Hierarchical Microstructure of Laser Powder Bed Fusion Produced Face-Centered-Cubic-Structured Equiatomic CrFeNiMn Multicomponent Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
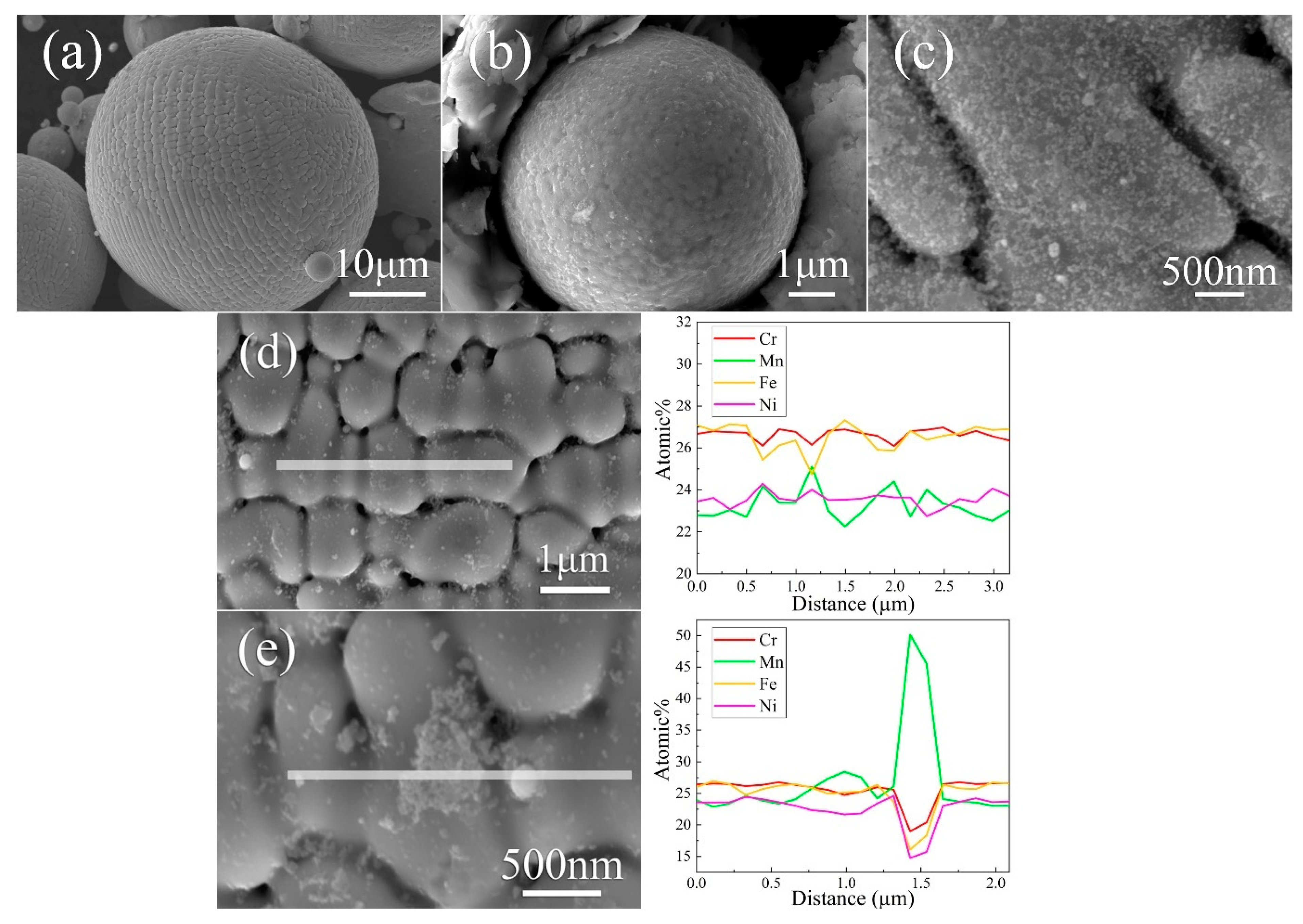
3.1. Microstructure of Gas-Atomized Powder
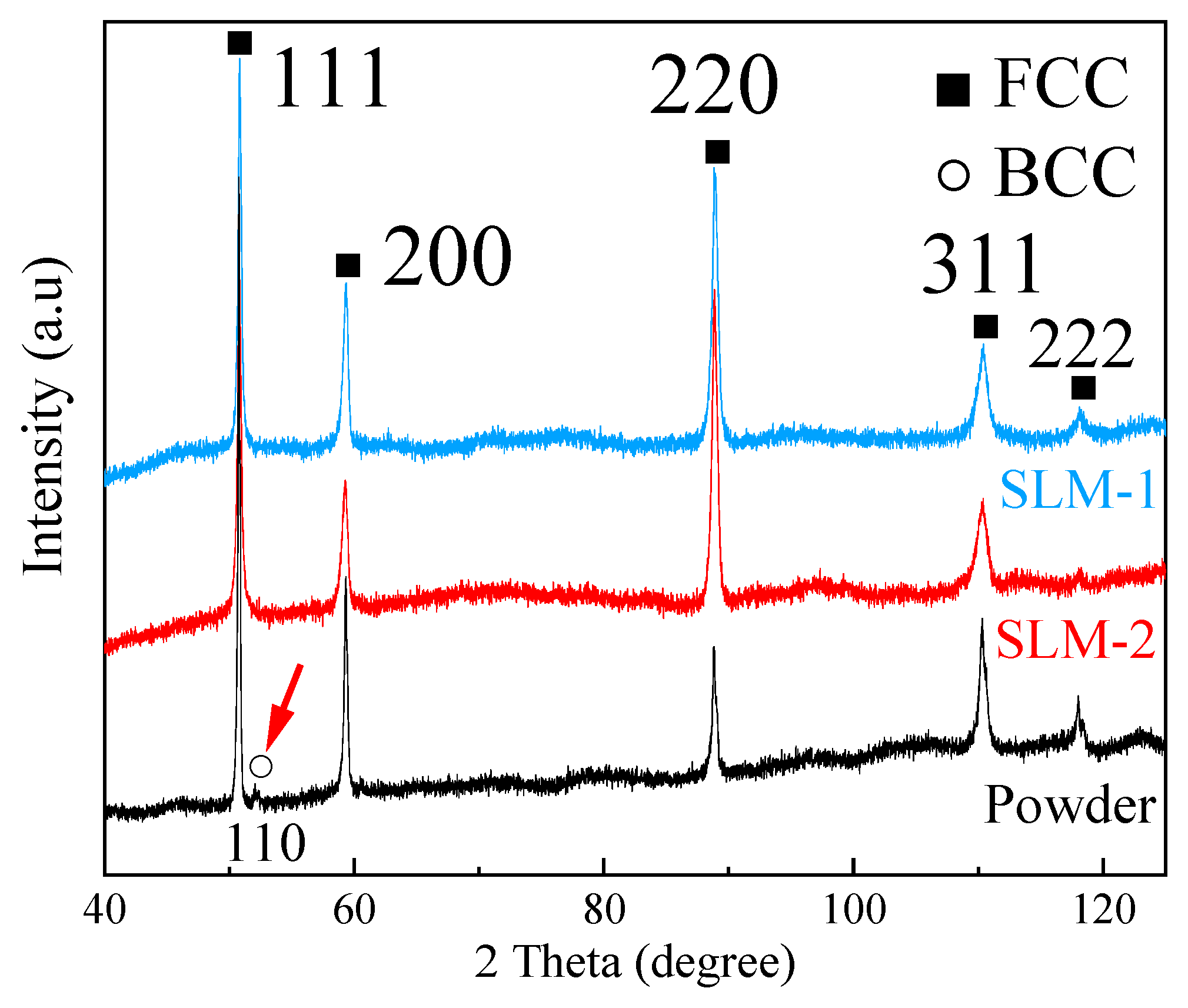
3.2. Density, Chemical Composition, and Phase Constituent
3.3. Microstructure of SLM CrFeNiMn Alloy
3.4. Hardness
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Huang, P.-K.; Yeh, J.-W.; Shun, T.-T.; Chen, S.-K. Multi-Principal-Element Alloys with Improved Oxidation and Wear Resistance for Thermal Spray Coating. Adv. Eng. Mater. 2004, 6, 74–78. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhý, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef] [Green Version]
- LaRosa, C.R.; Shih, M.; Varvenne, C.; Ghazisaeidi, M. Solid solution strengthening theories of high-entropy alloys. Mater. Charact. 2019, 151, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; MacDonald, B.E.; Zhang, D.; Wu, B.; Chen, W.; Ivanisenko, J.; Hahn, H.; Lavernia, E.J. Fcc nanostructured TiFeCoNi alloy with multi-scale grains and enhanced plasticity. Scr. Mater. 2018, 143, 108–112. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Sun, Z.; Li, J.; Li, L.; Song, X.; Wen, X.; Xie, L.; Yang, X. Effect of Mo and aging temperature on corrosion behavior of (CoCrFeNi)100-Mo high-entropy alloys. J. Alloys Compd. 2020, 812, 152139. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Lampke, T. Enhanced Wear Behaviour of Spark Plasma Sintered AlCoCrFeNiTi High-Entropy Alloy Composites. Materials 2018, 11, 2225. [Google Scholar] [CrossRef] [Green Version]
- Sahlberg, M.; Karlsson, D.; Zlotea, C.; Jansson, U. Superior hydrogen storage in high entropy alloys. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.C.; Yeh, J.-W.; Liaw, P.K.; Zhang, Y. (Eds.) High-Entropy Alloys; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-27011-1. [Google Scholar] [CrossRef]
