Influence of Titanium Surface Porosity on Adhesive Strength of Coatings Containing Calcium Silicate
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
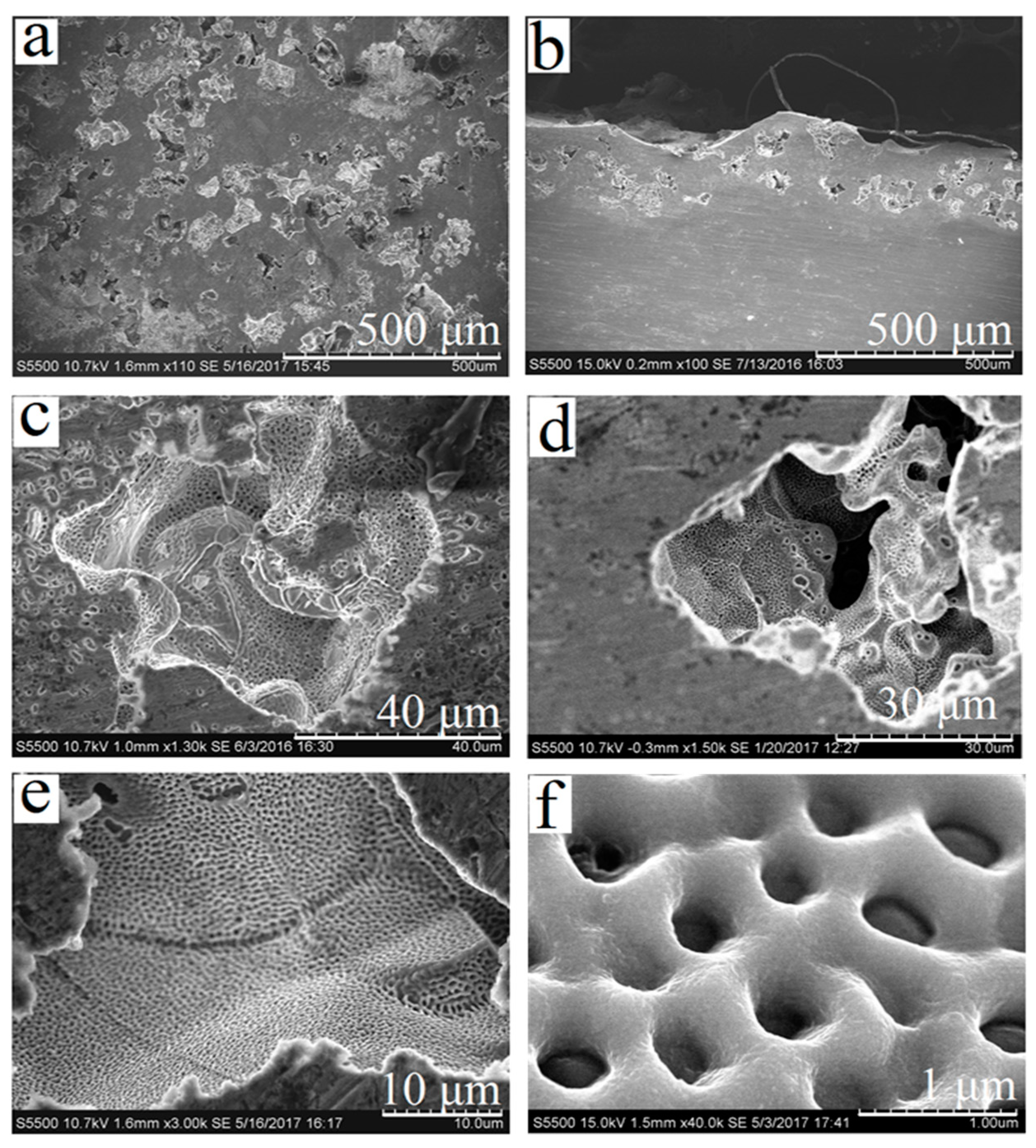
3.1. Forming Porous Surface Layer
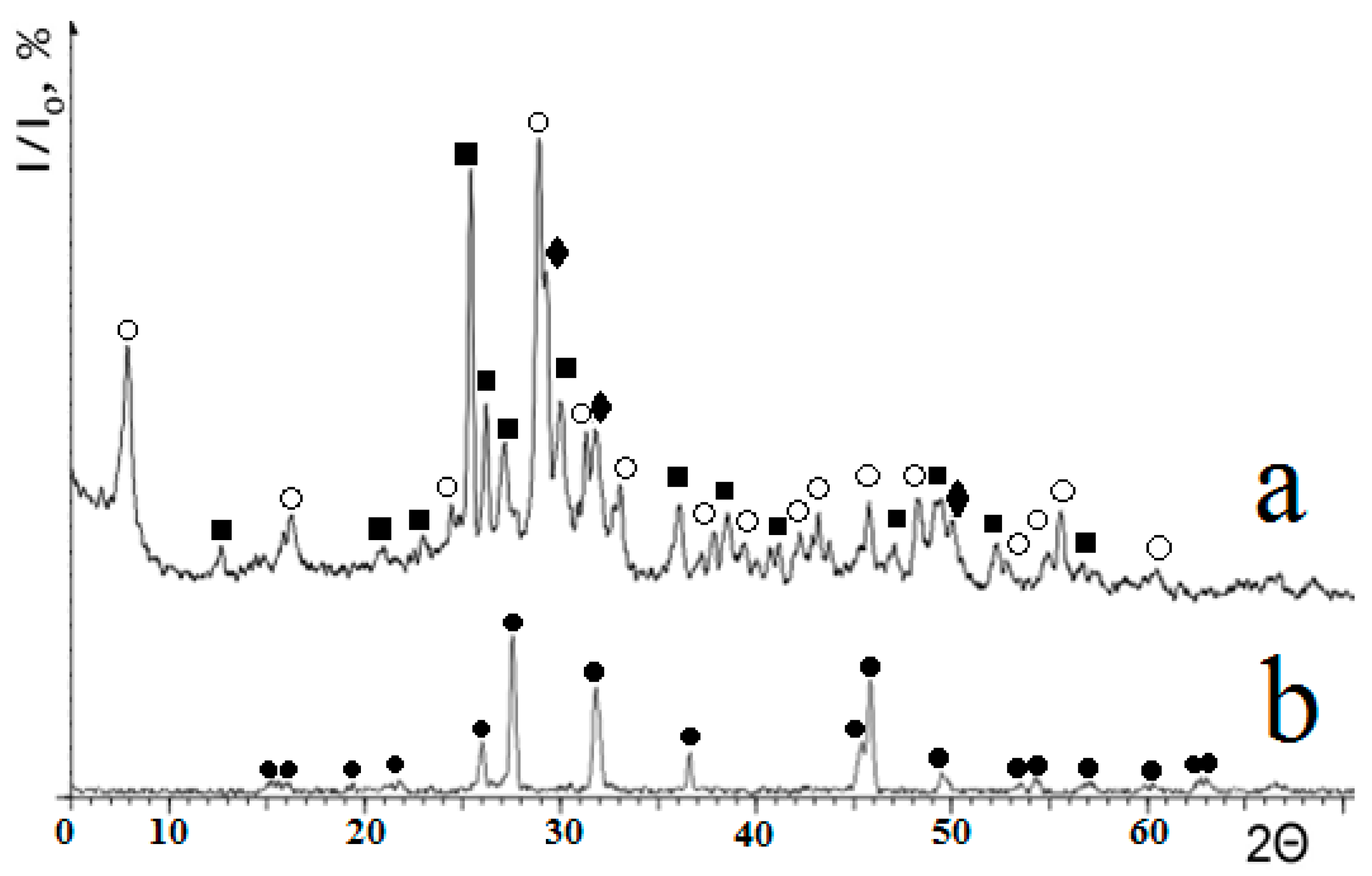
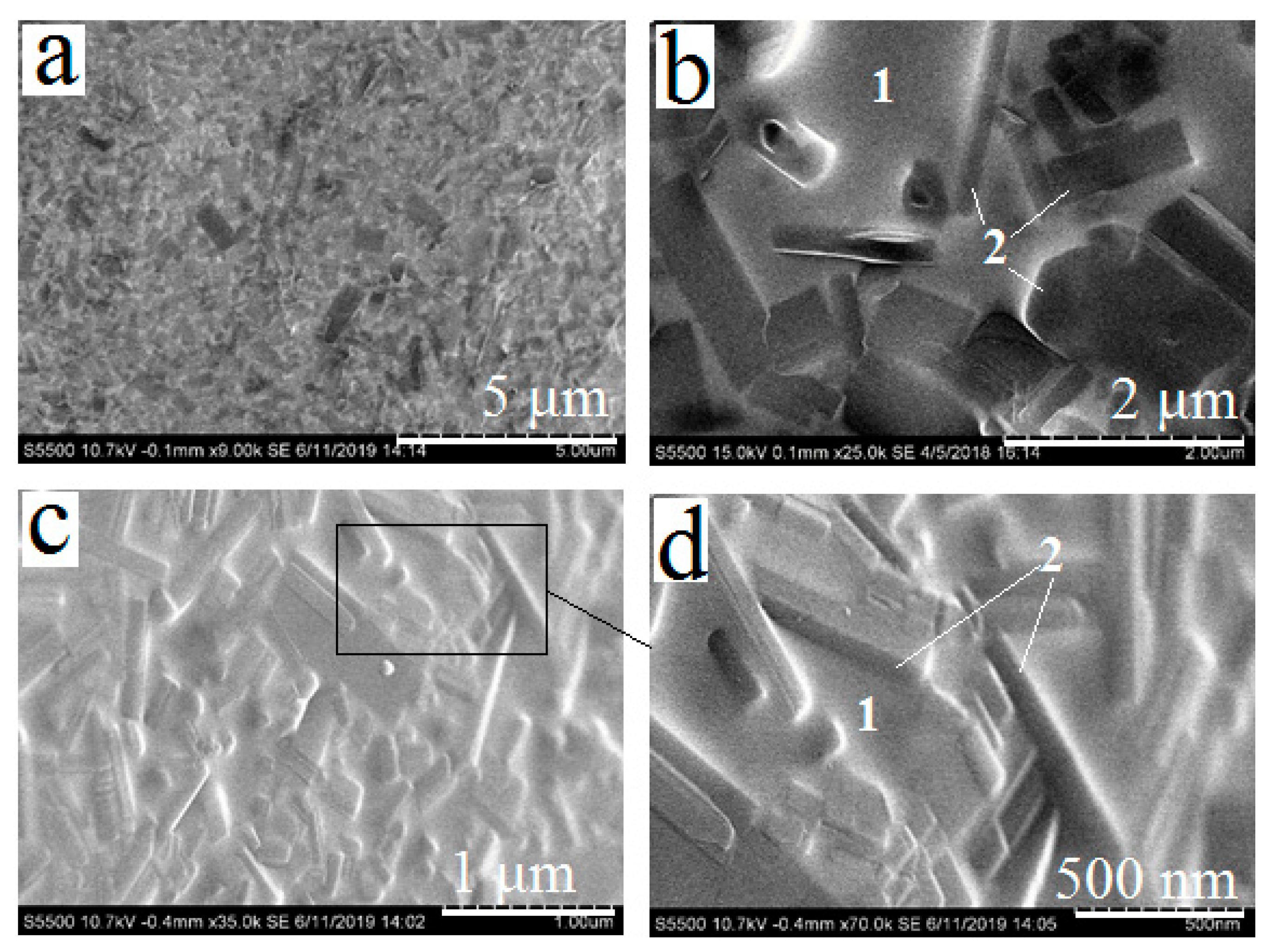
3.2. Synthesis of Calcium and Lead Silicates
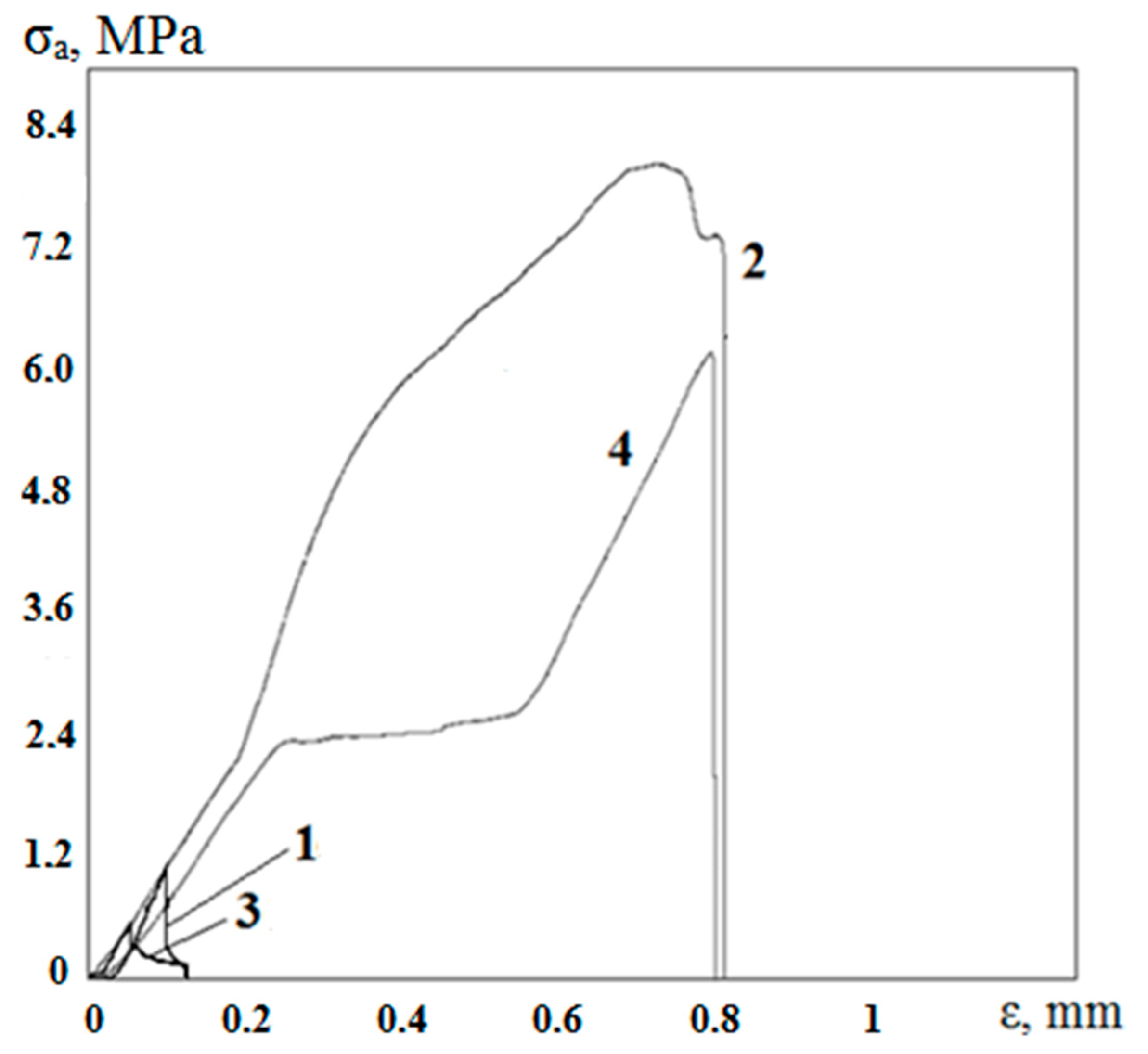
3.3. Obtaining and Studying the Coating
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gorynin, I.V.; Ushkov, S.S.; Khatuntsev, A.N.; Loshakova, N.I. Titanium alloys for marine engineering. Polytekhnica 2007, 387, 112–116. (In Russian) [Google Scholar]
- Leyens, C.; Peters, M. (Eds.) Titanium and Titanium Alloys. In Fundamentals and Applications; WILEY-VCH GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Hołyńska, M.; Tighe, A.; Semprimoschnig, C. Coatings and Thin Films for Spacecraft Thermo-Optical and Related Functional Applications. Adv. Mater. Interfaces 2018, 5, 1701644. [Google Scholar] [CrossRef]
