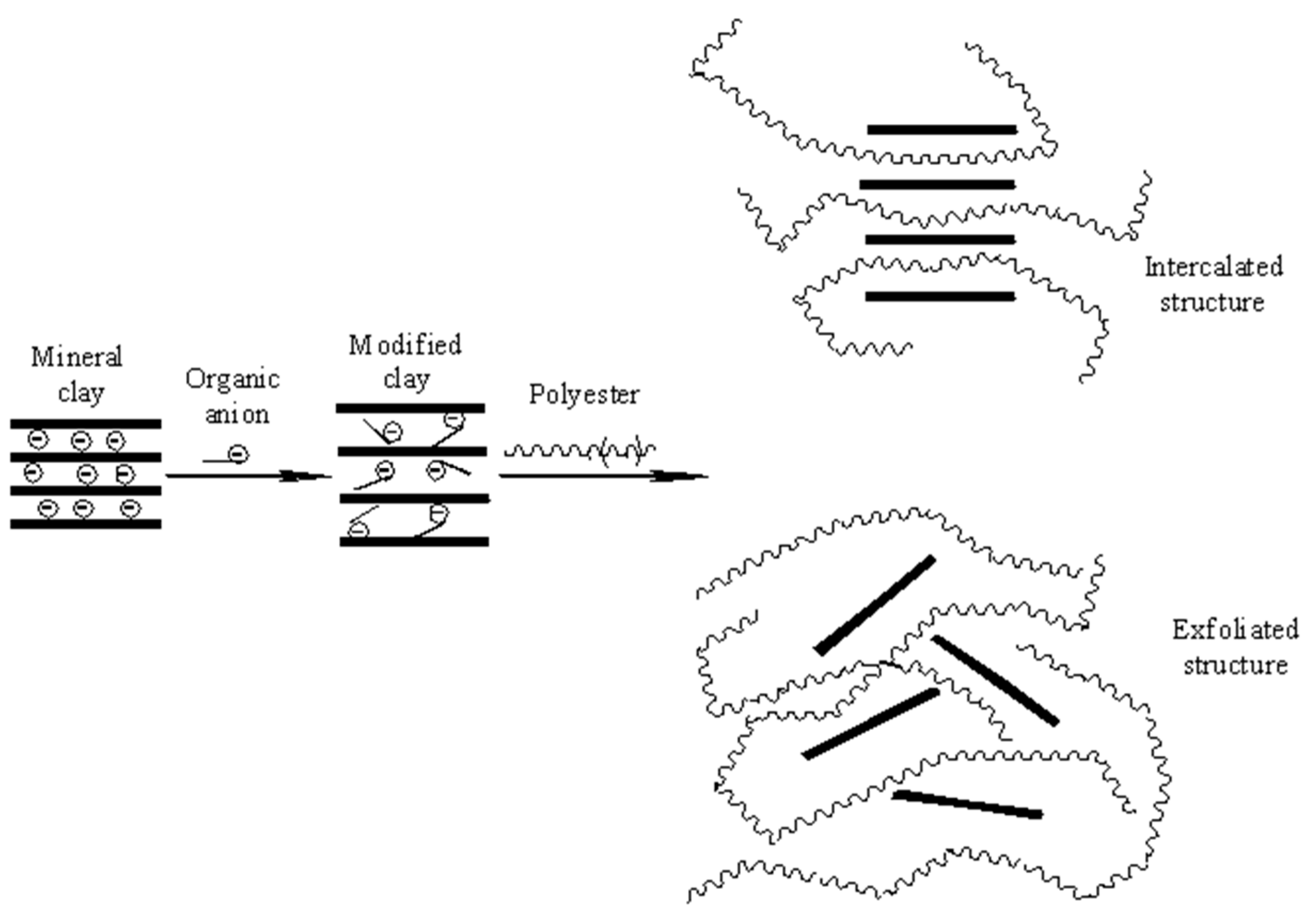
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Fabrication of PHBHV/LDH-SDS Plates
2.2.2. Characterization
2.2.3. Cell Culture Model
Fluorescent Labeling of Actin Filaments
MTT Assay
Lactate Dehydrogenase Assay
Statistical Analysis
3. Results and Discussions
3.1. X-ray Diffraction
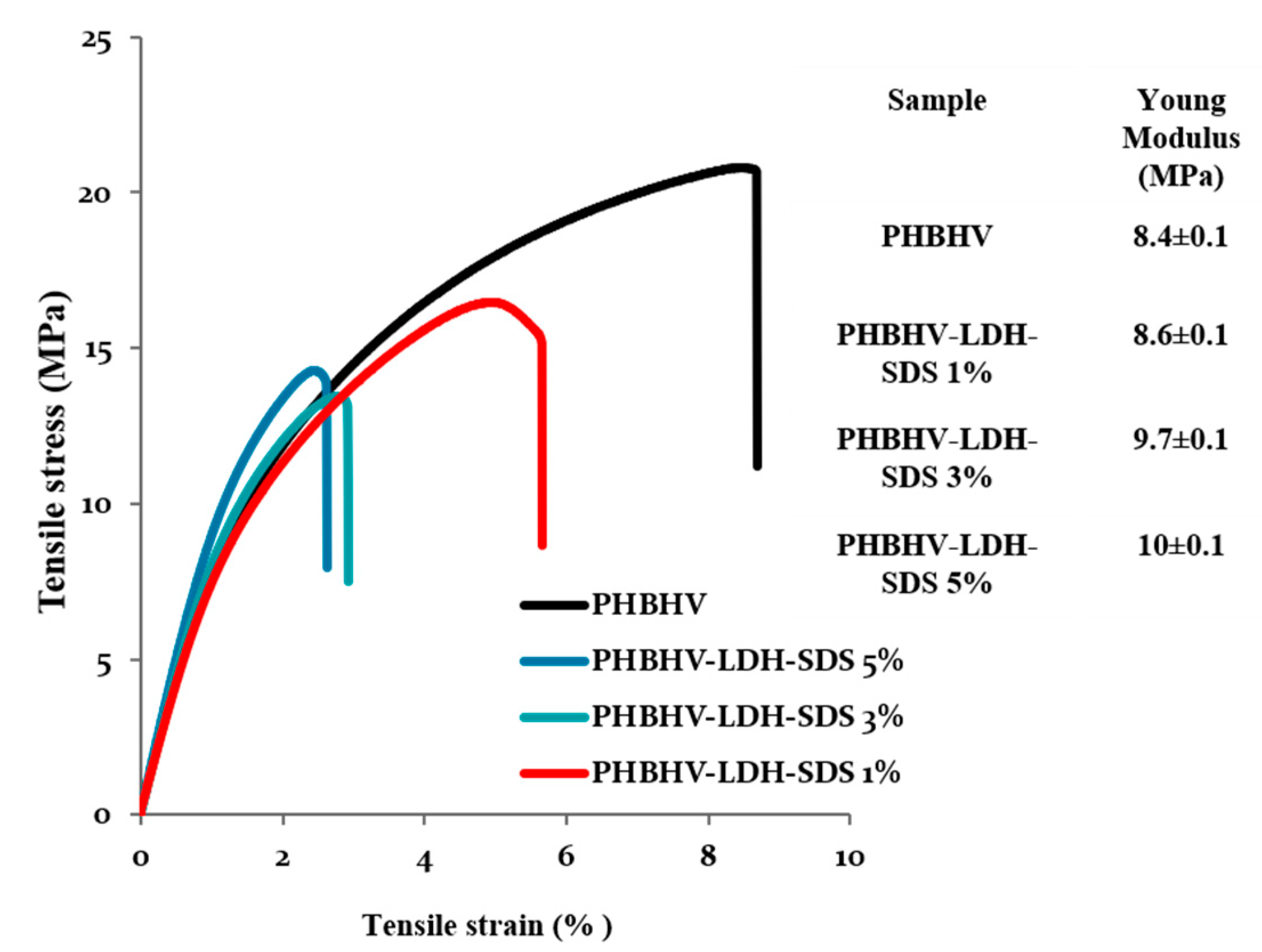
3.2. Tensile Strength
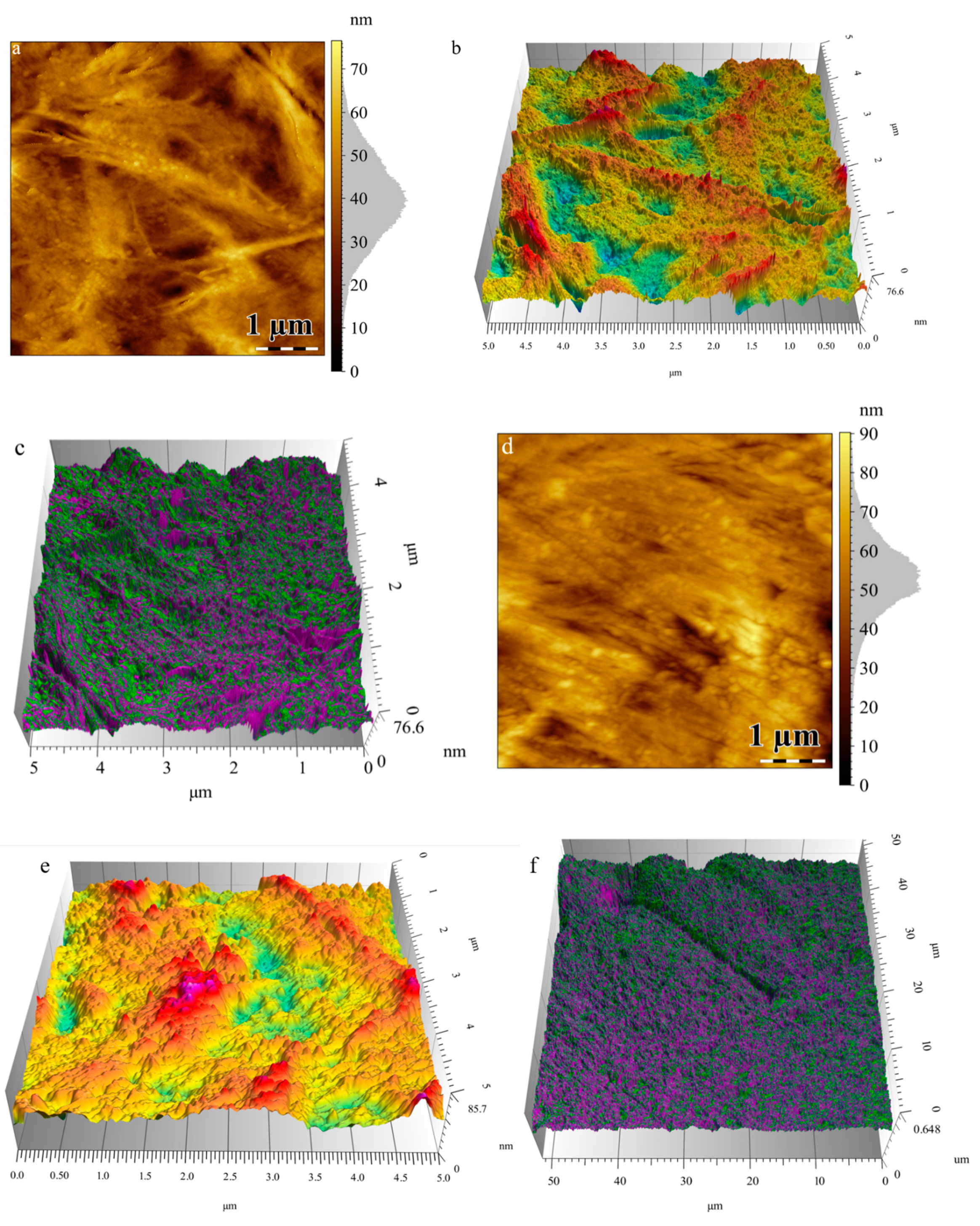
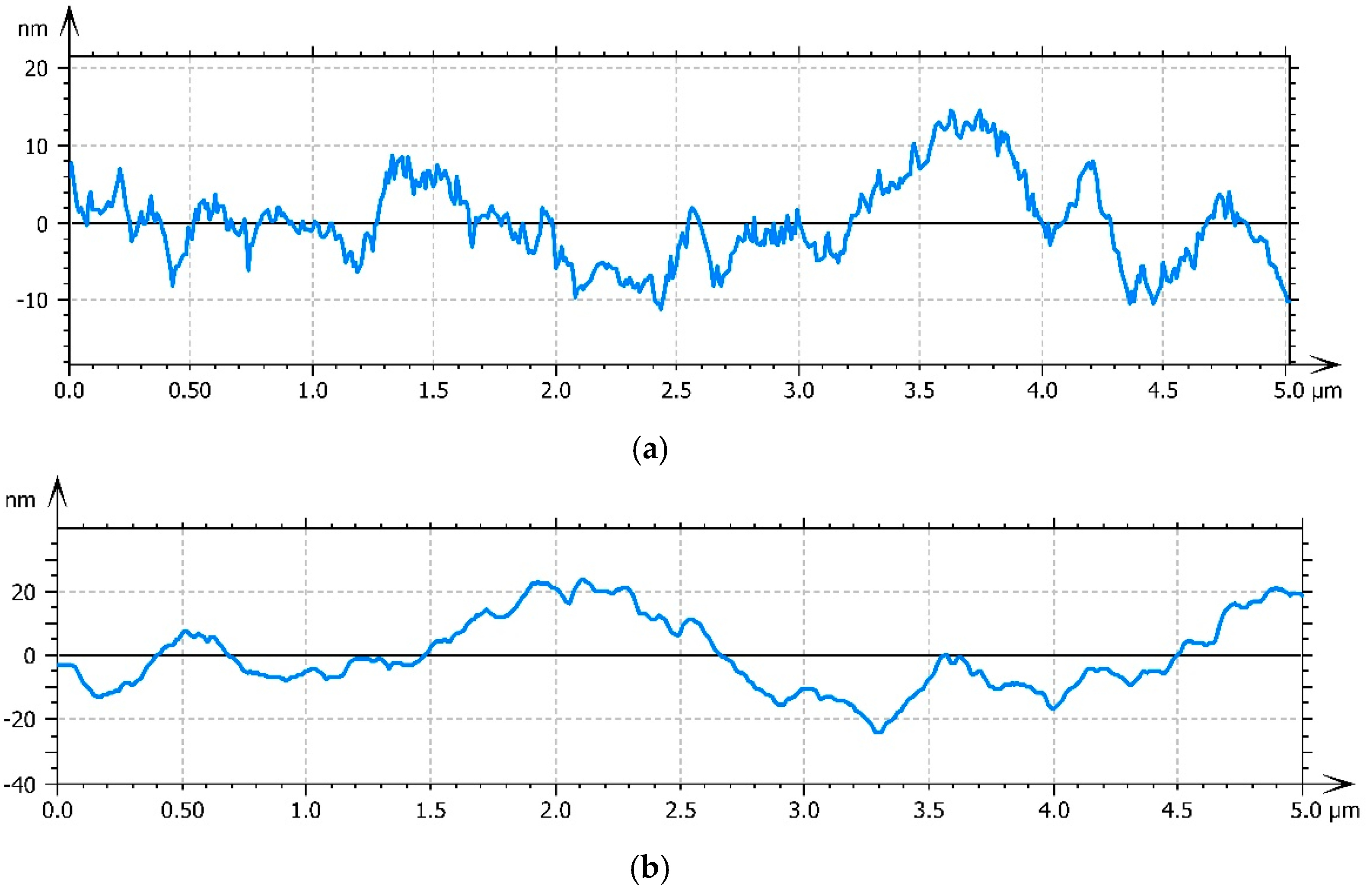
3.3. Atomic Force Microscopy (AFM)
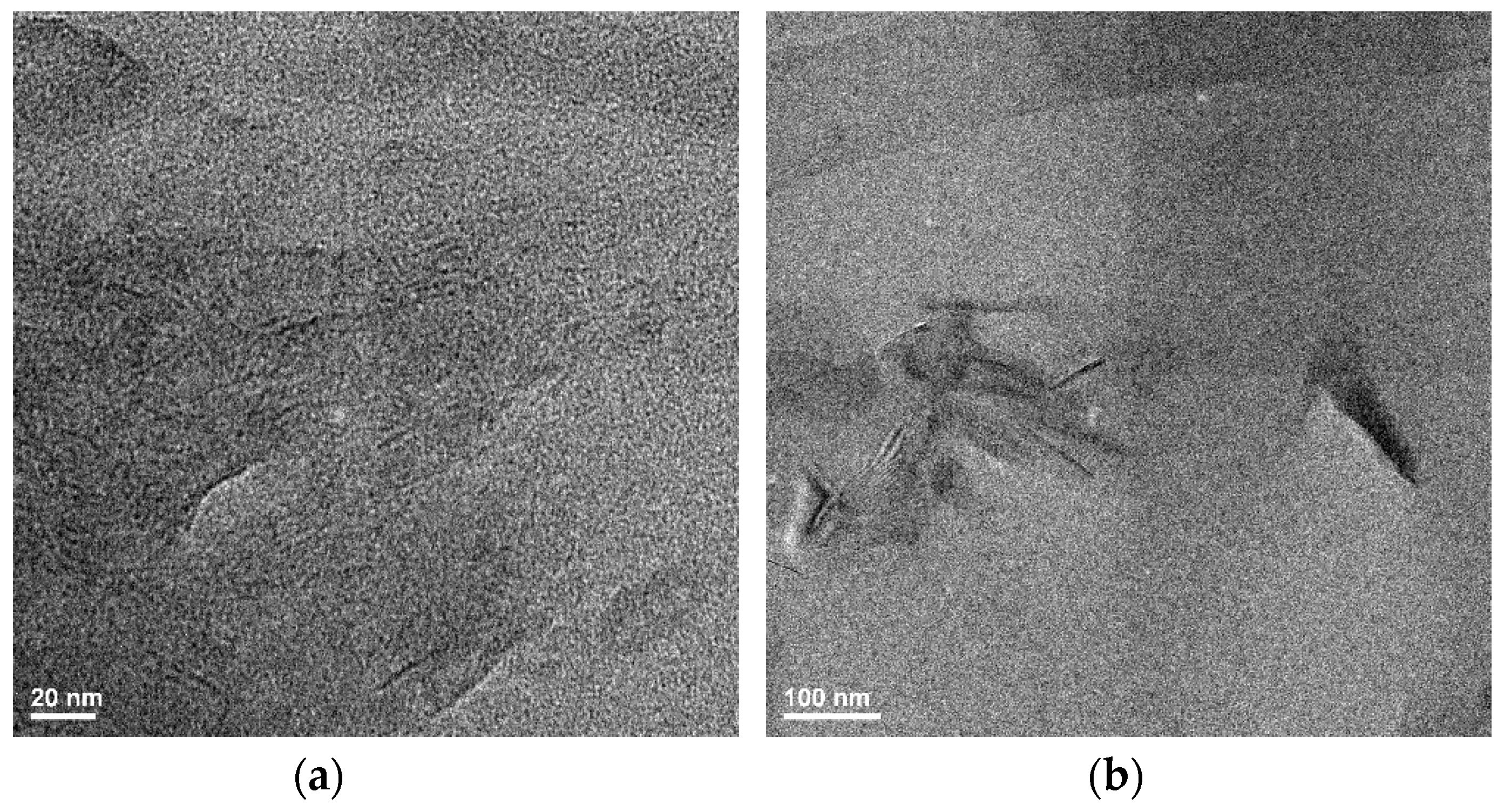
3.4. Transmission Electron Microscopy (TEM)
3.5. hASCs Morphology on PHBHV, PHBHV/LDH-SDS 1%, PHBHV/LDH-SDS 3%, and PHBHV/LDH-SDS 5% Biomaterials
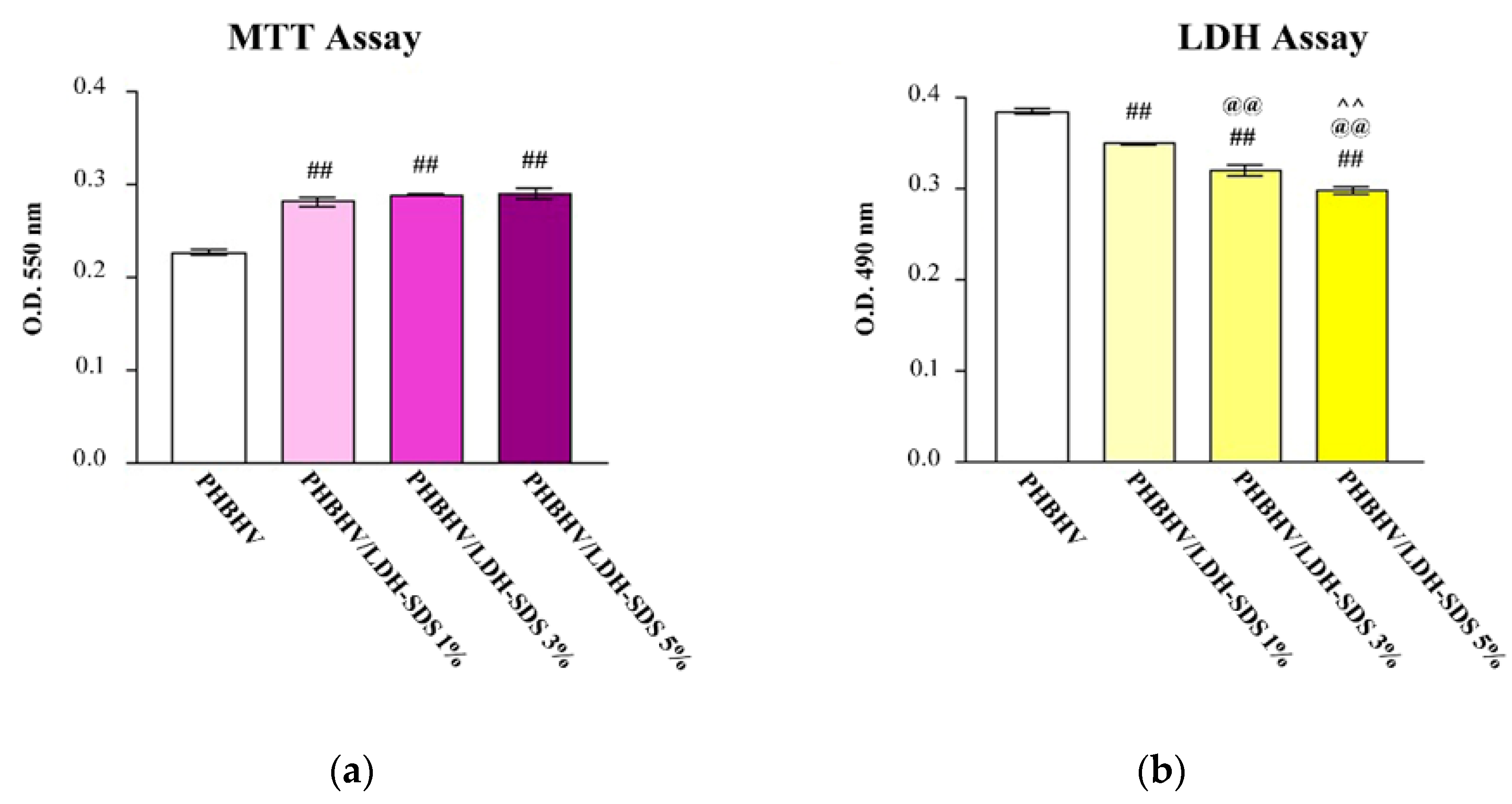
3.6. hASCs Viability in Contact with PHBHV, PHBHV/LDH-SDS 1%, PHBHV/LDH-SDS 3%, and PHBHV/LDH-SDS 5% Biomaterials
3.7. Biomaterials’ Cytotoxic Potential on hASCs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bikiaris, D.N. Nanocomposites of aliphatic polyesters: An overview of the effect of different nanofillers on enzymatic hydrolysis and biodegradation of polyesters. Polym. Degrad. Stab. 2013, 98, 1908–1928. [Google Scholar] [CrossRef]
