The Activation of Ti-Zr-V-Hf Non-Evaporable Getter Films with Open-Cell Copper Metal Foam Substrates
Abstract
:1. Introduction
2. Experiments and Methods
2.1. Characterization Methods
2.2. Sample Preparation
3. Results and Discussion
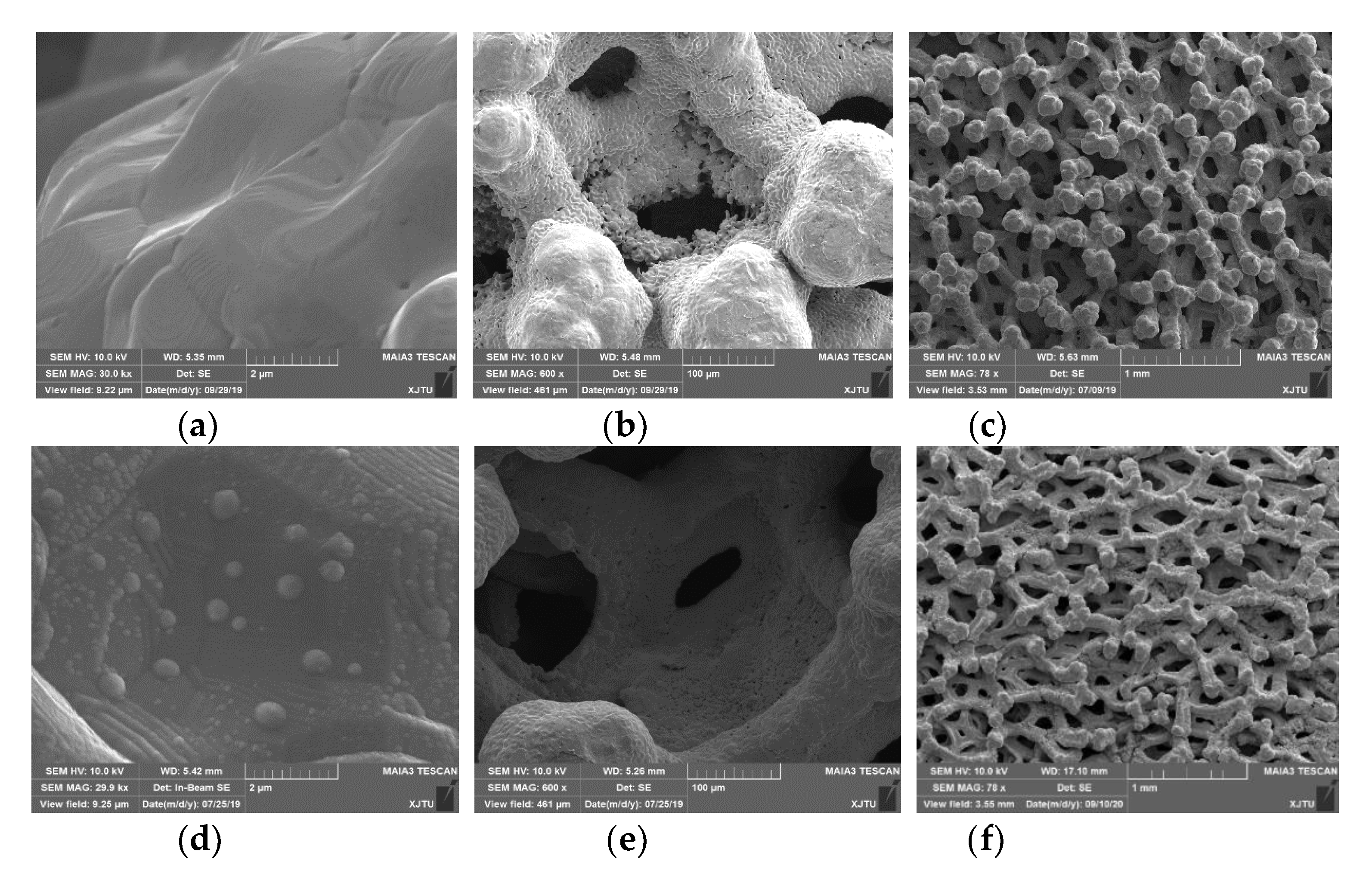
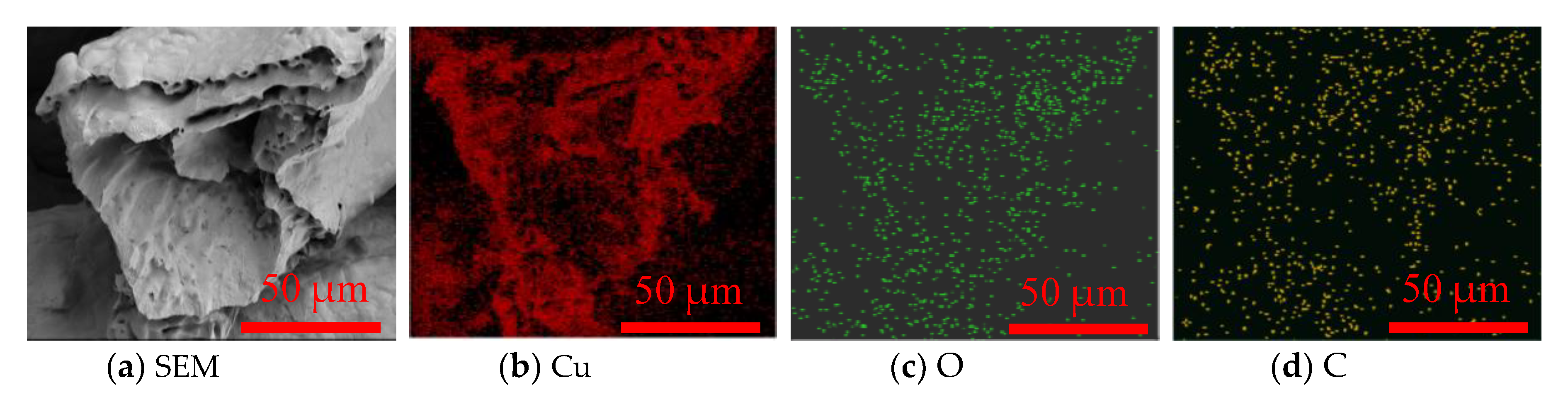
3.1. Surface Morphology
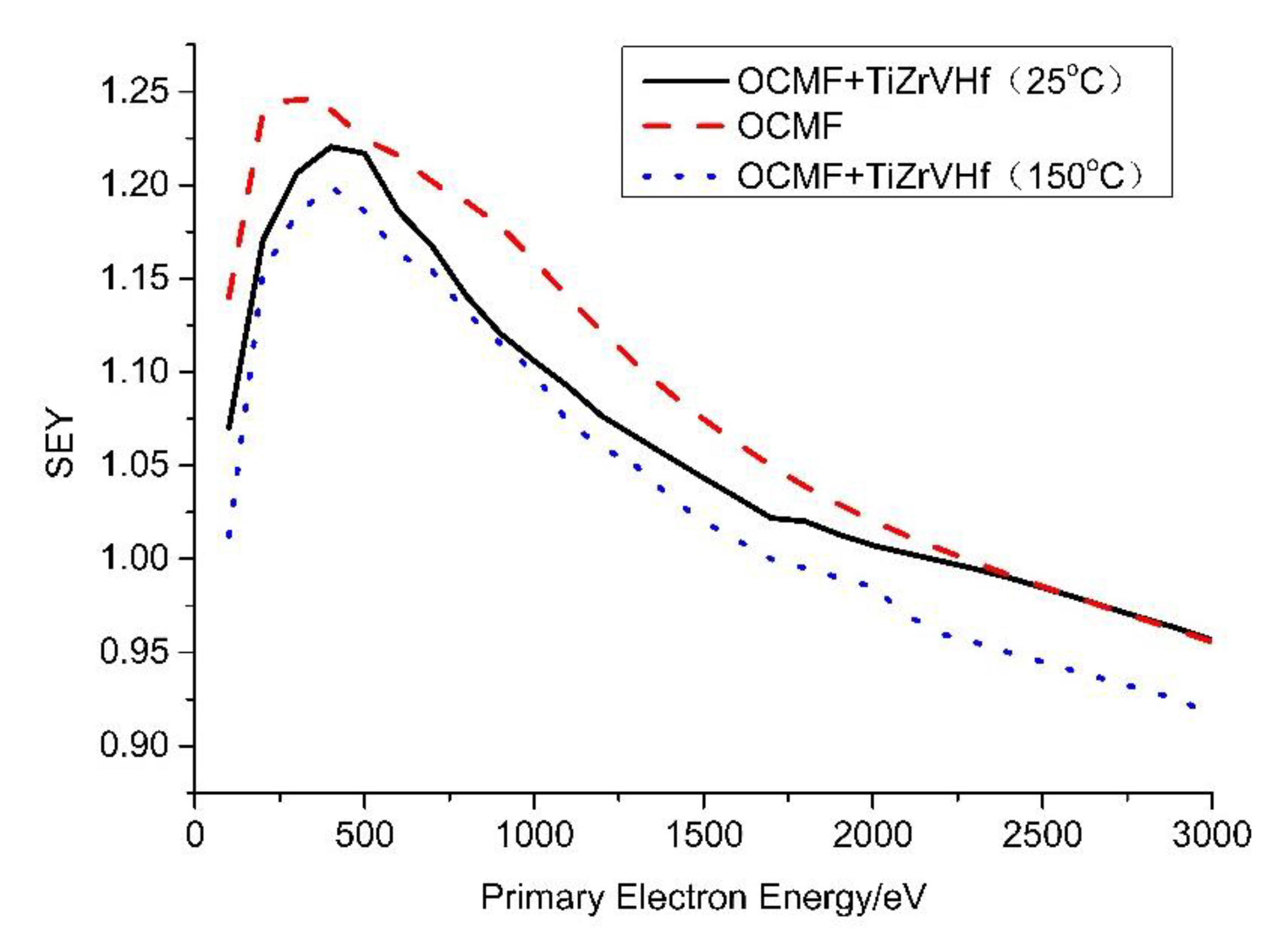
3.2. SEY
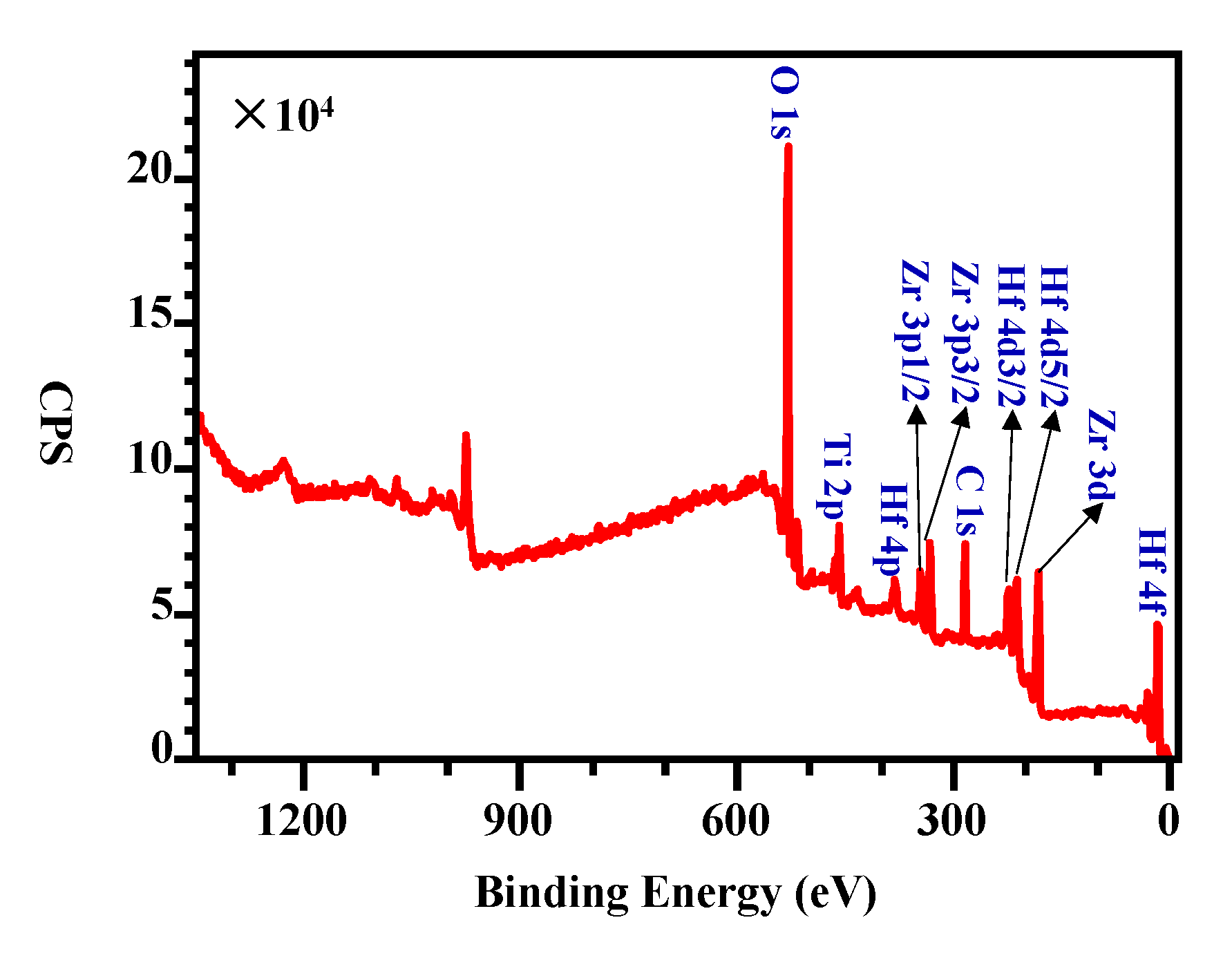
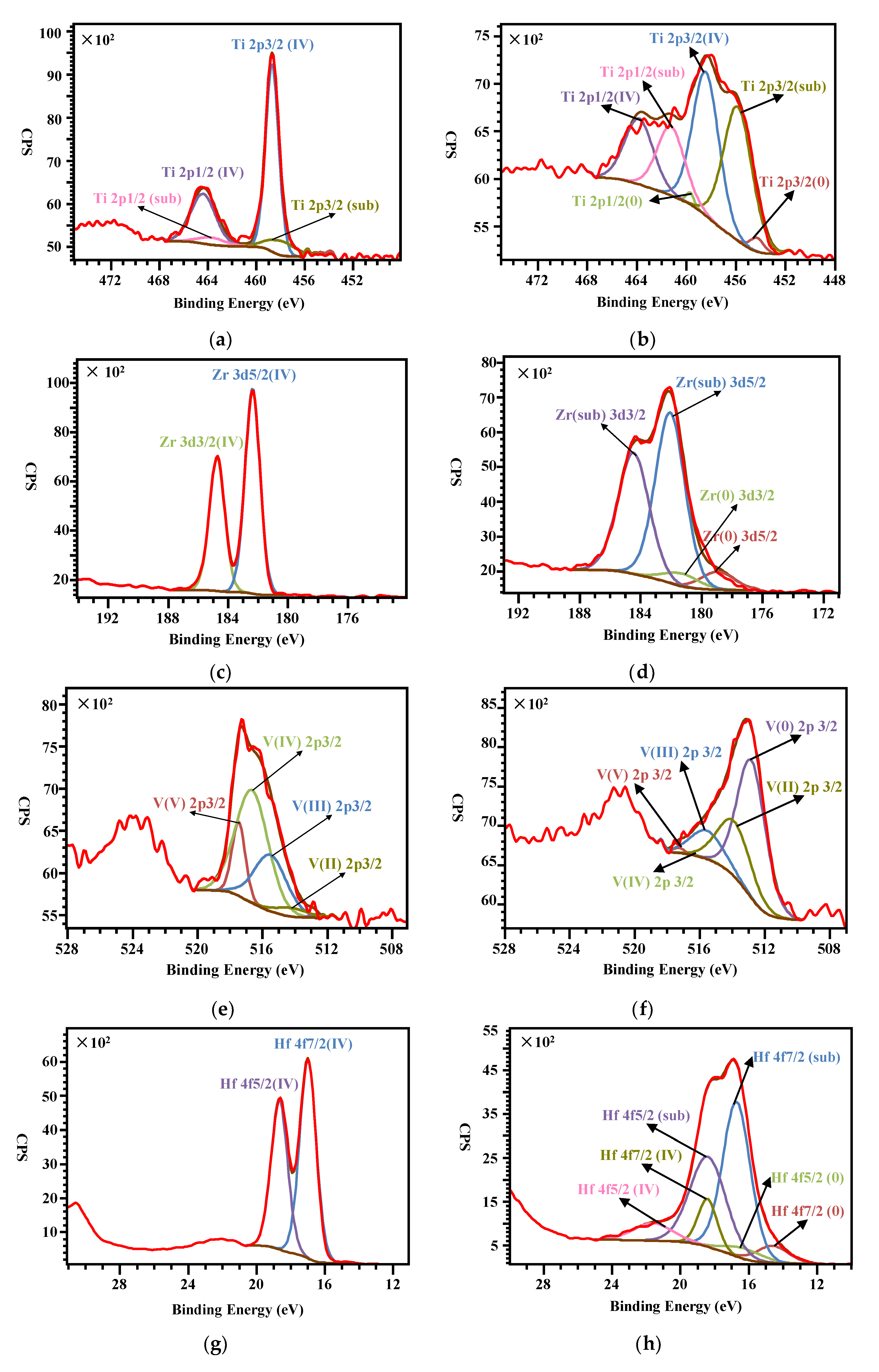
3.3. Surface Chemical States Variation during Activation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anashin, V.V.; Collins, I.R.; Dostovalov, R.V.; Fedorov, N.V.; Krasnov, A.A.; Malyshev, O.B.; Ruzinov, V.L. Comparative study of photodesorption from TiZrV coated and uncoated stainless steel vacuum chambers. Vacuum 2004, 75, 155–159. [Google Scholar] [CrossRef]
- Mahner, E.; Hansen, J.; Küchler, D.; Malabaila, M.; Taborelli, M. Ion-stimulated gas desorption yields of electropolished, chemically etched, and coated (Au, Ag, Pd, TiZrV) stainless steel vacuum chambers and St707 getter strips irradiated with 4.2 MeV/u lead ions. Phys. Rev. Spec. Top. Accel. Beams 2005, 8, 053201. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.J.; Seraphim, R.M.; Ramirez, A.J.; Tabacniks, M.H.; Nascente, P.A.P. Characterization and evaluation of Ti-Zr-V non-evaporable getter films used in vacuum systems. Phys. Proced. 2012, 32, 840–852. [Google Scholar] [CrossRef] [Green Version]