- Murty, K.L.; Charit, I. Structural materials for Gen-IV nuclear reactors: Challenges and opportunities. J. Nucl. Mater. 2008, 383, 189–195. [Google Scholar] [CrossRef]
- Raghavan, V. Effect of manganese on the stability of austenite in Fe-Cr-Ni alloys. Metall. Mater. Trans. A 1995, 26, 237–242. [Google Scholar] [CrossRef]
- Wu, Z.; Bei, H. Microstructures and mechanical properties of compositionally complex Co-free FeNiMnCr18 FCC solid solution alloy. Mater. Sci. Eng. 2015, 640, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.A.P.K.; Li, C.; Leonard, K.J.; Bei, H.; Zinkle, S.J. Microstructural stability and mechanical behavior of FeNiMnCr high entropy alloy under ion irradiation. Acta Mater. 2016, 113, 230–244. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Bei, H.; Otto, F.; Pharr, G.M.; George, E.P. Recovery, recrystallization, grain growth and phase stability of a family of FCC-structured multi-component equiatomic solid solution alloys. Intermetallics 2014, 46, 131–140. [Google Scholar] [CrossRef]
- Hooreweder, B.V.; Moens, D.; Boonen, R.; Kruth, J.-P.; Sas, P. Analysis of Fracture Toughness and Crack Propagation of Ti6Al4V Produced by Selective Laser Melting. Adv. Eng. Mater. 2012, 14, 92–97. [Google Scholar] [CrossRef]
- Lavernia, E.J.; Srivatsan, T.S. The rapid solidification processing of materials: Science, principles, technology, advances, and applications. J. Mater. Sci. 2010, 45, 287–325. [Google Scholar] [CrossRef]
- Brif, Y.; Thomas, M.; Todd, I. The use of high-entropy alloys in additive manufacturing. Scr. Mater. 2015, 99, 93–96. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Nguyen, Q.B.; Ng, F.L.; An, X.H.; Liao, X.Z.; Liaw, P.K.; Nai, S.M.L.; Wei, J. Hierarchical microstructure and strengthening mechanisms of a CoCrFeNiMn high entropy alloy additively manufactured by selective laser melting. Scr. Mater. 2018, 154, 20–24. [Google Scholar] [CrossRef]
- Luo, S.; Gao, P.; Yu, H.; Yang, J.; Wang, Z.; Zeng, X. Selective laser melting of an equiatomic AlCrCuFeNi high-entropy alloy: Processability, non-equilibrium microstructure and mechanical behavior. J. Alloys Compd. 2019, 771, 387–397. [Google Scholar] [CrossRef]
