Novel Eco-Friendly Tannic Acid-Enriched Hydrogels-Preparation and Characterization for Biomedical Application
Abstract
1. Introduction
2. Results
2.1. Scanning Electron Microscope
2.2. Bioerosion
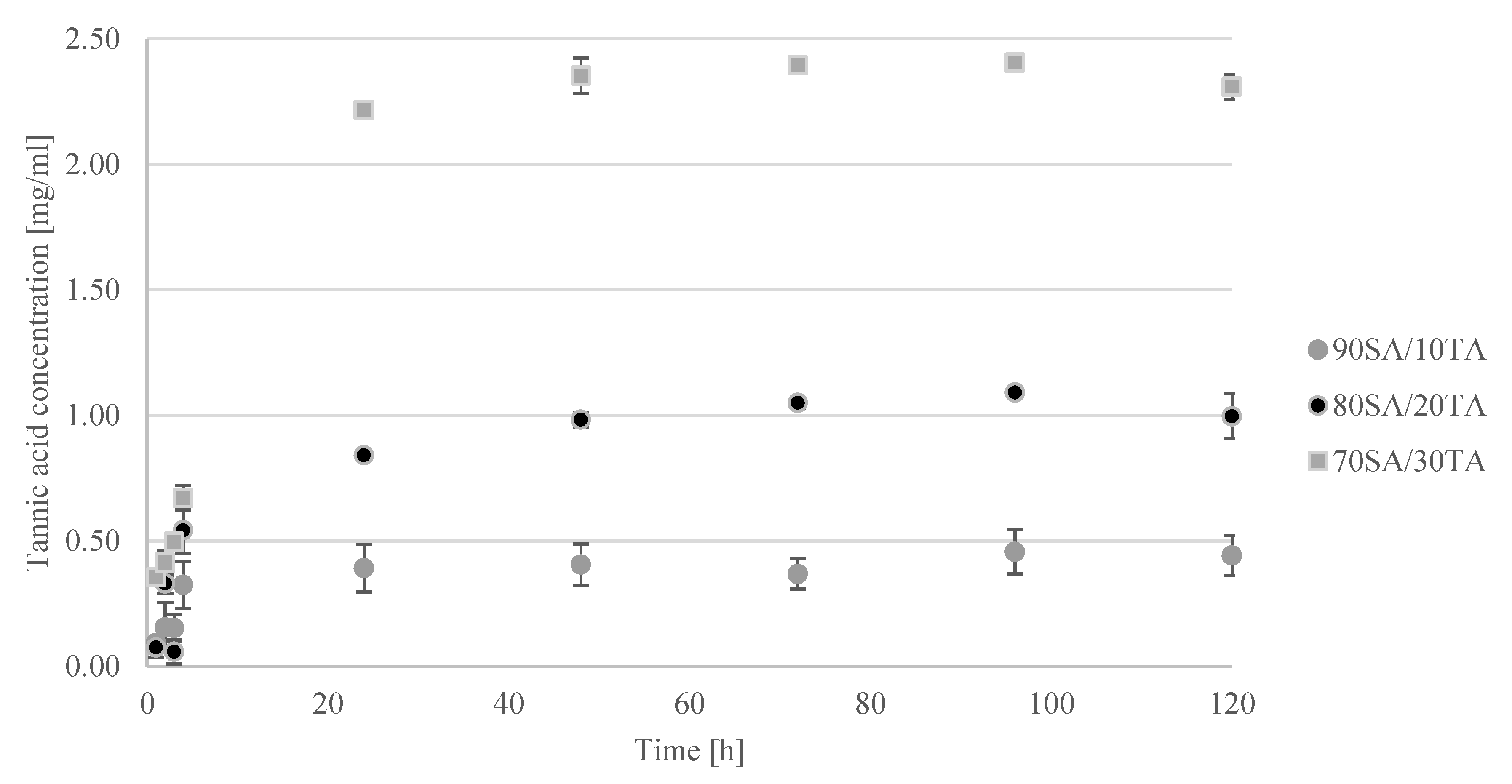
2.3. Tannic Acid Release
2.4. Dehydrogenase Activity
2.5. Blood Compatibility
2.6. Results of Human BMSC, HaCaT, and MNT-1 Growth/Survival on the Studied Materials
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Hydrogel Preparation
4.3. Scanning Electron Microscope
4.4. Bioerosion
4.5. Tannic Acid Release
4.6. Dehydrogenase Activity
4.7. Blood Compatibility
4.8. Cultures of Human Bone Marrow Stromal Cells (BMSC), HaCaT and MNT-1
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bi, Y.G.; Lin, Z.T.; Deng, S.T. Fabrication and characterization of hydroxyapatite/sodium alginate/chitosan composite microspheres for drug delivery and bone tissue engineering a scientific article. Mater. Sci. Eng. C 2019, 100, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing a scientific article. Biomaterial 2017, 122, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, D.; Viswanadha, V.P.; Elango, S. Wound dressings—A review. BioMedicine 2015, 5, 22. [Google Scholar]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds a scientific article. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Jose, G.; Shalumon, K.T.; Chen, J.P. Natural polymers based hydrogels for cell culture applications a scientific article. Curr. Med. Chem. 2020, 27, 2734–2776. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Hong, Y.; Zhang, X.; Yang, L.; Li, J.; Jiang, D.; Bunpetch, V.; Hu, Y.; Zhang, H.; Ouyang, S. Tough hydrogel with enhanced tissue integration and in situ forming capability for osteochondral defect repair a scientific article. Appl. Mater. Today 2018, 13, 32–44. [Google Scholar] [CrossRef]
- Kazi, G.A.S.; Yamamoto, O. Effectiveness of the sodium alginate as surgical sealant materials a scientific article. Wound Med. 2019, 24, 18–23. [Google Scholar] [CrossRef]
- Aluani, D.; Tzankova, V.; Kondeva-Burdina, M.; Yordanov, Y.; Nikolova, E.; Odzhakov, F.; Apostolov, A.; Markova, T.; Yoncheva, K. Еvaluation of biocompatibility and antioxidant efficiency of chitosan-alginate nanoparticles loaded with quercetin a scientific article. Int. J. Biol. Macromol. 2017, 103, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Aadil, K.R.; Nathani, A.; Sharma, C.S.; Lenka, N.; Gupta, P. Fabrication of biocompatible alginate-poly(vinyl alcohol) nanofibers scaffolds for tissue engineering applications a scientific article. Mater. Technol. 2018, 33, 507–512. [Google Scholar] [CrossRef]
- Mi, H.Y.; Jing, X.; Huang, H.X.; Peng, X.F.; Turng, L.S. Superhydrophobic graphene/cellulose/silica aerogel with hierarchical structure as superabsorbers for high efficiency selective oil absorption and recovery a scientific article. Ind. Eng. Chem. Res. 2018, 57, 1745–1755. [Google Scholar] [CrossRef]
