1. Introduction
“Global warming is one of the most important issues mankind is currently facing. It is increasing day by day due to the burning of fossil fuels in very large amounts around the world to meet energy demands. Nowadays nearly 60% of the world’s power generation depends upon fossil fuels. Thermal power plants are burning coal in huge amounts resulting in the emission of flue gases like carbon dioxide (CO
2), sulphur dioxide (SO
2) and carbon monoxide (CO) leading to many ecological problems like air pollution and global warming. To reduce emission of these harmful gases in the environment has become the most important concern of many countries. The scientists and engineers are deploying various strategies to reduce concentration of the flue gases (CO
2, SO
2) by increasing the efficiency of the thermal power plants currently running or using other renewable resources of energy generation [
1,
2]. Therefore, solar and wind energies are some of the most common renewable energy resources adopted by many countries to meet their energy requirement. Moreover, immense research has been carried out on an advanced ultra-super critical thermal power plant that can withstand temperatures beyond 700 °C. Generally, a 0.5% increase in the power generation can be achieved by increasing the steam temperature by 10 °C [
3,
4,
5,
6]. Therefore, in order to improve the efficiency of thermal power plants, there is the need for an appropriate material for the manufacturing of turbine rotor and blades, which can be utilized at extreme conditions i.e., above 700 °C and 250 bar temperature and pressure, respectively.
For this purpose, Nickel based alloys of grades 617, 625 and 740 are considered to be the most promising materials owing to their good mechanical and metallurgical properties [
7,
8]. During steam turbine operation, the stage of low pressure and high temperature is considered to be most destructive for material prospect. Accordingly, a material with high fatigue strength and low corrosion kinetics at higher temperatures is required. 12Cr steel with its low cost and high corrosion resistance is considered to be the suitable material to serve this purpose. However, the bottleneck to use alloy 617 and 12Cr as a rotor material requires the designing of dissimilar material welding technology. Therefore, a reliable welding technology is required to fabricate hybrid structures to withstand the extreme conditions. Few researchers have already investigated the welding of alloy 617 with various materials and the consequent effects on the properties were evaluated. For instance, Hosseini et al. investigated the microstructure and weldability of Inconel 617 and 310 austenitic steel using Inconel 82, 617 and 310 austenitic steel as a filler materials [
9]. The results revealed that Inconel 617/310 austenitic steel joints welded by using Inconel 617 filler metals demonstrated the highest solidification cracking resistance. While on the other hand, 310 steel filler joints showed the lowest cracking resistance, which was attributed to low melting point and secondary precipitate. Rando et al. studied the low cycle fatigue behavior of Inconel 617 weldments using gas tungsten arc welding (GTAW) at room temperature and at 800 °C in air [
10]. The fatigue tests showed the decrease in fatigue life with increased total strain and temperature. The inferior fatigue characteristics were attributed to the accumulation of the plastic strain and ductility of material at elevated temperature. Nonetheless, no comprehensive literature is available to thoroughly access the microstructural and corrosion properties of dissimilar material welding between 12Cr and Alloy 617 after the PWHT.