- Joseph, J.; Jarvis, T.; Wu, X.; Stanford, N.; Hodgson, P.; Fabijanic, D.M. Comparative study of the microstructures and mechanical properties of direct laser fabricated and arc-melted AlxCoCrFeNi high entropy alloys. Mater. Sci. Eng. 2015, 633, 184–193. [Google Scholar] [CrossRef]
- Fritsching, U.; Uhlenwinkel, V. Hybrid Gas Atomization for Powder Production. In Powder Metallurgy; Kondoh, K., Ed.; InTech: Rijeka, Croatia, 2012; pp. 99–124. ISBN 978-953-51-0071-3. [Google Scholar]
- Lehtonen, J.; Ge, Y.; Ciftci, N.; Heczko, O.; Uhlenwinkel, V.; Hannula, S.-P. Phase structures of gas atomized equiatomic CrFeNiMn high entropy alloy powder. J. Alloys Compd. 2020, 827, 154142. [Google Scholar] [CrossRef]
- Liu, B.; Wildman, R.; Tuck, C.; Ashcroft, I.; Hague, R. Investigation the effect of particle size distribution on processing parameters optimisation in selective laser melting process. In Proceedings of the International solid freeform fabrication symposium: An additive manufacturing conference, Austin, TX, USA, 8–10 August 2011; pp. 227–238. [Google Scholar]
- Kamath, C.; El-dasher, B.; Gallegos, G.F.; King, W.E. Density of additively-manufactured, 316L SS parts using laser powder-bed fusion at powers up to 400W. Int. J. Adv. Manuf. Technol. 2014, 74, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Lehto, P.; Remes, H.; Saukkonen, T.; Hänninen, H.; Romanoff, J. Influence of grain size distribution on the Hall–Petch relationship of welded structural steel. Mater. Sci. Eng. 2014, 592, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Metallic Materials—Vickers Hardness Test—Part 1: Test Method; ISO Standard. 6507-1:2018; ISO: Genava, Switzerland, 2018.
- Ali, U.; Esmaeilizadeh, R.; Ahmed, F.; Sarker, D.; Muhammad, W.; Keshavarzkermani, A.; Mahmoodkhani, Y.; Marzbanrad, E.; Toyserkani, E. Identification and characterization of spatter particles and their effect on surface roughness, density and mechanical response of 17-4 PH stainless steel laser powder-bed fusion parts. Mater. Sci. Eng. 2019, 756, 98–107. [Google Scholar] [CrossRef]
