Nickel-Embedded Carbon Materials Derived from Wheat Flour for Li-Ion Storage
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Bio-Derived Carbon Materials
2.2. Analysis and Characterization
2.3. Electrochemical Test
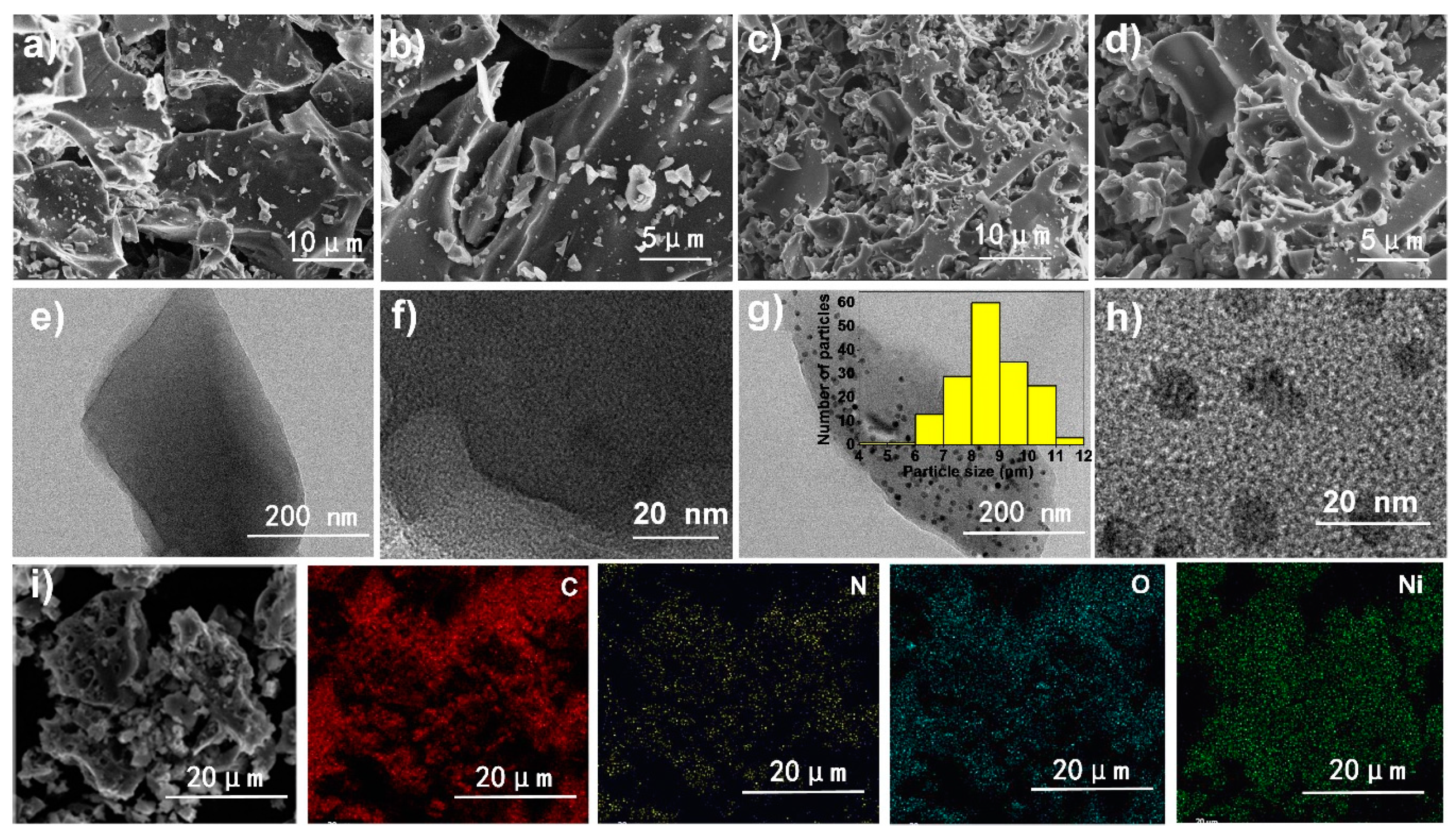
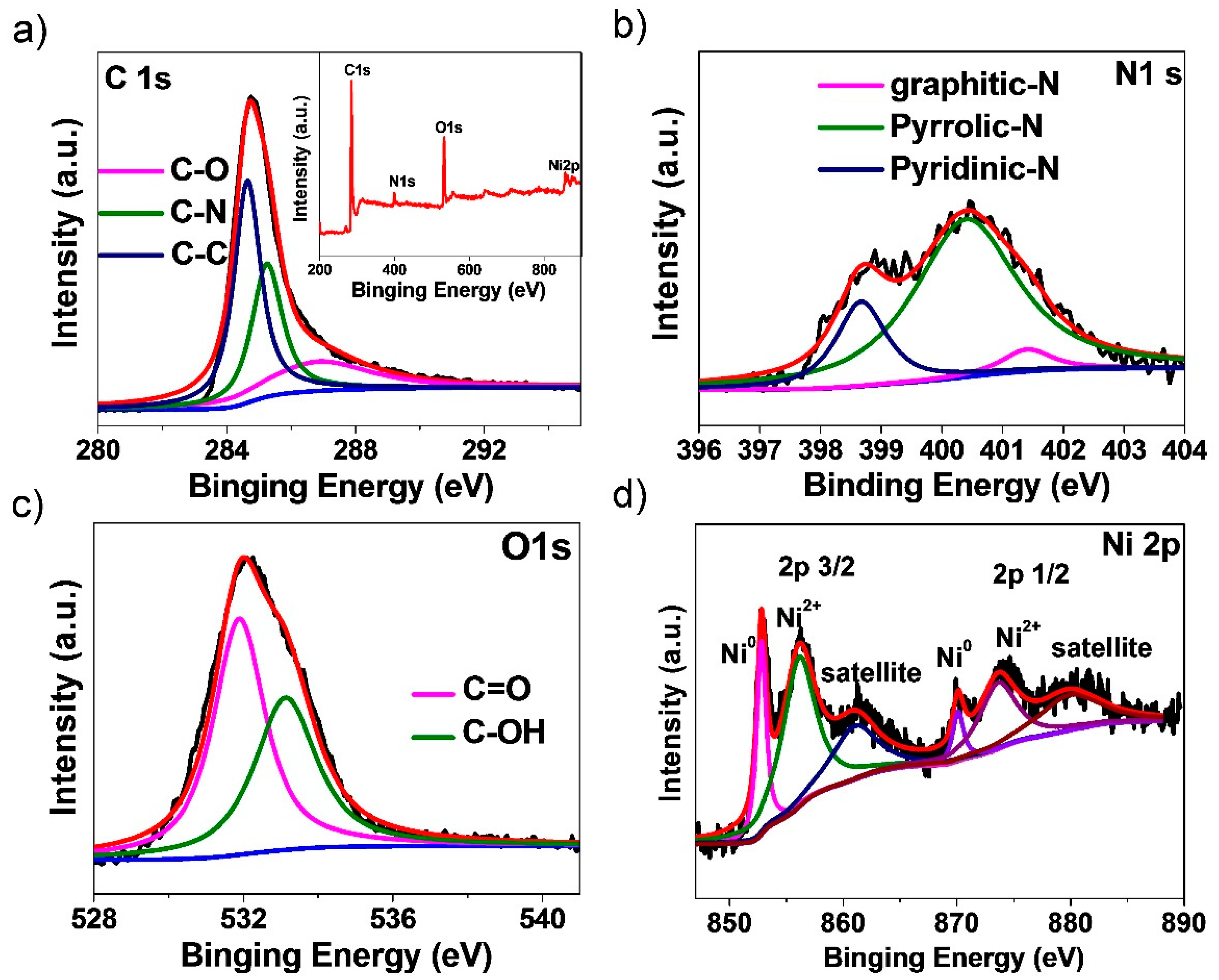
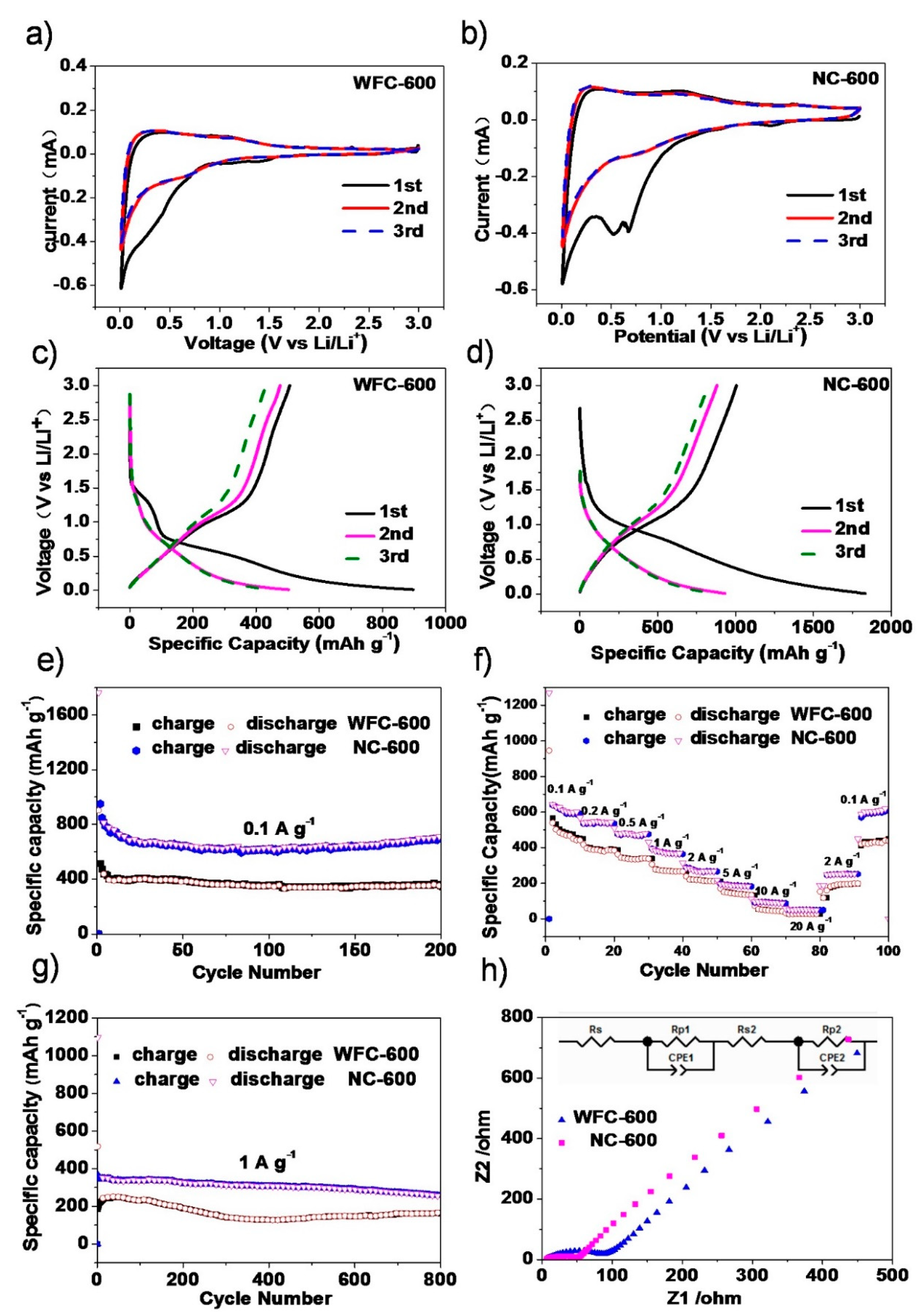
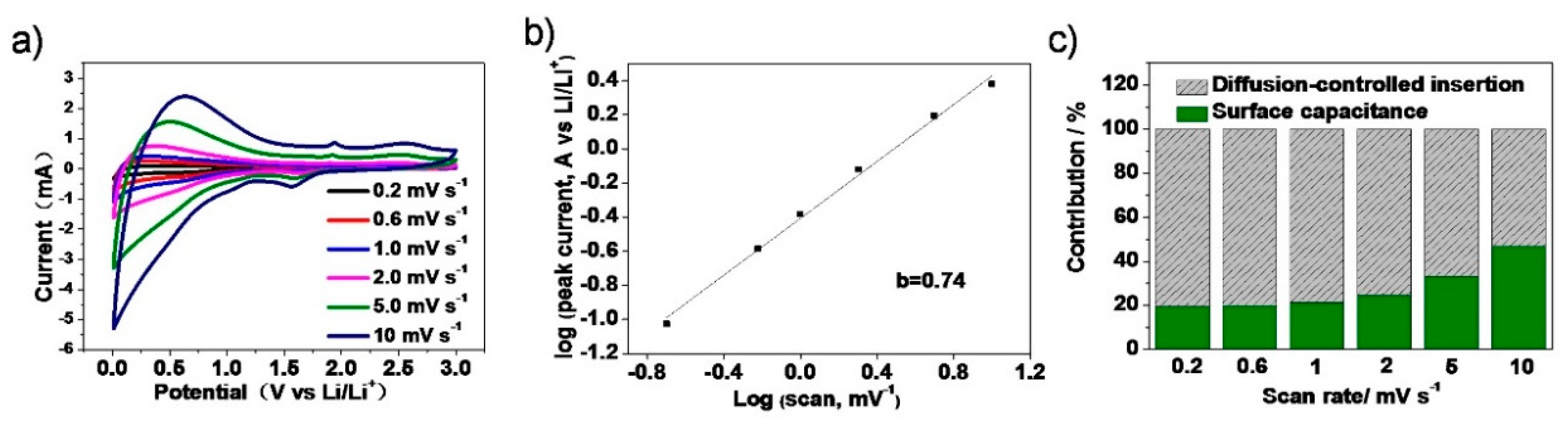
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, W.; Zhou, J.; Wang, Z.; Zhao, L.; Li, P.; Yang, Y.; Yang, C.; Huang, H.; Guo, S. Short-Range Order in Mesoporous Carbon Boosts Potassium-Ion Battery Performance. Adv. Energy Mater. 2017, 8, 1701648. [Google Scholar] [CrossRef]
- Di Lecce, D.; Verrelli, R.; Hassoun, J. Lithium-ion batteries for sustainable energy storage: Recent advances towards new cell configurations. Green Chem. 2017, 19, 3442–3467. [Google Scholar] [CrossRef]
- Liu, H.; Jia, M.; Yue, S.; Cao, B.; Zhu, Q.; Sun, N.; Xu, B. Creative utilization of natural nanocomposites: Nitrogen-rich mesoporous carbon for a high-performance sodium ion battery. J. Mater. Chem. A 2017, 5, 9572–9579. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, Y.; Key, J.; Shen, P.K. Three-dimensional graphene sheets with NiO nanobelt outgrowths for enhanced capacity and long term high rate cycling Li-ion battery anode material. J. Power Sour. 2018, 379, 362–370. [Google Scholar] [CrossRef]
- Li, Q.; Yi, Z.; Cheng, Y.; Wang, X.; Yin, D.; Wang, L. Microwave-assisted synthesis of the sandwich-like porous Al2O3/RGO nanosheets anchoring NiO nanocomposite as anode materials for lithium-ion batteries. Appl. Surf. Sci. 2018, 427, 354–362. [Google Scholar] [CrossRef]
- Jiao, Y.; Han, D.; Ding, Y.; Zhang, X.; Guo, G.; Hu, J.; Yang, D.; Dong, A. Fabrication of three-dimensionally interconnected nanoparticle superlattices and their lithium-ion storage properties. Nat. Commun. 2015, 6, 6420. [Google Scholar] [CrossRef]
- He, D.; Gao, Y.; Wang, Z.; Yao, Y.; Wu, L.; Zhang, J.; Huang, Z.-H.; Wang, M.-X. One-step green fabrication of hierarchically porous hollow carbon nanospheres (HCNSs) from raw biomass: Formation mechanisms and supercapacitor applications. J. Colloid Interface Sci. 2021, 581, 238–250. [Google Scholar] [CrossRef]
- Gao, H.; Zhou, T.; Zheng, Y.; Zhang, Q.; Liu, Y.; Chen, J.; Liu, H.; Guo, Z. CoS Quantum Dot Nanoclusters for High-Energy Potassium-Ion Batteries. Adv. Funct. Mater. 2017, 27, 1702634. [Google Scholar] [CrossRef]