- Mikhailov, M.; Vlasov, V.; Yuryev, S.; Neshchimenko, V.; Shcherbina, V. Optical properties and radiation stability of TiO2 powders modified by Al2O3, ZrO2, SiO2, TiO2, ZnO, and MgO nanoparticles. Dye. Pigment. 2015, 123, 72–77. [Google Scholar] [CrossRef]
- Heydari, V.; Bahreini, Z. Synthesis of silica-supported ZnO pigments for thermal control coatings and analysis of their reflection model. J. Coatings Technol. Res. 2017, 15, 223–230. [Google Scholar] [CrossRef]
- Mikhailov, M.M. Prediction of Optical Degradation of Temperature-Controlled Coatings of Spacecraft; “Nauka” Sib, Ed.; Russian Academy of Sciences: Novosibirsk, Russian, 1999; p. 192. (In Russian) [Google Scholar]
- Mikhailov, M.M.; Neshchimenko, V.V.; Li, C. The degradation kinetics of the optical properties under proton irradiation for ZnO pigments modified by Al2O3 and Al2O3·CeO2 nanopowders. Radiat. Eff. Defects Solids 2012, 167, 26–36. [Google Scholar] [CrossRef]
- Meseguer, J.; Perez-Grande, I.; Sanz-Andres, A. Spacecraft Thermal Control; Woodhead Publishing: Cambridge, UK, 2012. [Google Scholar]
- Mantovani, L.; Tribaudino, M.; Dondi, M.; Zanelli, C. Synthesis and color performance of CaCoSi2O6 pyroxene, a new ceramic colorant. Dye. Pigment. 2015, 120, 118–125. [Google Scholar] [CrossRef]
- Jose, S.; Reddy, M.L.P. Lanthanum–strontium copper silicates as intense blue inorganic pigments with high near-infrared reflectance. Dye. Pigment. 2013, 98, 540–546. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Feng, S. Hydrothermal synthesis and properties of pigments Chinese purple BaCuSi2O6 and dark blue BaCu2Si2O7. Dye. Pigment. 2014, 105, 167–173. [Google Scholar] [CrossRef]