- Singleton, J.H. Hydrogen pumping by sputter-ion pumps and getter pumps. J. Vac. Sci. Technol. 1971, 8, 275. [Google Scholar] [CrossRef]
- Sutara, F.; Skala, T.; Masek, K.; Matolin, V. Surface characterization of activated Ti-Zr-V NEG coatings. Vacuum 2009, 83, 824–827. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Tallarico, D.A.; Nascente, P.A.P. Preparation and characterization of Ti-Zr-V non-evaporable getter films to be used in ultra-high vacuum. AIP Conf. Proc. 2009, 1092, 168–172. [Google Scholar]
- Benvenuti, C.; Santana, A.E.; Ruzinov, V. Ultimate pressures achieved in TiZrV sputter-coated vacuum chambers. Vacuum 2001, 60, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gao, Y.; Fan, J.; You, Z.; Wang, S.; Xu, Z. Study on the effect of laser parameters on the SEY of aluminum alloy. IEEE Trans. Nucl. Sci. 2019, 66, 609–615. [Google Scholar] [CrossRef]
- Pivi, M.; King, F.K.; Kirby, R.E.; Raubenheimer, T.O.; Stupakov, G.; le Pimpec, F. Sharp reduction of the secondary electron emission yield from grooved surfaces. J. Appl. Phys. 2008, 104, 104904. [Google Scholar] [CrossRef] [Green Version]