- Bera, A.; Dubey, S.; Bhayani, K.; Mondal, D.; Mishra, S.; Ghosh, P.K. Microbial synthesis of polyhydroxyalkanoate using seaweed-derived crude levulinic acid as co-nutrient. Int. J. Biol. Macromol. 2015, 72, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, X.-L.; Peng, S.-W.; Guo, X.-Y.; Shang, G.-G.; Chen, J.-C.; Wu, Q.; Chen, G.-Q. Induced apoptosis of osteoblasts proliferating on polyhydroxyalkanoates. Biomaterials 2013, 34, 3737–3746. [Google Scholar] [CrossRef] [PubMed]
- Chanprateep, S.; Napathorn, S.C. Current trends in biodegradable polyhydroxyalkanoates. J. Biosci. Bioeng. 2010, 110, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Freier, T. Biopolyesters in Tissue Engineering Applications. In Polymers for Regenerative Medicine; Carsten, W., Ed.; Springer: Heidelberg/Berlin, Germany, 2006; pp. 1–61. [Google Scholar]
- Grage, K.; Jahns, A.C.; Parlane, N.; Palanisamy, R.; Rasiah, I.A.; Atwood, J.A.; Rehm, B.H.A. Bacterial Polyhydroxyalkanoate Granules: Biogenesis, Structure, and Potential Use as Nano-/Micro-Beads in Biotechnological and Biomedical Applications. Biomacromolecules 2009, 10, 660–669. [Google Scholar] [CrossRef]
- Brunel, D.G.; Pachekoski, W.M.; Dalmolin, C.; Agnelli, J.A.M. Natural additives for poly (hydroxybutyrate - CO - hydroxyvalerate) - PHBV: Effect on mechanical properties and biodegradation. Mater. Res. 2014, 17, 1145–1156. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, J.; Lan, J.; Liu, Z.; He, N.; Shen, L.; Li, Q. Biosynthesis of poly(hydroxybutyrate-hydroxyvalerate) from the acclimated activated sludge and microbial characterization in this process. Bioresour. Technol. 2013, 148, 61–69. [Google Scholar] [CrossRef]