- Tyulnin, V.A.; Tkach, V.R.; Eirich, V.I.; Starodubtsev, N.P. Wollastonite: A Unique Multi-Purpose Mineral Raw Material; Publishing House “Ruda i Metally”: Moscow, Russia, 2003; p. 144. (In Russian) [Google Scholar]
- Sedelnikova, M.; Sharkeev, Y.; Komarova, E.G.; Khlusov, I.A.; Chebodaeva, V. Structure and properties of the wollastonite–calcium phosphate coatings deposited on titanium and titanium–niobium alloy using microarc oxidation method. Surf. Coatings Technol. 2016, 307, 1274–1283. [Google Scholar] [CrossRef]
- Gordienko, P.S.; Mikhailov, M.M.; Banerjee, S.; Sharma, Y.C.; Yarusova, S.B.; Zhevtun, I.; Vlasov, V.; Shabalin, I.; Sushkov, Y. Effect of annealing conditions on the structure, phase and granulometry composition, and reflectance spectra and their changes on irradiation for calcium silicate powders. Mater. Chem. Phys. 2017, 197, 266–271. [Google Scholar] [CrossRef]
- Neshchimenko, V.; Li, C.; Mikhailov, M.M. Radiation stability of TiO2 hollow particles pigments and coatings synthesis by hydrothermal methods from TTIP. Dye. Pigment. 2017, 145, 354–358. [Google Scholar] [CrossRef]
- Tokar, S.V.; Barinova, O.P. Inorganic coatings based on alkali metal silicates and their resistance to proton irradiation. Tekhnika i Tekhnologiya Silicatov. 2019, 26, 6–8. (In Russian) [Google Scholar]
- Comyn, J. Adhesion Science; Royal Society of Chemistry (RSC): Cambridge, UK, 2007. [Google Scholar]
- Zhevtun, I.; Gordienko, P.; Яpycoвa, C.; Yarusova, S. Formation of Wear-Resistant Composite Coatings on Titanium Alloys in the Electric arc Treatment of Aqueous Electrolytes; RIOR; INFRA-M: Moscow, Russia, 2018; 155p. [Google Scholar]
- Zhevtun, I.G.; Gordienko, P.S.; Yarusova, S.B.; Kul’Chin, Y.N.; Subbotin, E.; Pivovarov, D.S.; Yatsko, D.S. Micro- and Nanoporous Structure Formed on the Titanium Surface by Laser Treatment. Phys. Met. Met. 2018, 119, 491–496. [Google Scholar] [CrossRef]