- Sun, N.; Guan, Z.; Liu, Y.; Cao, Y.; Zhu, Q.; Liu, H.; Wang, Z.; Zhang, P.; Xu, B. Extended “Adsorption–Insertion” Model: A New Insight into the Sodium Storage Mechanism of Hard Carbons. Adv. Energy Mater. 2019, 9, 1901351. [Google Scholar] [CrossRef]
- Rybarczyk, M.K.; Li, Y.; Qiao, M.; Hu, Y.-S.; Titirici, M.-M.; Lieder, M. Hard carbon derived from rice husk as low cost negative electrodes in Na-ion batteries. J. Energy Chem. 2019, 29, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Hu, M.; He, Y.-B.; Yang, L.; Han, C.; Lv, R.; Kang, F.; Li, B. Transition metal assisted synthesis of tunable pore structure carbon with high performance as sodium/lithium ion battery anode. Carbon 2018, 129, 667–673. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, J.; Liu, S.; Cheng, B.; Xu, Y.; Zhang, P.; Zhang, Q.; Li, Y.; Zhong, S. Improved electrochemical performance of bagasse and starch-modified LiNi0.5Mn0.3Co0.2O2 materials for lithium-ion batteries. J. Mater. Sci. 2017, 53, 5242–5254. [Google Scholar] [CrossRef]
- Zheng, P.; Liu, T.; Zhang, J.; Zhang, L.; Liu, Y.; JianFeng, H.; Guo, S. Sweet potato-derived carbon nanoparticles as anode for lithium ion battery. RSC Adv. 2015, 5, 40737–40741. [Google Scholar] [CrossRef]
- Xu, X.; Gao, J.; Tian, Q.; Zhai, X.; Liu, Y. Walnut shell derived porous carbon for a symmetric all-solid-state supercapacitor. Appl. Surf. Sci. 2017, 411, 170–176. [Google Scholar] [CrossRef]
- Wahid, M.; Gawli, Y.; Puthusseri, D.; Kumar, A.; Shelke, M.V.; Ogale, S. Nutty Carbon: Morphology Replicating Hard Carbon from Walnut Shell for Na Ion Battery Anode. ACS Omega 2017, 2, 3601–3609. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, S.; Jain, A.; Ulaganathan, M.; Edison, E.; Srinivasan, M.; Balasubramanian, R.; Aravindan, V.; Madhavi, S. Li-ion vs. Na-ion capacitors: A performance evaluation with coconut shell derived mesoporous carbon and natural plant based hard carbon. Chem. Eng. J. 2017, 316, 506–513. [Google Scholar] [CrossRef]
- Lv, W.; Wen, F.; Xiang, J.; Zhao, J.; Li, L.; Wang, L.; Liu, Z.; Tian, Y. Peanut shell derived hard carbon as ultralong cycling anodes for lithium and sodium batteries. Electrochim. Acta 2015, 176, 533–541. [Google Scholar] [CrossRef]