- Visakh, P.M. CHAPTER 1. Polyhydroxyalkanoates (PHAs), their Blends, Composites and Nanocomposites: State of the Art, New Challenges and Opportunities. In Polyhydroxyalkanoate (PHA) Based Blends, Composites and Nanocomposites, Green Chemistry Series; Royal Society of Chemistry (RSC), Thomas Graham House (290): Cambridge, UK, 2014; pp. 1–17. [Google Scholar]
- Bhati, R.; Mallick, N. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production by the diazotrophic cyanobacterium Nostoc muscorum Agardh: Process optimization and polymer characterization. Algal Res. 2015, 7, 78–85. [Google Scholar] [CrossRef]
- Gunning, M.A.; Geever, L.M.; Killion, J.A.; Lyons, J.G.; Higginbotham, C.L. Mechanical and biodegradation performance of short natural fibre polyhydroxybutyrate composites. Polym. Test. 2013, 32, 1603–1611. [Google Scholar] [CrossRef]
- Peschel, G.; Konrad, A.; Wieland, G.D.; Martin, D.P.; Roth, M.; Dahse, H.-M.; Mueller, P.-J. Growth of keratinocytes on porous films of poly(3-hydroxybutyrate) and poly(4-hydroxybutyrate) blended with hyaluronic acid and chitosan. J. Biomed. Mater. Res. Part A 2008, 85, 1072–1081. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, K.; Zhang, X.-F.; Hu, P.; Chen, G.