- Wang, F.; Lu, X.; Li, X.Y. Selective removals of heavy metals (Pb2+, Cu2+, and Cd2+) from wastewater by gelation with alginate for effective metal recovery a scientific article. J. Hazard. Mater. 2016, 308, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, M.; Shukla, P.; Bajpai, S.K. Ca (II) plus Ba (II) ions crosslinked alginate gels prepared by a novel diffusion through dialysis tube (DTDT) approach and preliminary BSA release study a scientific article. Polym. Degrad. Stabil. 2016, 134, 22–29. [Google Scholar] [CrossRef]
- Kaygusuz, H.; Torlak, E.; Akm-Evingur, G.; Ozen, I.; von Klitzing, R.; Erim, F.B. Antimicrobial cerium ion-chitosan crosslinked alginate biopolymer films: A novel and potential wound dressing a scientific article. Int. J. Biol. Macromol. 2017, 105, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.P.; Melman, G.; Letourneau, N.J.; Mendelson, N.L.; Melman, A. Photodegradable iron (III) cross-linked alginate gels a scientific article. Biomacromolecules 2012, 13, 2465–2471. [Google Scholar] [CrossRef]
- Kaygusuz, H.; Erim, F.B.; Pekcan, O.; Evingur, G.A. Cation effect on slow release from alginate beads: A fluorescence study a scientific article. J. Fluoresc. 2014, 24, 161–167. [Google Scholar] [CrossRef][Green Version]
- Prabhu, S.M.; Meenakshi, S. Novel one-pot synthesis of dicarboxylic acids mediated alginate-zirconium biopolymeric complex for defluoridation of water a scientific article. Carbohydr. Polym. 2015, 120, 60–68. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Y.; Yao, J. Construction of hydrophobic alginate-based foams induced by zirconium ions for oil and organic solvent cleanup a scientific article. J. Coll. Interface Surf. 2019, 533, 182–189. [Google Scholar] [CrossRef]
- Ngobili, T.A.; Shah, H.; Park, J.P.; Kwist, K.W.; Inskeep, B.; Burg, K.J.L.; Booth, B.W. Remodeling of tannic acid crosslinked collagen type 1 induces apoptosis in er+ breast cancer cells a scientific article. Anticancer Res. 2015, 35, 1285–1290. [Google Scholar]
- Brazdaru, L.; Micutz, M.; Staicu, T.; Albu, M.; Sulea, D.; Leca, M. Structural and rheological properties of collagen hydrogels containing tannic acid and chlorhexidine digluconate intended for topical applications a scientific article. Comptes Rendus Chim. 2015, 18, 160–169. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Mazur, O.; Miłek, O.; Michalska-Sionkowska, M.; Osyczka, A.M.; Kleszczyński, K. Development of tannic acid-enriched materials modified by poly(ethylene glycol) for potential applications as wound dressing. Prog. Biomater. 2020, 9, 115–123. [Google Scholar] [CrossRef]
- Wu, J.; Liao, W.; Zhang, J.; Chen, W. Thermal behavior of collagen crosslinked with tannic acid under microwave heating a scientific article. J. Therm. Anal. Calorim. 2018, 135, 2329–2335. [Google Scholar] [CrossRef]