- Xiang, J.; Lv, W.; Mu, C.; Zhao, J.; Wang, B. Activated hard carbon from orange peel for lithium/sodium ion battery anode with long cycle life. J. Alloys Compd. 2017, 701, 870–874. [Google Scholar] [CrossRef]
- Li, F.; Qin, F.; Zhang, K.; Fang, J.; Lai, Y.; Li, J. Hierarchically porous carbon derived from banana peel for lithium sulfur battery with high areal and gravimetric sulfur loading. J. Power Sour. 2017, 362, 160–167. [Google Scholar] [CrossRef]
- Gao, Z.; Song, N.; Zhang, Y.; Schwab, Y.; He, J.; Li, X. Carbon Nanotubes Derived from Yeast-Fermented Wheat Flour and Their Energy Storage Application. ACS Sustain. Chem. Eng. 2018, 6, 11386–11396. [Google Scholar] [CrossRef]
- Hu, M.; Wang, J.; Liu, J.; Wang, P.; Feng, Y.; Wang, H.; Nie, N.; Wang, Y.; Huang, Y. A flour-based one-stop supercapacitor with intrinsic self-healability and stretchability after self-healing and biodegradability. Energy Storage Mater. 2019, 21, 174–179. [Google Scholar] [CrossRef]
- Lan, G.; Wang, Y.; Qiu, Y.; Wang, X.; Liang, J.; Han, W.; Tang, H.; Liu, H.; Liu, J.; Li, Y. Wheat flour-derived N-doped mesoporous carbon extrudate as superior metal-free catalysts for acetylene hydrochlorination. Chem. Commun. 2018, 54, 623–626. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, K.; Wei, R.; Yuan, M.; Zhang, Y.; Liang, F.; Yao, Y. High-capacity flour-based nano-Si/C composite anode materials for lithium-ion batteries. Ionics 2019, 26, 1–11. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, X.; Zhong, X.; Li, S.; Liu, T.; Zhuang, G.; Li, X.; Deng, S.; Mei, D.; Wang, J.-G. Hierarchical Porous NC@CuCo Nitride Nanosheet Networks: Highly Efficient Bifunctional Electrocatalyst for Overall Water Splitting and Selective Electrooxidation of Benzyl Alcohol. Adv. Funct. Mater. 2017, 27, 1704169. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, F.; Bai, T.; Long, B.; Liao, Q.; Ren, Y.; Yang, J. Interconnected highly graphitic carbon nanosheets derived from wheat stalk as high performance anode materials for lithium ion batteries. Green Chem. 2016, 18, 2078–2088. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, Y.; Ji, G.; Zhang, B.; Ji, G.; Du, Y. Achieving Sustainable Ultralight Electromagnetic Absorber from Flour by Turning Surface Morphology of Nanoporous Carbon. ACS Sustain. Chem. Eng. 2018, 6, 15850–15857. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, Z.; Zheng, L.; Teng, F.; Hu, L.; Fang, X. A Novel Sustainable Flour Derived Hierarchical Nitrogen-Doped Porous Carbon/Polyaniline Electrode for Advanced Asymmetric Supercapacitors. Adv. Energy Mater. 2016, 6, 1601111. [Google Scholar] [CrossRef]