-Q. Study on the three-dimensional proliferation of rabbit articular cartilage-derived chondrocytes on polyhydroxyalkanoate scaffolds. Biomaterials 2002, 23, 4049–4056. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Qiong, W.; Chen, G.-Q. Attachment, proliferation and differentiation of osteoblasts on random biopolyester poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds. Biomaterials 2004, 25, 669–675. [Google Scholar] [CrossRef]
- Nogueira, T.; Botan, R.; Wypych, F.; Lona, L.M.F. Study of thermal and mechanical properties of PMMA/LDHs nanocomposites obtained by in situ bulk polymerization. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1025–1030. [Google Scholar] [CrossRef]
- Delhoyo, C. Layered double hydroxides and human health: An overview. Appl. Clay Sci. 2007, 36, 103–121. [Google Scholar] [CrossRef]
- Rives, V.; Del Arco, M.; Martín, C. Intercalation of drugs in layered double hydroxides and their controlled release: A review. Appl. Clay Sci. 2014, 88, 239–269. [Google Scholar] [CrossRef]
- Chiang, M.-F.; Wu, T.-M. Synthesis and characterization of biodegradable poly(l-lactide)/layered double hydroxide nanocomposites. Compos. Sci. Technol. 2010, 70, 110–115. [Google Scholar] [CrossRef]
- Lennerová, D.; Kovanda, F.; Brožek, J. Preparation of Mg–Al layered double hydroxide/polyamide 6 nanocomposites using Mg–Al–taurate LDH as nanofiller. Appl. Clay Sci. 2015, 114, 265–272. [Google Scholar] [CrossRef]