- Nilsén, F.; Ituarte, I.F.; Salmi, M.; Partanen, J.; Hannula, S.-P. Effect of process parameters on non-modulated Ni-Mn-Ga alloy manufactured using powder bed fusion. Addit. Manuf. 2019, 28, 464–474. [Google Scholar] [CrossRef] [Green Version]
- Kelly, T.F.; Cohen, M.; Sande, J.B. Rapid solidification of a droplet-processed stainless steel. Metall. Trans. A 1984, 15, 819–833. [Google Scholar] [CrossRef]
- Tan, J.H.K.; Sing, S.L.; Yeong, W.Y. Microstructure modelling for metallic additive manufacturing: A review. Virtual. Phys. Prototyp. 2020, 15, 87–105. [Google Scholar] [CrossRef]
- Bi, G.; Sun, C.-N.; Chen, H.; Ng, F.L.; Ma, C.C.K. Microstructure and tensile properties of superalloy IN100 fabricated by micro-laser aided additive manufacturing. Mater. Des. 2014, 60, 401–408. [Google Scholar] [CrossRef]
- Kruth, J.P.; Froyen, L.; Van Vaerenbergh, J.; Mercelis, P.; Rombouts, M.; Lauwers, B. Selective laser melting of iron-based powder. J. Mater. Process. Technol. 2004, 149, 616–622. [Google Scholar] [CrossRef]
- Kannatey-Asibu, E. Principles of Laser Materials Processing; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; ISBN 978-0-470-45930-0. [Google Scholar]
- Wilson, P.R.; Chen, Z. The effect of manganese and chromium on surface oxidation products formed during batch annealing of low carbon steel strip. Corros. Sci. 2007, 49, 1305–1320. [Google Scholar] [CrossRef]
- Saeidi, K.; Gao, X.; Zhong, Y.; Shen, Z.J. Hardened austenite steel with columnar sub-grain structure formed by laser melting. Mater. Sci. Eng. 2015, 625, 221–229. [Google Scholar] [CrossRef]
- Kou, S. Welding Metallurgy, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2003; ISBN 978-0-471-43491-7. [Google Scholar]