- Costa Pinto, P.; Calatroni, S.; Neupert, H.; Letant-Delrieux, D.; Edwards, P.; Chiggiato, P.; Taborelli, M.; Vollenberg, W.; Yin-Vallgren, C.; Colaux, J.L.; et al. Carbon coatings with low secondary electron yield. Vacuum 2013, 98, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Le Pimpec, F.; Kirby, R.E.; King, F.K.; Pivi, M. The effect of gas ion bombardment on the secondary electron yield of TiN, TiCN and TiZrV coatings for suppressing collective electron effects in storage rings. Nucl. Instrum. Methods Phys. Res. Sect. A 2006, 564, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.C.; Huh, S. Porous carbon-based supercapacitors directly derived from metal-organic frameworks. Materials 2020, 13, 4215. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, Y.C. Pore structure characteristics of foam composite with active carbon. Materials 2020, 13, 4038. [Google Scholar] [CrossRef]
- Monkova, K.; Vasina, M.; Monka, P.P.; Kozak, D.; Vanca, J. Effect of the pore shape and size of 3D-Printed open-porous ABS materials on sound absorption performance. Materials 2020, 13, 4474. [Google Scholar] [CrossRef]
- Davies, G.J.; Zhen, S. Metallic foams their production properties and applications. J. Mater. Sci. 1983, 18, 1899–1911. [Google Scholar] [CrossRef]
- Calmidi, V.V.; Mahajan, R.L. Forced convection in high porosity metal foams. J. Heat Transfer 2000, 122, 557–565. [Google Scholar] [CrossRef]
- Gaitanaros, S.; Kyriakides, S. Dynamic crushing of aluminum foams: Part II–Analysis. Int. J. Solids Struct. 2014, 51, 1646–1661. [Google Scholar] [CrossRef] [Green Version]