- Hu, J.; Gan, M.; Ma, L.; Zhang, J.; Xie, S.; Xu, F.; Shen, J.Z.X.; Yin, H. Preparation and enhanced properties of polyaniline/grafted intercalated ZnAl-LDH nanocomposites. Appl. Surf. Sci. 2015, 328, 325–334. [Google Scholar] [CrossRef]
- Senapati, S.; Thakur, R.; Verma, S.P.; Duggal, S.; Mishra, D.P.; Das, P.; Shripathi, T.; Kumar, M.; Rana, D.; Maiti, P. Layered double hydroxides as effective carrier for anticancer drugs and tailoring of release rate through interlayer anions. J. Control. Release 2016, 224, 186–198. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Liu, H.; Li, S.-P.; Li, X.-D. Synthesis of methotrexatum intercalated zinc–aluminum-layered double hydroxides and the corresponding cell studies. Appl. Clay Sci. 2016, 121-122, 103–110. [Google Scholar] [CrossRef]
- Guo, B.; Zhao, Y.; Huang, Q.; Jiao, Q. A new method to prepare exfoliated UV-cured polymer/LDH nanocomposites via nanoplatelet-like LDHs modified with N-Lauroyl-glutamate. Compos. Sci. Technol. 2013, 81, 37–41. [Google Scholar] [CrossRef]
- Basu, D.; Das, A.; Salaeh, S.; Wagenknecht, U.; Heinrich, G. Advances in layered double hydroxide (LDH)-based elastomer composites. Prog. Polym. Sci. 2014, 39, 594–626. [Google Scholar] [CrossRef]
- Costa, F.R.; Leuteritz, A.; Wagenknecht, U.; Jehnichen, D.; Häußler, L.; Heinrich, G. Intercalation of Mg–Al layered double hydroxide by anionic surfactants: Preparation and characterization. Appl. Clay Sci. 2008, 38, 153–164. [Google Scholar] [CrossRef]