- Lim, D.G.; Kim, K.; Razdan, M.; Diaz, R.; Osswald, S.; Pol, V.G. Lithium storage in structurally tunable carbon anode derived from sustainable source. Carbon 2017, 121, 134–142. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, L.; Long, C.; Fan, Z. From flour to honeycomb-like carbon foam: Carbon makes room for high energy density supercapacitors. Nano Energy 2015, 13, 527–536. [Google Scholar] [CrossRef]
- Hong, X.; Liub, Y.; Fub, J.; Wangb, X.; Zhangc, T.; Wangd, S.; Houc, F.; Liangce, J. A Wheat Flour Derived Hierarchical Porous Carbon/Graphitic Carbon Nitride Composite for High-Performance Lithium-Sulfur Batteries. Carbon 2020, 170, 119–126. [Google Scholar] [CrossRef]
- Parekh, M.H.; Sediako, A.D.; Naseri, A.; Thomson, M.J.; Pol, V.G. In Situ Mechanistic Elucidation of Superior Si-C-Graphite Li-Ion Battery Anode Formation with Thermal Safety Aspects. Adv. Energy Mater. 2019, 10, 1902799. [Google Scholar] [CrossRef]
- Zhan, C.; Yu, X.; Kang, F.; Liang, Q.; Liu, W.; Wang, Y.; Lv, R.; Huang, Z.-H. Flour food waste derived activated carbon for high-performance supercapacitors. RSC Adv. 2016, 6, 89391–89396. [Google Scholar] [CrossRef]
- Yi, Z.; Han, Q.; Zan, P.; Wu, Y.; Cheng, Y.; Wang, L. Sb nanoparticles encapsulated into porous carbon matrixes for high-performance lithium-ion battery anodes. J. Power Sour. 2016, 331, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ma, L.; Ji, Q.; Meng, J.; Liang, S.; Xu, Z.; Wang, M.; Zuo, X.; Xiao, Y.; Zhu, J.; et al. MnO/Metal/Carbon Nanohybrid Lithium-Ion Battery Anode With Enhanced Electrochemical Performance: Universal Facile Scalable Synthesis and Fundamental Understanding. Adv. Mater. Interfaces 2019, 6, 1900335. [Google Scholar] [CrossRef]
- Ma, L.; Meng, J.; Cheng, Y.; Gao, J.; Wang, X.; Ji, Q.; Wang, M.; Zuo, X.; Zhu, J.; Xia, Y. Epoxy Resin Enables Facile Scalable Synthesis of CuO/C Nanohybrid Lithium-Ion Battery Anode with Enhanced Electrochemical Performance. ChemistrySelect 2020, 5, 5479–5487. [Google Scholar] [CrossRef]