- An, X.; Kang, Y.; Li, G. The interaction between chitosan and tannic acid calculated based on the density functional theory a scientific article. Chem. Phys. 2019, 520, 100–107. [Google Scholar] [CrossRef]
- Kumorek, M.; Minisy, I.M.; Krunclava, T.; Vorsilakova, M.; Venclikova, K.; Chanova, E.M.; Janouskova, O.; Kubies, D. pH-responsive and antibacterial properties of self-assembled multilayer films based on chitosan and tannic acid a scientific article. Mater. Sci. Eng. C 2020, 109, 110493. [Google Scholar] [CrossRef]
- Popa, M.; Constantin, C.B.; Ochiuz, L.; Desbrieres, J.; Peptu, C.A. Controlling the release kinetics of calcein loaded liposomes from chitosan/tannic acid and chitosan/poly(vinyl alcohol)/tannic acid hydrogels a scientific article. Cellul. Chem. Technol. 2018, 52, 353–370. [Google Scholar]
- Kaczmarek, B.; Nadolna, K.; Owczarek, A.; Michalska-Sionkowska, M.; Sionkowska, A. The characterization of thin films based on chitosan and tannic acid mixture for potential applications as wound dressings a scientific article. Polym. Test. 2019, 78, 106007. [Google Scholar] [CrossRef]
- Zhang, Y.; He, F.; Li, X. Three-dimensional composite hydrogel based on polyamine zirconium oxide, alginate and tannic acid with high performance for Pb(II), Hg(II) and Cr(VI) trapping. J. Taiwan Inst. Chem. Eng. 2016, 65, 304–311. [Google Scholar] [CrossRef]
- Sabaeifard, P.; Abdi-Ali, A.; Soudi, M.R.; Dinarvand, R.; Abdi-Ali, A.; Soudi, M.R. Dinarvand Optimization of tetrazolium salt assay for Pseudomonas aeruginosa biofilm using microtiter plate method a scientific article. J. Microbiol. Methods 2014, 105, 134–140. [Google Scholar] [CrossRef]
- Walczak, M.; Michalska-Sionkowska, M.; Kaczmarek, B.; Sionkowska, A. Surface and antibacterial properties of thin films based on collagen and thymol. Mater. Today Comm. 2020, 22, 100949. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and antiinflammatory pH-responsive tannic acid-carboxylated agarose composite hydrogels for wound healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef]
- Du, X.; Hou, Y.; Wu, L. An anti-infective hydrogel adhesive with non-swelling and robust mechanical properties for sutureless wound closure a scientific article. J. Mater. Chem. B 2020, 8, 5682–5693. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Aktas, N. Single step natural poly(tannic acid) particle preparation as multitalented biomaterial. Mater. Sci. Eng. C 2015, 49, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Qi, P.; Zhang, X.X. Multiple Weak H-Bonds Lead to Highly Sensitive, Stretchable, Self-Adhesive, and Self-Healing Ionic Sensors a scientific article. ACS Appl. Mater. Interfaces 2019, 11, 7755–7763. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, M.; Shamloo, A.; Nokoorani, Y.D. Development of a polyvinyl alcohol/sodium alginate hydrogel-based scaffold incorporating bFGF-encapsulated microspheres for accelerated wound healing. Sci. Rep. 2020, 10, 7342. [Google Scholar] [CrossRef]