- Mun, J.; Jeon, H.; Jeong, B.; Jung, J. Decomposition of endothermic fuel using washcoated HZSM-5 on metal foam. Catal. Today 2020. [Google Scholar] [CrossRef]
- August, A.; Nestler, B. About the surface area to volume relations of open cell foams. Eng. Res. Express 2020, 2, 015021. [Google Scholar] [CrossRef]
- Liu, R.; Tan, Y.; Gong, F.; Li, X.; Dai, L.; Yang, J.; Xu, S.; Li, G. The application of porous foam structure cooling arrangement system for a thin disk laser. Optik 2020, 200, 163423. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Fan, Y.; Zhou, W. Fabrication of porous metal by selective laser melting as catalyst support for hydrogen production microreactor. Int. J. Hydrogen Energy 2020, 45, 10–22. [Google Scholar] [CrossRef]
- Mohamed Moussa, E.I.; Karkri, M. A numerical investigation of the effects of metal foam characteristics and heating/cooling conditions on the phase change kinetic of phase change materials embedded in metal foam. J. Energy Storage 2019, 26, 100985. [Google Scholar] [CrossRef]
- Banhart, J.; Ashby, M.; Fleck, N. Metal foams and porous metal structures. In Proceedings of the International Conference on Cellular Metals and Metal Foaming Technology, Bremen, Germany, 14–16 June 1999. [Google Scholar]
