Understanding the Role of Silver Nanostructures and Graphene Oxide Applied as Surface Modification of TiO2 in Photocatalytic Transformations of Rhodamine B under UV and Vis Irradiation
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Titanium Dioxide Coatings
2.3. Surface Modification of TiO2 Coatings with Graphene Oxide (GO) and Silver Nanostructures (AgNSs)
2.4. Characterization
2.5. Analysis of Photocatalytic Activity
3. Results and Discussion
3.1. Titanium Dioxide Coatings Characterization
3.2. Surface Modifications with AgNSs and GO
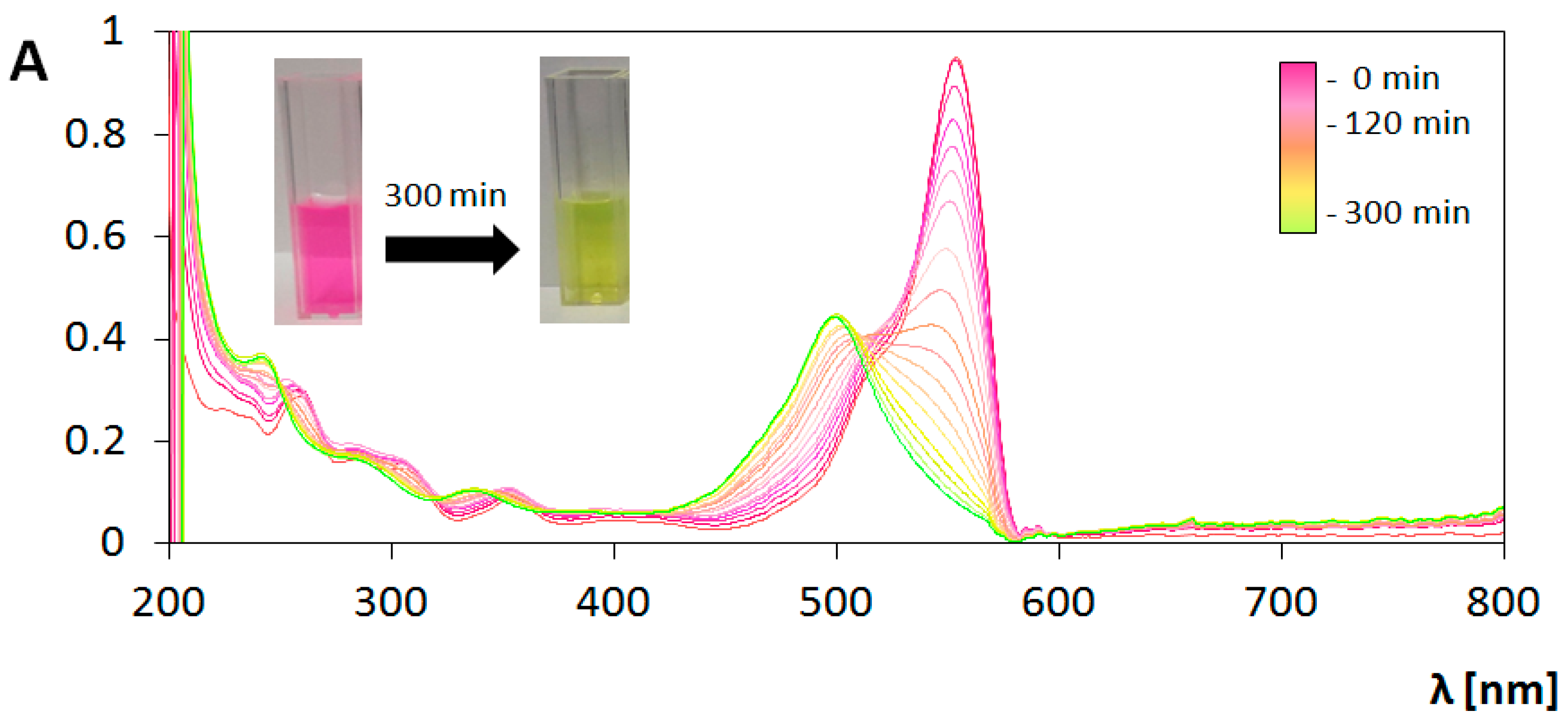
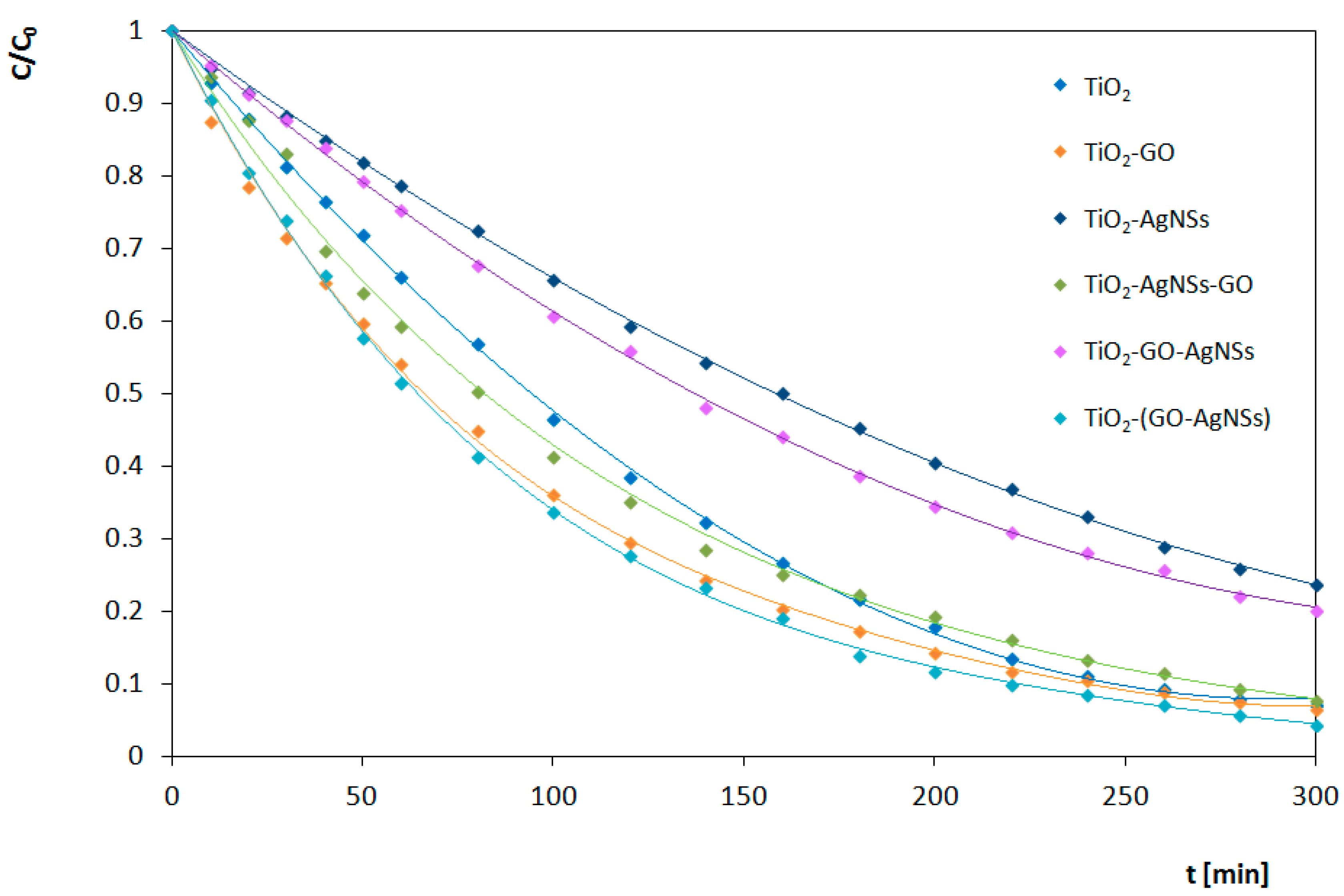
3.3. Photocatalytic Transformations of Rhodamine B
3.4. Analysis of Photocatalytic Properties
3.4.1. Under UV Irradiation
3.4.2. Under Visible Light (Vis) Irradiation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, H.; Qu, Y.; Zeid, T.; Duan, X. Towards highly efficient photocatalysts using semiconductor nanoarchitectures. Energy Environ. Sci. 2012, 5, 6732–6743. [Google Scholar] [CrossRef]
- Wang, R.; Lan, K.; Liu, B.; Yu, Y.; Chen, A.; Li, W. Confinement synthesis of hierarchical ordered macro-/mesoporous TiO2 nanostructures with high crystallization for photodegradation. Chem. Phys. 2019, 516, 48–54. [Google Scholar] [CrossRef]
- Guo, H.; Sen, T.; Zhang, J.; Wang, L. Hierarchical porous TiO2 single crystals templated from partly glassified polystyrene. J. Colloid Interface Sci. 2019, 538, 248–255. [Google Scholar] [CrossRef]
- Mikrut, P.; Kobielusz, M.; Macyk, W. Spectroelectrochemical characterization of euhedral anatase TiO2 crystals—Implications for photoelectrochemical and photocatalytic properties of {001} {100} and {101} facets. Electrochim. Acta 2019, 310, 256–265. [Google Scholar] [CrossRef]
- Ren, L.; Li, Y.; Hou, J.; Bai, J.; Mao, M.; Zeng, M.; Zhao, X.; Li, N. The pivotal effect of the interaction between reactant and anatase TiO2 nanosheets with exposed {001} facets on photocatalysis for the photocatalytic purification of VOCs. Appl. Catal. B 2016, 181, 625–634. [Google Scholar] [CrossRef]