- Du, L.; Qu, B.; Zhang, M. Thermal properties and combustion characterization of nylon 6/MgAl-LDH nanocomposites via organic modification and melt intercalation. Polym. Degrad. Stab. 2007, 92, 497–502. [Google Scholar] [CrossRef]
- Gómez-Fernández, S.; Ugarte, L.; Peña-Rodriguez, C.; Zubitur, M.; Corcuera, M.A.; Eceiza, A. Flexible polyurethane foam nanocomposites with modified layered double hydroxides. Appl. Clay Sci. 2016, 123, 109–120. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Ogawa, M.; Kuroda, K. Photofunctions of Intercalation Compounds. Chem. Rev. 1995, 95, 399–438. [Google Scholar] [CrossRef]
- Vaccari, A. Preparation and catalytic properties of cationic and anionic clays. Catal. Today 1998, 41, 53–71. [Google Scholar] [CrossRef]
- Gorchakov, V.; Dragun, G.; Kolmogorov, Y.; Smelova, V.; Tikhonova, L.; Tysjachnova, Y. The using of SR XRF for estimation of macro- and microelement contents of biological objects at the clay treatment. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2001, 470, 437–440. [Google Scholar] [CrossRef]
- Choy, J.-H.; Choi, S.-J.; Oh, J.-M.; Park, T. Clay minerals and layered double hydroxides for novel biological applications. Appl. Clay Sci. 2007, 36, 122–132. [Google Scholar] [CrossRef]