- Banhart, J. Manufacture, characterisation and application of cellular metals and metal foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Kulshreshtha, A.; Dhakad, S.K. Preparation of metal foam by different methods: A review. Mater. Today Proc. 2020, 26, 1784–1790. [Google Scholar] [CrossRef]
- Köhl, M.; Habijan, T.; Bram, M.; Buchkremer, H.P.; Stöver, D.; Köller, M. Powder metallurgical near-net-shape fabrication of porous NiTi shape memory alloys for use as long-term implants by the combination of the metal injection molding process with the space-holder technique. Adv. Eng. Mater. 2009, 11, 959–968. [Google Scholar] [CrossRef]
- Levine, B.R.; Fabi, D.W. Porous metals in orthopedic applications—A review. Materialwiss. Werkstofftech. 2010, 41, 1001–1010. [Google Scholar] [CrossRef]
- Kaz’mina, O.V.; Suslyaev, V.I.; Dorozhkin, K.V.; Kaimonov, M.R.; Stebeneva, V.I. Effect of a coating deposited on foam glass on the ability to absorb high-frequency electromagnetic radiation. Glass Ceram. 2018, 75, 230–233. [Google Scholar] [CrossRef]
- Leong, K.C.; Jin, L.W. Effect of oscillatory frequency on heat transfer in metal foam heat sinks of various pore densities. Int. J. Heat Mass Transfer 2006, 49, 671–681. [Google Scholar] [CrossRef]
- Malyshev, O.B.; Valizadeh, R.; Hogan, B.T.; Hannah, A.N. Electron-stimulated desorption from polished and vacuum fired 316LN stainless steel coated with Ti-Zr-Hf-V. J. Vac. Sci. Technol. A 2014, 32, 061601. [Google Scholar] [CrossRef] [Green Version]