- Bellardita, M.; Garlisi, C.; Ozer, L.Y.; Venezia, A.M.; Sá, J.; Mamedov, F.; Palmisano, L.; Palmisano, G. Highly stable defective TiO2-x with tuned exposed facets induced by fluorine: Impact of surface and bulk properties on selective UV/visible alcohol photo-oxidation. Appl. Surf. Sci. 2020, 510, 145419. [Google Scholar] [CrossRef]
- Zhao, T.; Xing, Z.; Xiu, Z.; Li, Z.; Yang, S.; Zhu, Q.; Zhou, W. Surface defect and rational design of TiO2−x nanobelts/g-C3N4 nanosheets/CdS quantum dots hierarchical structure for enhanced visible-light-driven photocatalysis. Int. J. Hydrog. Energy 2019, 44, 1586–1596. [Google Scholar] [CrossRef]
- Zhou, S.; Bao, N.; Zhang, Q.; Jie, X.; Jin, Y. Engineering hierarchical porous oxygen-deficient TiO2 fibers decorated with BiOCl nanosheets for efficient photocatalysis. Appl. Surf. Sci. 2019, 471, 96–107. [Google Scholar] [CrossRef]
- Jiang, Q.; Huang, J.; Ma, B.; Yang, Z.; Zhang, T.; Wang, X. Recyclable, hierarchical hollow photocatalyst TiO2@SiO2 composite microsphere realized by raspberry-like SiO2. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125112. [Google Scholar] [CrossRef]
- Song, J.; Sun, G.; Yu, J.; Si, Y.; Ding, B. Construction of ternary Ag@ZnO/TiO2 fibrous membranes with hierarchical nanostructures and mechanical flexibility for water purification. Ceram. Int. 2020, 46, 468–475. [Google Scholar] [CrossRef]
- Sanzone, G.; Zimbone, M.; Cacciato, G.; Ruffino, F.; Carles, R.; Privitera, V.; Grimaldi, M.G. Ag/TiO2 nanocomposite for visible light-driven photocatalysis. Superlattice. Microst. 2018, 123, 394–402. [Google Scholar] [CrossRef]
- Li, Y.; Lv, K.; Ho, W.; Dong, F.; Wu, X.; Xia, Y. Hybridization of rutile TiO2 (rTiO2) with g-C3N4 quantum dots (CN QDs): An efficient visible-light-driven Z-scheme hybridized photocatalyst. Appl. Catal. B 2017, 202, 611–619. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Chen, Z.; Qin, L.; Yang, L.; Zhu, L.; Tang, P.; Gao, T.; Huang, Y.; Sha, Z. Visible light induced photocatalysis on CdS quantum dots decorated TiO2 nanotube arrays. Appl. Catal. A 2015, 498, 159–166. [Google Scholar] [CrossRef]