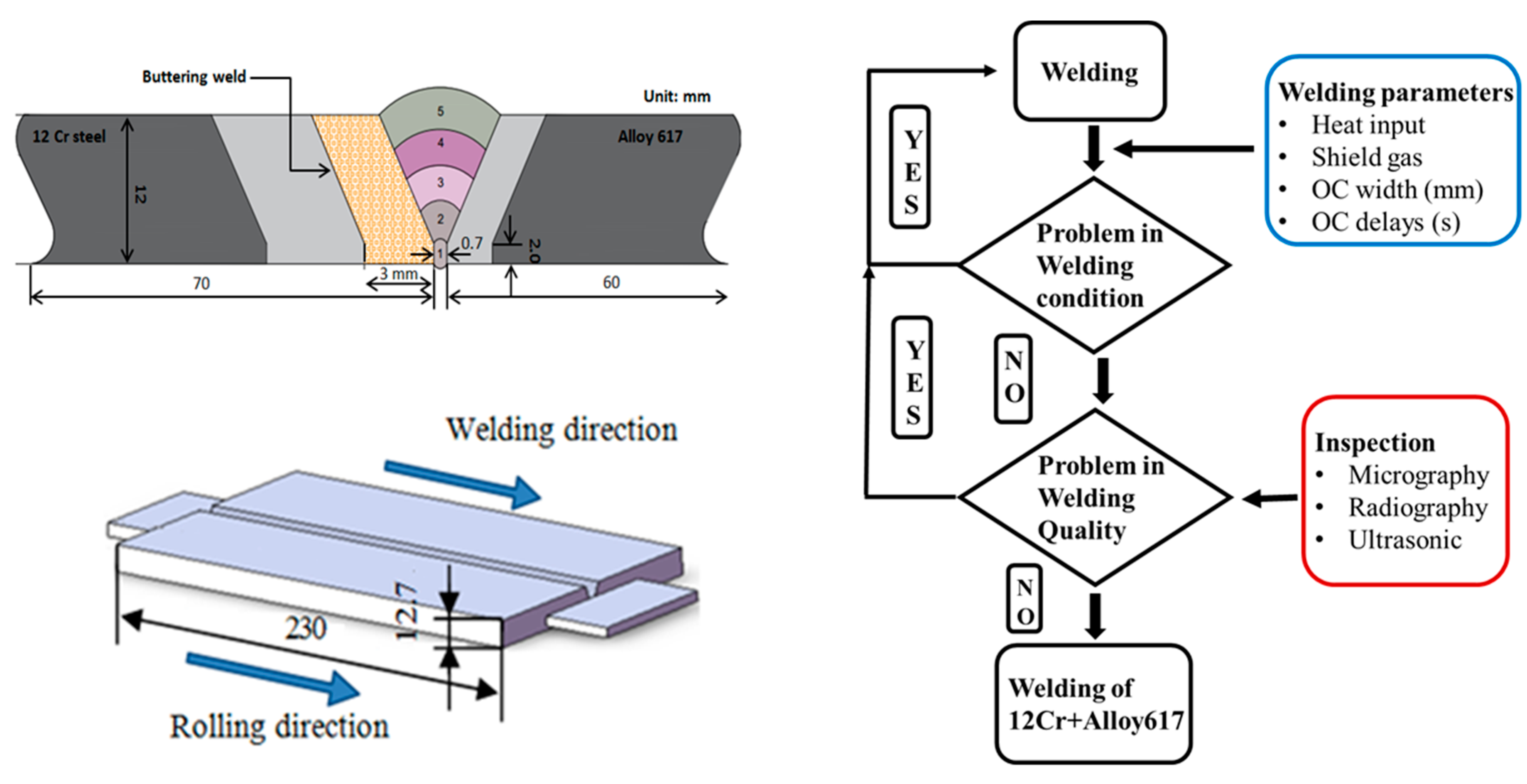
Accordingly, in this study, a dissimilar welding was carried out between Nickel Alloy 617 and 12Cr steel using buttering welding technique. The PWHT was carried out on the butter weld to relieve the residual stresses and its consequent effects on microstructure and corrosion kinetics were evaluated.
3. Results and Discussion
To investigate the effect of PWHT on the microstructure evolution of the important constituents of the dissimilar butter weld, optical microscopy was carried out.