- Stognij, A.; Novitskii, N.; Trukhanov, S.; Panina, L.; Sharko, S.; Serokurova, A.; Poddubnaya, N.; Ketsko, V.; Dyakonov, V.; Szymczak, H.; et al. Interface magnetoelectric effect in elastically linked Co/PZT/Co layered structures. J. Magn. Magn. Mater. 2019, 485, 291–296. [Google Scholar] [CrossRef]
- Bajpai, K.; Sreenivas, K.; Gupta, A.K.; Shukla, A.K. Cr-doped lead lanthanum zirconate titanate (PLZT) ceramics for pyroelectric and energy harvesting device applications. Ceram. Int. 2019, 45, 14111–14120. [Google Scholar] [CrossRef]
- Azarov, G.M.; Maiorova, E.V.; Oborina, M.A.; Belyakov, A.V. Wollastonite raw materials and their applications (a review). Glas. Ceram. 1995, 52, 237–240. [Google Scholar] [CrossRef]

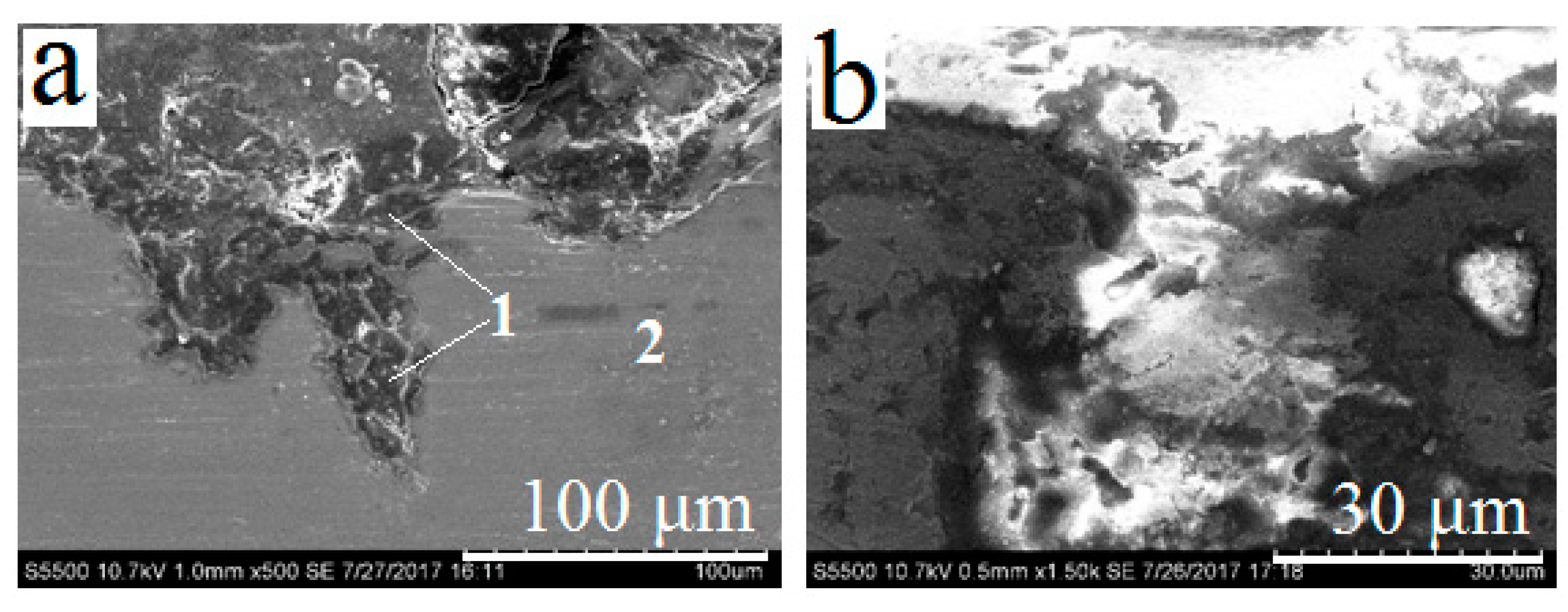


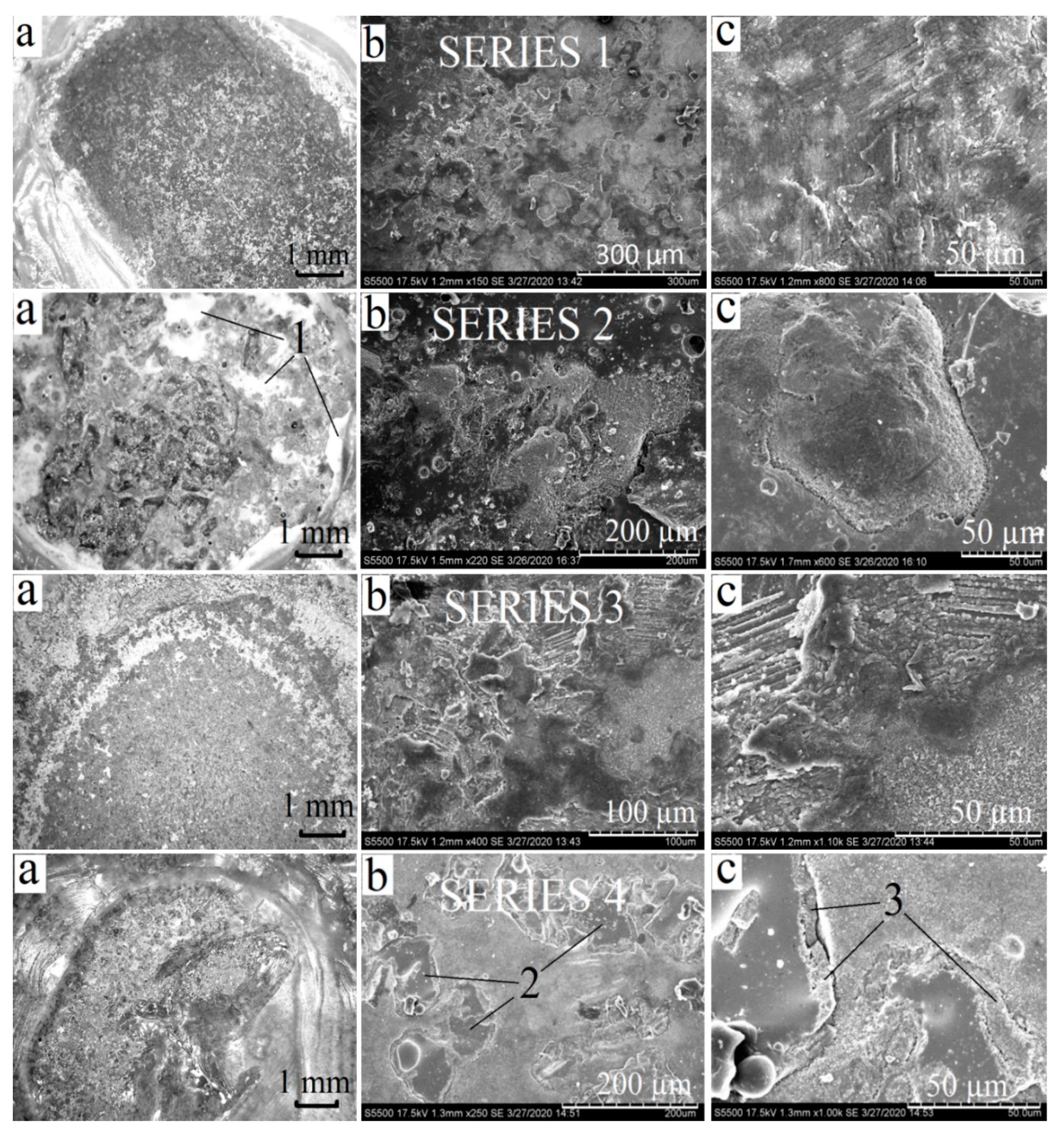
| No. of Area | Ca | Pb | Si | O |
|---|---|---|---|---|