- Jia, J.; Xue, P.; Hu, X.; Wang, Y.; Liu, E.; Fan, J. Electron-transfer cascade from CdSe@ZnSe core-shell quantum dot accelerates photoelectrochemical H2 evolution on TiO2 nanotube arrays. J. Catal. 2019, 375, 81–94. [Google Scholar] [CrossRef]
- Xu, Y.; Li, A.; Yao, T.; Ma, C.; Zhang, X.; Shah, J.H.; Han, H. Strategies for Efficient Charge Separation and Transfer in Artificial Photosynthesis of Solar Fuels. ChemSusChem 2017, 10, 4277–4305. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Recent progress in enhancing photocatalytic efficiency of TiO2-based materials. Appl. Catal. A-Gen. 2015, 495, 131–140. [Google Scholar] [CrossRef]
- Shuang, S.; Lv, R.; Cui, X.; Xie, Z.; Zheng, J.; Zhang, Z. Efficient photocatalysis with graphene oxide/Ag/Ag2S–TiO2 nanocomposites under visible light irradiation. RSC Adv. 2018, 8, 5784–5791. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.; Sun, Z. A mechanism for enhanced hydrophilicity of silver nanoparticles modified TiO2 thin films deposited by RF magnetron sputtering. Appl. Surf. Sci. 2009, 255, 6715–6720. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid. State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Yu, F.; Bai, X.; Yang, C.; Xu, L.; Ma, J. Reduced Graphene Oxide–P25 Nanocomposites as Efficient Photocatalysts for Degradation of Bisphenol A in Water. Catalysts 2019, 9, 607. [Google Scholar] [CrossRef] [Green Version]
- Akhavan, O.; Abdolahad, M.; Esfandiar, A.; Mohatashamifar, M. Photodegradation of graphene oxide sheets by TiO2 nanoparticles after a photocatalytic reduction. J. Phys. Chem. 2010, 114, 12955–12959. [Google Scholar] [CrossRef]
- Radich, J.G.; Krenselewski, A.L.; Zhu, J.; Kamat, P.V. Is graphene a stable platform for photocatalysis? Mineralization of reduced graphene oxide with UV-irradiated TiO2 nanoparticles. Chem. Mater. 2014, 26, 4662–4668. [Google Scholar] [CrossRef]
- Sim, L.C.; Leong, K.H.; Ibrahim, S.; Saravanan, P. Graphene oxide and Ag engulfed TiO2 nanotube arrays for enhanced electron mobility and visible-light-driven photocatalytic performance. J. Mater. Chem. A 2014, 2, 5315–5322. [Google Scholar] [CrossRef]