- Naghash, A.R.; Etsell, T.H.; Xu, S. XRD and XPS Study of Cu−Ni Interactions on Reduced Copper−Nickel−Aluminum Oxide Solid Solution Catalysts. Chem. Mater. 2006, 18, 2480–2488. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Yan, B.; Xiong, D.; Li, D.; Lawes, S.; Sun, X. Recent Developments and Understanding of Novel Mixed Transition-Metal Oxides as Anodes in Lithium Ion Batteries. Adv. Energy Mater. 2016, 6, 1502175. [Google Scholar] [CrossRef]
- Zou, F.; Chen, Y.M.; Liu, K.; Yu, Z.; Liang, W.; Bhaway, S.M.; Zhu, Y. Metal Organic Frameworks Derived Hierarchical Hollow NiO/Ni/Graphene Composites for Lithium and Sodium Storage. ACS Nano 2016, 10, 377–386. [Google Scholar] [CrossRef]
- Ma, Y.; Liang, X.; Tong, S.; Bao, G.; Ren, Q.; Dai, Z. Gold Nanoshell Nanomicelles for Potential Magnetic Resonance Imaging, Light-Triggered Drug Release, and Photothermal Therapy. Adv. Funct. Mater. 2012, 23, 815–822. [Google Scholar] [CrossRef]
- Song, Y.; Feng, A.; Liu, Z.; Li, D. Zeta potentials of PDMS surfaces modified with poly(ethylene glycol) by physisorption. Electrophoresis 2019, 41, 761–768. [Google Scholar] [CrossRef]
- Liao, C.; Yang, B.; Zhang, N.; Liu, M.; Chen, G.; Jiang, X.; Chen, G.; Yang, J.; Liu, X.; Chan, T.; et al. Constructing Conductive Interfaces between Nickel Oxide Nanocrystals and Polymer Carbon Nitride for Efficient Electrocatalytic Oxygen Evolution Reaction. Adv. Funct. Mater. 2019, 29, 1904020. [Google Scholar] [CrossRef]
- Yu, Z.-L.; Xin, S.; You, Y.; Yu, L.; Lin, Y.; Xu, D.-W.; Qiao, C.; Huang, Z.-H.; Yang, N.; Yu, S.-H.; et al. Ion-Catalyzed Synthesis of Microporous Hard Carbon Embedded with Expanded Nanographite for Enhanced Lithium/Sodium Storage. J. Am. Chem. Soc. 2016, 138, 14915–14922. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Sushko, M.L.; Wang, W.; Schwenzer, B.; Xiao, J.; Nie, Z.; Saraf, L.V.; Yang, Z.; Liu, J. Sodium Ion Insertion in Hollow Carbon Nanowires for Battery Applications. Nano Lett. 2012, 12, 3783–3787. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hwang, J.-Y.; Park, S.-M.; Sun, Y.-K. Superior lithium/potassium storage capability of nitrogen-rich porous carbon nanosheets derived from petroleum coke. J. Mater. Chem. A 2018, 6, 12551–12558. [Google Scholar] [CrossRef]
- Ou, J.; Zhang, Y.; Chen, L.; Zhao, Q.; Meng, Y.; Guo, Y.; Xiao, D. Nitrogen-rich porous carbon derived from biomass as a high performance anode material for lithium ion batteries. J. Mater. Chem. A 2015, 3, 6534–6541. [Google Scholar] [CrossRef]