- Iborra, C.V.; Aguzzi, C.; Cerezo, P.; Bedmar, M.C. Biopolymer–clay nanocomposites for controlled drug delivery. Mater. Sci. Technol. 2008, 24, 1020–1026. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimian, T.; Pouzoulet, F.; Squiban, C.; Buard, V.; André, M.; Cousin, B.; Gourmelon, P.; Benderitter, M.; Casteilla, L.; Tamarat, R. Cell Therapy Based on Adipose Tissue-Derived Stromal Cells Promotes Physiological and Pathological Wound Healing. Arter. Thromb. Vasc. Biol. 2009, 29, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-S.; Park, B.-S.; Sung, J.-H.; Yang, J.-M.; Park, S.-B.; Kwak, S.-J.; Park, J.-S. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J. Dermatol. Sci. 2007, 48, 15–24. [Google Scholar] [CrossRef]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef]
- Galateanu, B.; Dinescu, S.; Cimpean, A.; Dinischiotu, A.; Costache, M. Modulation of Adipogenic Conditions for Prospective Use of hADSCs in Adipose Tissue Engineering. Int. J. Mol. Sci. 2012, 13, 15881–15900. [Google Scholar] [CrossRef] [PubMed]
- Galateanu, B.; Dimonie, D.; Vasile, E.; Nae, S.; Cimpean, A.; Costache, M. Layer-shaped alginate hydrogels enhance the biological performance of human adipose-derived stem cells. BMC Biotechnol. 2012, 12, 35. [Google Scholar] [CrossRef]