Figure 2 and
Figure 3 show the optical micrographs (OM) of all the regions of the dissimilar butter weld before and after the PWHT, respectively. It is evident from
Figure 2a that Ni 617 consists of austenitic grains, which can also be observed in
Figure 3a but with a less homogeneous microstructure. However, no significant effect of PWHT on Ni 617 was observed due to its excellent heat resistant characteristics. Moreover, the HAZ of Alloy 617 (before and after the PWHT in
Figure 2b and
Figure 3b, respectively) showed a sudden increase in the grain size, which can be attributed to the grain growth due to welding heat input. The micrograph of the weld metal (
Figure 2c) confirmed the presence of the dendrite grains with the largest grain size compared to the other regions based on the highest tendency for grain growth due to maximum heat exposure. The dendritic microstructure suppresses the extreme segregation of alloying elements and impurities in the interdendritic regions, which can consequently lead to the enhanced strength and ductility of the weld metal [
12]. The martensitic grains were observed in the 12Cr-HAZ before the PWHT (
Figure 2d), and they were found to be deformed due to solid phase transformation by welding heat. The martensitic grains were detected in 12Cr for before and after the PWHT (
Figure 2e and
Figure 3e, respectively). Moreover, to determine the effect of PWHT on the elemental distribution in all the important regions of the dissimilar butter weld, SEM with EDS was performed on the weld before and after the PWHT. The SEM-EDS plots taken from the before and after PWHT butter welded samples line scan and the results are presented in
Table 2. It can be noticed that no significant difference was observed in the compositions of major constituents (Alloy 617, 12Cr) as a result of PWHT. However, a slight decrease in the Ni concentration was detected in the weld metal compared to the base metal. This can be attributed to the use of Thyssen 617 as a filler metal, which is almost identical to Alloy 617 as can be seen from
Table 1. Moreover, the buttering weld was found to consist of molybdenum (Mo), chromium (Cr), iron (Fe) and cobalt (Co), which was due the dilution of 12Cr and Thyssen 617 during the buttering weld process. Furthermore, no remarkable difference in the concentration of major elements (Fe, Ni and Mo) was noticed after the PWHT. However, the concentration of Cr was found to be decreased in the 12Cr-HAZ after the PWHT, which was due to the sensitization effect imparted by the PWHT [
13].
To determine the effect of PWHT on the electrochemical behavior of the weldment couple sample, the sample before and after the PWHT were immersed in 5 wt.% NaCl + 0.5 wt.% CH
3COOH electrolyte at different temperatures (RT, 30 °C, 50 °C and 70 °C).
Figure 4 and
Figure 5 show the potentiodynamic polarization curves for the weldment couple before and after PWHT at various temperatures, respectively. The Tafel extrapolation approach was used to calculate the corrosion potential (
Ecorr) and corrosion current density (
icorr) while corrosion rate was calculated using formula [
14] as shown in Equation (1). The values of kinetic parameters corrosion rates for both samples at various temperatures are illustrated in
Table 3. The electrochemical properties of base alloys (alloy 617 and 12Cr) also illustrated in
Table 3 for comparison purpose. It can be seen from the
Table 3 that alloy 617 showed low corrosion rates at various temperatures as compared to the 12Cr owing to its excellent corrosion resistant. The corrosion rate of both base alloys increased with the increase in temperature.
where ‘
EW’ is the equivalent weight of the alloy in grams (g), ‘
ρ’ is the density of alloy in grams per centimeter cubed (g∙cm
−3) and ‘
icorr’ is the corrosion current density in ampere per centimeter squared (A∙cm
−2). E.W. can be calculated by the following equation:
here W
i, f
i and n
i are the atomic weight, weight fraction and valence of the ith element in the alloy.
The purpose to conduct the potentiodynamic polarization test in corrosive electrolyte of 5 wt.% NaCl solution (brine) of concentration up to saturation level and buffer acidification through 0.5 wt.% CH
3COOH at different temperature was used to simulate the service conditions. The potentiodynamic polarization scans with anodic branches and cathodic branches shifted towards the high current density value with the increase in the temperature while the corrosion potential (
Ecorr) value almost remain same as shown in
Figure 4 and
Figure 5. Even the PWHT also does not affect the
Ecorr value as there was a slight shift in the value when compared to that recorded at room temperature (RT). However, when we compare the results after PWHT (
Figure 5) with the increase in temperature from RT to 70 °C, the value of
Ecorr shifted towards a more active potential. The hydrogen reduction was occurred at the cathodic polarization branch due to the de-aerated acidic condition of the electrolyte according to the following reaction [
15]:
The evolution of hydrogen gas bubbles occurred on the sample surface during cathodic polarization. In the anodic polarization region, there was no sign of passivation or formation of any barrier film as all the curves show the rapid dissolution behavior. The nickel and chromium metals show the passive behavior in the acidic electrolyte but due to the presence of the high concentration of Cl