- Zhang, K.; Li, X.; Liang, J.; Zhu, Y.; Hu, L.; Cheng, Q.; Guo, C.; Lin, N.; Qian, Y. Nitrogen-doped porous interconnected double-shelled hollow carbon spheres with high capacity for lithium ion batteries and sodium ion batteries. Electrochim. Acta 2015, 155, 174–182. [Google Scholar] [CrossRef]
- Yang, C.C.; Zhang, D.M.; Du, L.; Jiang, Q. Hollow Ni–NiO nanoparticles embedded in porous carbon nanosheets as a hybrid anode for sodium-ion batteries with an ultra-long cycle life. J. Mater. Chem. A 2018, 6, 12663–12671. [Google Scholar] [CrossRef]
- Subramaniyam, C.M.; Srinivasan, N.; Tai, Z.; Liu, H.-K.; Goodenough, J.B.; Dou, S.X. Self-assembled porous carbon microparticles derived from halloysite clay as a lithium battery anode. J. Mater. Chem. A 2017, 5, 7345–7354. [Google Scholar] [CrossRef]
- Tang, T.; Zhang, T.; Li, W.; Huang, X.; Wang, X.; Qiu, H.; Hou, Y. Mesoporous N-doped graphene prepared by a soft-template method with high performance in Li–S batteries. Nanoscale 2019, 11, 7440–7446. [Google Scholar] [CrossRef]
- Hao, R.; Lan, H.; Kuang, C.; Wang, H.; Guo, L. Superior potassium storage in chitin-derived natural nitrogen-doped carbon nanofibers. Carbon 2018, 128, 224–230. [Google Scholar] [CrossRef]
- Jae, W.; Song, J.; Hong, J.J.; Kim, J. Raspberry-like hollow Ni/NiO nanospheres anchored on graphitic carbon sheets as anode material for lithium-ion batteries. J. Alloys Compd. 2019, 805, 957–966. [Google Scholar] [CrossRef]
- Marchetti, L.; Miserque, F.; Perrin, S.; Pijolat, M. XPS study of Ni-base alloys oxide films formed in primary conditions of pressurized water reactor. Surf. Interface Anal. 2015, 47, 632–642. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zhang, Y.; Lin, C.; Yang, W.; Meng, Y.; Guo, Y.; Li, M.; Xiao, D. Hierarchically porous nitrogen-rich carbon derived from wheat straw as an ultra-high-rate anode for lithium ion batteries. J. Mater. Chem. A 2014, 2, 9684–9690. [Google Scholar] [CrossRef]
- Tian, J.; Shao, Q.; Dong, X.; Zheng, J.; Pan, D.; Zhang, X.; Cao, H.; Hao, L.; Liu, J.; Mai, X.; et al. Bio-template synthesized NiO/C hollow microspheres with enhanced Li-ion battery electrochemical performance. Electrochim. Acta 2018, 261, 236–245. [Google Scholar] [CrossRef]