| 1 | 1.01 | 22.96 | 36.61 | 39.42 |
| 2 | 24.57 | 3.36 | 23.93 | 48.14 |
| No. of Area | Ca | Pb | Ti | Si | O | C |
|---|---|---|---|---|---|---|
| 1 | 1.36 | 30.12 | 20.40 | 8.31 | 21.16 | 18.65 |
| 2 | – | – | 80.14 | – | 1.24 | 18.62 |
| No. of Area | Pb | Ti | Si | O | C |
|---|---|---|---|---|---|
| 1 | 18.39 | 1.38 | 22.03 | 34.79 | 23.41 |
| 2 | – | 32.81 | – | 42.96 | 24.23 |
| 3 | – | 82.71 | – | 0.21 | 17.08 |
| Series No. | Adhesive Strength σa, MPa | Maximum Deviations, MPa |
|---|---|---|
| 1 | 1.06 | 0.13 |
| 2 | 8.06 | 0.49 |
| 3 | 0.55 | 0.09 |
| 4 | 6.2 | 0.57 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhevtun, I.; Gordienko, P.; Kulchin, Y.; Nikitin, A.; Pivovarov, D.; Yarusova, S.; Golub, A.; Nikiforov, P.; Timchenko, V. Influence of Titanium Surface Porosity on Adhesive Strength of Coatings Containing Calcium Silicate. Materials 2020, 13, 4493. https://doi.org/10.3390/ma13204493
Zhevtun I, Gordienko P, Kulchin Y, Nikitin A, Pivovarov D, Yarusova S, Golub A, Nikiforov P, Timchenko V. Influence of Titanium Surface Porosity on Adhesive Strength of Coatings Containing Calcium Silicate. Materials. 2020; 13(20):4493. https://doi.org/10.3390/ma13204493
Chicago/Turabian StyleZhevtun, Ivan, Pavel Gordienko, Yury Kulchin, Alexander Nikitin, Dmitry Pivovarov, Sofia Yarusova, Andrey Golub, Pavel Nikiforov, and Vadim Timchenko. 2020. "Influence of Titanium Surface Porosity on Adhesive Strength of Coatings Containing Calcium Silicate" Materials 13, no. 20: 4493. https://doi.org/10.3390/ma13204493
APA StyleZhevtun, I., Gordienko, P., Kulchin, Y., Nikitin, A., Pivovarov, D., Yarusova, S., Golub, A., Nikiforov, P., & Timchenko, V. (2020). Influence of Titanium Surface Porosity on Adhesive Strength of Coatings Containing Calcium Silicate. Materials, 13(20), 4493. https://doi.org/10.3390/ma13204493