- Abouzeid, R.E.; Khiari, R.; Salama, A.; Diab, M.; Beneveti, D.; Dufresne, A. In situ mineralization of nano-hydroxyapatite on bifunctional cellulose nanofiber/polyvinyl alcohol/sodium alginate hydrogel using 3D printing. Int. J. Biol. Macromol. 2020, 160, 538–547. [Google Scholar] [CrossRef]
- Benedini, L.; Laiuppa, J.; Santillian, G.; Baldini, M.; Messina, P. Antibacterial alginate/nano-hydroxyapatite composites for bone tissue engineering: Assessment of their bioactivity, biocompatibility, and antibacterial activity. Mater. Sci. Eng. C 2020, 115, 111101. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Wekwejt, M.; Nadolna, K.; Owczarek, A.; Mazur, O.; Pałubicka, A. The mechanical properties and bactericidal degradation effectiveness of tannic acid-based thin films for wound care a scientific article. J. Mech. Behav. Biomed. Mater. 2020, 110, 103916. [Google Scholar] [CrossRef] [PubMed]
- Pamula, E.; Kokoszka, J.; Cholewa-Kowalska, K.; Laczka, M.; Kantor, L.; Niedzwiecki, L.; Reilly, G.C.; Filipowska, J.; Madej, W.; Kolodziejczyk, M.; et al. Degradation, bioactivity, and osteogenic potential of composites made of PLGA and two different sol-gel bioactive glasses. Annu. Rev. Biomed. Eng. 2011, 39, 2114. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Miłek, O.; Nadolna, K.; Owczarek, A.; Kleszczyński, K.; Osyczka, A.M. Normal and cancer cells response on the thin films based on chitosan and tannic acid. Toxicol. In Vitro 2020, 62, 104668. [Google Scholar] [CrossRef]




| Specimen | Hemolysis Rate (%) |
|---|---|
| 100SA | 0.56 ± 0.14 |
| 90SA/10TA | 0.32 ± 0.20 |
| 80SA/20TA | 0.92 ± 0.45 |
| 70SA/30TA | 2.19 ± 1.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarek, B.; Miłek, O.; Michalska-Sionkowska, M.; Zasada, L.; Twardowska, M.; Warżyńska, O.; Kleszczyński, K.; Osyczka, A.M. Novel Eco-Friendly Tannic Acid-Enriched Hydrogels-Preparation and Characterization for Biomedical Application. Materials 2020, 13, 4572. https://doi.org/10.3390/ma13204572
Kaczmarek B, Miłek O, Michalska-Sionkowska M, Zasada L, Twardowska M, Warżyńska O, Kleszczyński K, Osyczka AM. Novel Eco-Friendly Tannic Acid-Enriched Hydrogels-Preparation and Characterization for Biomedical Application. Materials. 2020; 13(20):4572. https://doi.org/10.3390/ma13204572
Chicago/Turabian StyleKaczmarek, Beata, Oliwia Miłek, Marta Michalska-Sionkowska, Lidia Zasada, Marta Twardowska, Oliwia Warżyńska, Konrad Kleszczyński, and Anna Maria Osyczka. 2020. "Novel Eco-Friendly Tannic Acid-Enriched Hydrogels-Preparation and Characterization for Biomedical Application" Materials 13, no. 20: 4572. https://doi.org/10.3390/ma13204572
APA StyleKaczmarek, B., Miłek, O., Michalska-Sionkowska, M., Zasada, L., Twardowska, M., Warżyńska, O., Kleszczyński, K., & Osyczka, A. M. (2020). Novel Eco-Friendly Tannic Acid-Enriched Hydrogels-Preparation and Characterization for Biomedical Application. Materials, 13(20), 4572. https://doi.org/10.3390/ma13204572