- Baglin, V.; Collins, I.; Henrist, B.; Hilleret, N.; Vorlaufer, G. A summary of main experimental results concerning the secondary electron emission of copper. LHC Proj. Rep. 2002, 472, 15. [Google Scholar]
- Bojko, I.; Hilleret, N.; Scheuerlein, C. Influence of air exposures and thermal treatments on the secondary electron yield of copper. J. Vac. Sci. Technol. A 2000, 18, 972–979. [Google Scholar] [CrossRef] [Green Version]
- Furman, M.A.; Chaplin, V.H. Update on electron-cloud power deposition for the LHC arc dipoles. Phys. Rev. Spec. Top. Accel. Beams 2006, 9, 034403. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, J.; Xu, Y.; Zhang, Y.; Zhang, B.; Wei, W.; Zhang, T. Research on Low Secondary Electron Yield Materials for Future Accelerators; JACOW: Geneva, Switzerland, 2016; pp. 3284–3286. [Google Scholar]
- Ermolieff, A.; Bernard, P.; Marthon, S.; Wittmer, P. Nitridation of polycrystalline titanium as studied by in situ angle-resolved X-ray photoelectron spectroscopy. Surf. Interface Anal. 1988, 11, 563–568. [Google Scholar] [CrossRef]
- Badrinarayanan, S.; Sinha, S.; Mandale, A.B. XPS studies of nitrogen ion implanted zirconium and titanium. J. Electron Spectrosc. Relat. Phenom. 1989, 49, 303–309. [Google Scholar] [CrossRef]