- Liu, H.; Jia, M.; Sun, N.; Cao, B.; Chen, R.; Zhu, Q.; Wu, F.; Qiao, N.; Xu, B. Nitrogen-Rich Mesoporous Carbon as Anode Material for High-Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 27124–27130. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, Y.; Hu, Y.-S.; Li, H.; Chen, L.; Huang, X. A waste biomass derived hard carbon as a high-performance anode material for sodium-ion batteries. J. Mater. Chem. A 2016, 4, 13046–13052. [Google Scholar] [CrossRef]
- Xiao, Y.; Su, D.; Wang, X.; Wu, S.; Zhou, L.; Shi, Y.; Fang, S.; Cheng, H.-M.; Li, F. CuS Microspheres with Tunable Interlayer Space and Micropore as a High-Rate and Long-Life Anode for Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1800930. [Google Scholar] [CrossRef]
- Mai, Y.; Tu, J.; Gu, C.; Wang, X. Graphene anchored with nickel nanoparticles as a high-performance anode material for lithium ion batteries. J. Power Sour. 2012, 209, 1–6. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X.-Y.; Li, J.; Zou, Y.-L.; Tang, J. Study of nano-porous hard carbons as anode materials for lithium ion batteries. Mater. Chem. Phys. 2012, 135, 445–450. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Q.; Sun, B.; Gu, J.; Yu, B.; Zhang, W.; Zhang, D. N-doped catalytic graphitized hard carbon for high-performance lithium/sodium-ion batteries. Sci. Rep. 2018, 8, 9934. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Schnepp, Z.; Titirici, M.M. Rice husk-derived carbon anodes for lithium ion batteries. J. Mater. Chem. A 2013, 1, 5269–5273. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, L.; Huang, J.; Zou, J.; Chen, S.; Cheng, H.; Jiang, C.; Gao, P.; Niu, X. Loofah-derived carbon as an anode material for potassium ion and lithium ion batteries. Electrochim. Acta 2019, 306, 446–453. [Google Scholar] [CrossRef]
- Mai, Y.; Shi, S.; Zhang, D.; Lu, Y.; Gu, C.; Tu, J. NiO–Graphene hybrid as an anode material for lithium ion batteries. J. Power Sour. 2012, 204, 155–161. [Google Scholar] [CrossRef]
- Soler-Piña, F.J.; Hernández-Rentero, C.; Caballero, Á.; Morales, J.; Rodríguez-Castellón, E.; Canales-Vázquez, J. Highly graphitized carbon nanosheets with embedded Ni nanocrystals as anode for Li-ion batteries. Nano Res. 2019, 13, 86–94. [Google Scholar] [CrossRef]
- Bin, D.S.; Duan, S.Y.; Lin, X.J.; Liu, L.; Liu, Y.; Xu, Y.S.; Wan, L.J. Structural engineering of SnS2/Graphene nanocomposite for high-performance K-ion battery anode. Nano Energy 2019, 60, 912–918. [Google Scholar] [CrossRef]