- ISO 4287:1997. Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Terms, Definitions and Surface Texture Parameters; International Organization for Standardization: Geneve, Switzerland, 1997. [Google Scholar]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasile, E.; Radu, I.-C.; Galateanu, B.; Rapa, M.; Hudita, A.; Jianu, D.; Stanescu, P.-O.; Cioflan, H.; Zaharia, C. Novel Nanocomposites Based on Bacterial Polyester/LDH-SDS Clay for Stem Cells Delivery in Modern Wound Healing Management. Materials 2020, 13, 4488. https://doi.org/10.3390/ma13204488
Vasile E, Radu I-C, Galateanu B, Rapa M, Hudita A, Jianu D, Stanescu P-O, Cioflan H, Zaharia C. Novel Nanocomposites Based on Bacterial Polyester/LDH-SDS Clay for Stem Cells Delivery in Modern Wound Healing Management. Materials. 2020; 13(20):4488. https://doi.org/10.3390/ma13204488
Chicago/Turabian StyleVasile, Eugeniu, Ionut-Cristian Radu, Bianca Galateanu, Maria Rapa, Ariana Hudita, Dana Jianu, Paul-Octavian Stanescu, Horia Cioflan, and Catalin Zaharia. 2020. "Novel Nanocomposites Based on Bacterial Polyester/LDH-SDS Clay for Stem Cells Delivery in Modern Wound Healing Management" Materials 13, no. 20: 4488. https://doi.org/10.3390/ma13204488
APA StyleVasile, E., Radu, I.-C., Galateanu, B., Rapa, M., Hudita, A., Jianu, D., Stanescu, P.-O., Cioflan, H., & Zaharia, C. (2020). Novel Nanocomposites Based on Bacterial Polyester/LDH-SDS Clay for Stem Cells Delivery in Modern Wound Healing Management. Materials, 13(20), 4488. https://doi.org/10.3390/ma13204488