- Schuh, B.; Mendez-Martin, F.; Völker, B.; George, E.P.; Clemens, H.; Pippan, R.; Hohenwarter, A. Mechanical properties, microstructure and thermal stability of a nanocrystalline CoCrFeMnNi high-entropy alloy after severe plastic deformation. Acta Mater. 2015, 96, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Ding, Q.; Zhong, Y.; Zou, J.; Wu, J.; Chiu, Y.-L.; Li, J.; Zhang, Z.; Yu, Q.; Shen, Z. Dislocation network in additive manufactured steel breaks strength-ductility trade-off. Mater. Today 2018, 21, 354–361. [Google Scholar] [CrossRef] [Green Version]
- Barkia, B.; Aubry, P.; Haghi-Ashtiani, P.; Auger, T.; Gosmain, L.; Schuster, F.; Maskrot, H. On the origin of the high tensile strength and ductility of additively manufactured 316L stainless steel: Multiscale investigation. J. Mater. Sci. Technol. 2020, 41, 209–218. [Google Scholar] [CrossRef]



| Specimen | Cr | Mn | Fe | Ni | Relative Density |
|---|---|---|---|---|---|
| Powder | 26.9 | 25.1 | 24.5 | 23.5 | / |
| SLM-1 | 25.5 | 24.9 | 24.8 | 24.8 | 98.0% ± 0.1% |
| SLM-2 | 25.8 | 24.0 | 25.3 | 24.9 | 97.9% ± 0.2% |
| Scanning Speed mm/s | Average Cell Size/nm | Relative Cell Size Dispersion | Volume-Weighted Average Cell Size/nm | Cell Wall Proportion | Vickers Hardness/HV0.5 | |
|---|---|---|---|---|---|---|
| SLM-1 | 200 | 390 | 1.85 | 450 | 14.70% | 248 ± 8 |
| SLM-2 | 150 | 520 | 2.16 | 680 | 15.17% | 221 ± 14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Ge, Y.; Lehtonen, J.; Hannula, S.-P. Hierarchical Microstructure of Laser Powder Bed Fusion Produced Face-Centered-Cubic-Structured Equiatomic CrFeNiMn Multicomponent Alloy. Materials 2020, 13, 4498. https://doi.org/10.3390/ma13204498
Yang X, Ge Y, Lehtonen J, Hannula S-P. Hierarchical Microstructure of Laser Powder Bed Fusion Produced Face-Centered-Cubic-Structured Equiatomic CrFeNiMn Multicomponent Alloy. Materials. 2020; 13(20):4498. https://doi.org/10.3390/ma13204498
Chicago/Turabian StyleYang, Xuan, Yanling Ge, Joonas Lehtonen, and Simo-Pekka Hannula. 2020. "Hierarchical Microstructure of Laser Powder Bed Fusion Produced Face-Centered-Cubic-Structured Equiatomic CrFeNiMn Multicomponent Alloy" Materials 13, no. 20: 4498. https://doi.org/10.3390/ma13204498