- Mariappan, V.K.; Krishnamoorthy, K.; Pazhamalai, P.; Natarajan, S.; Sahoo, S.; Nardekar, S.S.; Kim, S.-J. Antimonene dendritic nanostructures: Dual-functional materials for high-performance energy storage and harvesting devices. Nano Energy 2020, 77, 105248. [Google Scholar] [CrossRef]
- Li, X.-L.; Bai, S.; Yue, X.-Y.; Chen, D.; Qiu, Q.-Q.; Song, Y.; Wu, X.-J.; Zhou, Y.-N. Rod-shaped monoclinic CoMo2S4 with exceptionally reversible phase conversion for sodium storage. J. Alloys Compd. 2020, 838, 155613. [Google Scholar] [CrossRef]

| Sample | Porosity Parameters | Element Composition (atom %) | ||||||
|---|---|---|---|---|---|---|---|---|
| SBET (m2g−1) | VT (cm3g−1) | Vmeso (cm3g−1) | Vmicro (cm3g−1) | C | N | O | Ni | |
| WFC-600 | 3.3 | / | / | / | 87.0 | 4.2 | 8.8 | / |
| NC-600 | 470.5 | 0.3 | 0.1 | 0.2 | 85.1 | 3.1 | 9.2 | 2.6 |
| WFC-800 | 3.5 | / | / | / | 84.1 | 9.7 | 6.2 | / |
| NC-800 | 321.3 | 0.2 | 0.08 | 0.1 | 83.4 | 2.9 | 9.7 | 2.7 |
| Materials | Specific Capacity (mAh g−1) | Current Density (A g−1) | Cycle Stability | References |
|---|---|---|---|---|
| glucosamine-based porous carbon | 264 | 0.1 | 100 | [11] |
| structurally tunable carbon | 217 | 1C | 100 | [28] |
| nano-porous hard carbons | 453.1 | 0.2C | 50 | [59] |
| nitrogen-doped porous hollow carbon | 512 | 1.5C | 500 | [46] |
| sweet potato-derived carbon | 320 | 0.1 | 200 | [13] |
| N-doped graphitized hard carbon | 389 | 0.1 | 100 | [60] |
| rice husk-derived carbon | 403 | 0.2C | 100 | [61] |
| Loofah-derived carbon | 225 | 0.1 | 200 | [62] |
| NiO/C hollow microspheres | 628 | 0.1 | 100 | [54] |
| Ni-graphene | 675 | 0.1 | 35 | [58] |
| NiO-graphene | 646.1 | 0.1 | 35 | [63] |
| C/Ni700 | 205 | 1C | 200 | [64] |
| NC-600 | 700.8 | 0.1 | 200 | this work |
| 257.2 | 1 | 800 | ||
| 51.3 | 20 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Wu, X.; Li, Y.; Wang, S.; Zhuo, S. Nickel-Embedded Carbon Materials Derived from Wheat Flour for Li-Ion Storage. Materials 2020, 13, 4611. https://doi.org/10.3390/ma13204611
Ding W, Wu X, Li Y, Wang S, Zhuo S. Nickel-Embedded Carbon Materials Derived from Wheat Flour for Li-Ion Storage. Materials. 2020; 13(20):4611. https://doi.org/10.3390/ma13204611
Chicago/Turabian StyleDing, Wen, Xiaozhong Wu, Yanyan Li, Shuo Wang, and Shuping Zhuo. 2020. "Nickel-Embedded Carbon Materials Derived from Wheat Flour for Li-Ion Storage" Materials 13, no. 20: 4611. https://doi.org/10.3390/ma13204611