− ions which act as a de-polarizer and weldment couple which act as a galvanic couple. The anodic polarization from
Figure 4 and
Figure 5 show that the dissolution was due to the Fe which was present in the weldment couple portion of 12Cr steel in large wt.% according to the following reaction [
15]:
The corrosion rates were increased with the increase in temperature for both the samples (before and after PWHT). The corrosion rate of the weldment couple before heat treatment was measured to be 0.0121 mm/y at RT, which was increased to 0.0164 mm/y at the solution temperature of 30 °C. Moreover, the corrosion rate was remarkably increased to 0.0924 mm/y (~660 % higher compared to RT) when the solution temperature was maintained at 70 °C. It can be attributed to the fact that when the temperature is increased, the pH of the electrolyte shifted towards more an acidic value and the mobility of the anion species, i.e., Cl
− ions, increases. The Cl
− ions are a strong oxidizing agent and cause pitting or localized corrosion in passive alloys like nickel alloys; they penetrate through the porous oxide film and can be absorbed on surface resulting in higher deterioration of the surface of those alloys [
16,
17,
18,
19]. After absorbing on the surface, these ions can promote the diffusion of metal ions from the surface to the corrosive medium leading to formation of metal hydrides [
20]. As in
Figure 4 at 70 °C, the cathodic curve shows the diffusion control behavior on the sample surface during the reduction process [
21,
22]. Moreover, it can be seen from
Table 3 that the weldment after the PWHT demonstrated an identical trend, as the corrosion rates increased with the increase in solution temperature (0.0156 and 0.1037 at RT and 70 °C, respectively). However, the samples with PWHT showed higher corrosion rates compared to the samples without PWHT in similar testing conditions. This may be attributed to the reason that PWHT led to the sensitization of Cr, which reduced the concentration of Cr in the 12Cr-HAZ and 12Cr region (
Table 2), resulting in high corrosion susceptibility. Although the temperature effect is evident, the basic polarization curve remained the same, indicating that the temperature only heavily effected the corrosion rate not the corrosion mechanism.
Furthermore, the activation energy (
Ea) was calculated to estimate the adsorption behavior of Cl
− ions on the surface of the weldment couple before and after the PWHT. The dependence of corrosion rate on the temperature was determined using the following Arrhenius equation [
23]:
where ‘
A’ is the pre-exponential factor, ‘
Ea’ is the activation energy for surface dissolution in joules per mole (J mol
−1), ‘
R’ is the universal gas constant whose value is 8.314 J mol
−1 K
−1, ‘
T’ is the absolute temperature in kelvin (K) and ‘
icorr’ is the corrosion current density in amperes per centimeter squared (A cm
−2). The value of
Ea for both the samples was calculated from the slope of the plot between ln
icorr and the inverse of the absolute temperature (1/
T). Moreover, transition state theory was utilized to calculate the enthalpy (Δ
H) and the entropy (Δ
S) from the following equation of the corrosion rate process [
22]:
Where ‘
N’ is the Avogadro’s number whose value is 6.023 × 10
23 mole
−1, ‘
h’ is the Planck’s constant whose value is 6.626 × 10
−32 Js. The values for Δ
H and Δ
S can be calculated from the slope (−Δ
S/
R) and intercept (ln (
R/
Nh) + Δ
S/
R) from the graph between (ln
icorr/
T) and inverse of absolute temperature (1/T).
Figure 6a–c shows the various plots for calculating the activation parameters for the corrosion process of both the samples and results are summarized in
Table 4.
Figure 6a presents the variation of the corrosion rate as a function of temperature and it is evident that the corrosion rate is in direct relation with the temperature of the corrosion solution.
Figure 6b illustrates the Arrhenius plot and the
Ea values were calculated to be 35.34 ± 1.51 kJ/mol and 32.62 ± 1.85 kJ/mol for the weldment couple before and after the PWHT, respectively. As it is well understood that the
Ea is the resistance offered by the material to the initiation of corrosion so lower activation energy reflects the lower resistance for metal corrosion. In addition, after the formation of the passive layer, the corrosion process will be further pushed to the regions of higher
Ea values, indicating that corrosion of the alloy will occur on the uncovered areas which will lower
Ea [
22]. Accordingly, it implies that the corrosion is thermodynamically more convenient for the weldment after the PWHT rather than before the heat treatment. In addition, the entropy of activation (Δ
S) is negative for both the samples, which can be attributed to the reason that the activated complexes in the rate determining step need lower energy for completion of the reaction. It can be seen from
Table 4 that the weldment couple after the PWHT has lower Δ
S values compared to the sample without the heat treatment, which reflects the high kinetics for the corrosion reaction.