- Siemensmeyer, B.; Bade, K.; Schultze, J.W. XPS and electrochemical studies of thin TiN Layers. Ber. Bunsenges. Phys. Chem. 1991, 95, 1461–1469. [Google Scholar] [CrossRef]
- Slinkard, W.E.; Degroot, P.B. Vanadium-titanium oxide catalysts for oxidation of butene to acetic acid. J. Catal. 1981, 68, 423–432. [Google Scholar] [CrossRef]
- Burke, A.R.; Brown, C.R.; Bowling, W.C.; Glaub, J.E.; Kapsch, D.; Love, C.M.; Whitaker, R.B.; Moddeman, W.E. Ignition mechanism of the titanium-boron pyrotechnic mixture. Surf. Interface Anal. 1988, 11, 353–358. [Google Scholar] [CrossRef]
- Leinen, D.; Lassaletta, G.; Fernández, A.; Caballero, A.; González-Elipe, A.R.; Martín, J.M.; Vacher, B. Ion beam induced chemical vapor deposition procedure for the preparation of oxide thin films. II. Preparation and characterization of AlxTiyOz thin films. J. Vac. Sci. Technol. A 1996, 14, 2842–2848. [Google Scholar] [CrossRef]
- Balaceanu, M.; Braic, M.; Braic, V.; Vladescu, A.; Negrila, C.C. Surface chemistry of plasma deposited ZrC hard coatings. J. Optoelectron. Adv. Mater. 2005, 7, 2557–2560. [Google Scholar]
- Chourasia, A.R.; Hickman, J.L.; Miller, R.L.; Nixon, G.A.; Seabolt, M.A. X-Ray photoemission study of the oxidation of Hafnium. Int. J. Spectrosc. 2009, 2009, 439065. [Google Scholar] [CrossRef] [Green Version]
- Dey, S.K.; Das, A.; Tsai, M.; Gu, D.; Floyd, M.; Carpenter, R.W.; de Waard, H.; Werkhoven, C.; Marcus, S. Relationships among equivalent oxide thickness, nanochemistry, and nanostructure in atomic layer chemical-vapor-deposited Hf–O films on Si. J. Appl. Phys. 2004, 95, 5042–5048. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, J.; Gao, Y.; Hu, Y.; You, Z.; Xie, Y.; Li, H.; Wu, Y.; Yang, S.; Wang, D.; et al. The Activation of Ti-Zr-V-Hf Non-Evaporable Getter Films with Open-Cell Copper Metal Foam Substrates. Materials 2020, 13, 4650. https://doi.org/10.3390/ma13204650
Wang J, Zhang J, Gao Y, Hu Y, You Z, Xie Y, Li H, Wu Y, Yang S, Wang D, et al. The Activation of Ti-Zr-V-Hf Non-Evaporable Getter Films with Open-Cell Copper Metal Foam Substrates. Materials. 2020; 13(20):4650. https://doi.org/10.3390/ma13204650
Chicago/Turabian StyleWang, Jie, Jing Zhang, Yong Gao, Yaocheng Hu, Zhiming You, Yupeng Xie, Haipeng Li, Yue Wu, Shanghui Yang, Dengwang Wang, and et al. 2020. "The Activation of Ti-Zr-V-Hf Non-Evaporable Getter Films with Open-Cell Copper Metal Foam Substrates" Materials 13, no. 20: 4650. https://doi.org/10.3390/ma13204650
APA StyleWang, J., Zhang, J., Gao, Y., Hu, Y., You, Z., Xie, Y., Li, H., Wu, Y., Yang, S., Wang, D., Wang, S., & Xu, Z. (2020). The Activation of Ti-Zr-V-Hf Non-Evaporable Getter Films with Open-Cell Copper Metal Foam Substrates. Materials, 13(20), 4650. https://doi.org/10.3390/ma13204650