- Liu, L.; Bai, H.; Liu, J.; Sun, D.D. Multifunctional graphene oxide-TiO2-Ag nanocomposites for high performance water disinfection and decontamination under solar irradiation. J. Hazard. Mater. 2013, 261, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.-P.; Wang, H.-L.; Zhao, D.-Y.; Jiang, W.-F. Preparation and photocatalytic activity of Ag-modified GO-TiO2 mesocrystals under visible light irradiation. Appl. Surf. Sci. 2019, 480, 105–114. [Google Scholar] [CrossRef]
- Alsharaeh, E.H.; Bora, T.; Soliman, A.; Ahmed, F.; Bharath, G.; Ghoniem, M.G.; Abu-Salah, K.M.; Dutta, J. Sol-gel-assisted microwave-derived synthesis of anatase Ag/TiO2/GO nanohybrids toward efficient visible light phenol degradation. Catalysts 2017, 7, 133. [Google Scholar] [CrossRef]
- Savarese, M.; Raucci, U.; Adamo, C.; Netti, P.A.; Ciofinic, I.; Rega, N. Non-radiative decay paths in rhodamines: New theoretical insights. Phys. Chem. Chem. Phys. 2014, 16, 20681–20688. [Google Scholar] [CrossRef]
- Savarese, M.; Raucci, U.; Netti, P.A.; Adamo, C.; Ciofini, I.; Rega, N. Modeling of charge transfer processes to understand photophysical signatures: The case of Rhodamine 110. Chem. Phys. Lett. 2014, 610–611, 148–152. [Google Scholar] [CrossRef]
- Rochkind, M.; Pasternak, S.; Paz, Y. Using Dyes for Evaluating Photocatalytic Properties: A Critical Review. Molecules 2015, 20, 88–110. [Google Scholar] [CrossRef] [Green Version]
- Piwoński, I.; Kądzioła, K.; Kisielewska, A.; Soliwoda, K.; Wolszczak, M.; Lisowska, K.; Wrońska, N.; Felczak, A. The effect of the deposition parameters onsize, distribution and antimicrobial properties of photoinduced silvernanoparticles on titania coatings. Appl. Surf. Sci. 2011, 257, 7076–7082. [Google Scholar] [CrossRef]
- Kądzioła, K.; Piwoński, I.; Kisielewska, A.; Szczukocki, D.; Krawczyk, B.; Sielski, J. The photoactivity of titanium dioxide coatings with silver nanoparticlesprepared by sol–gel and reactive magnetron sputteringmethods—comparative studies. Appl. Surf. Sci. 2014, 288, 503–512. [Google Scholar] [CrossRef]
- Spilarewicz-Stanek, K.; Kisielewska, A.; Ginter, J.; Bałuszyńska, K.; Piwoński, I. Elucidation of the function of oxygen moieties on graphene oxide and reduced graphene oxide in the nucleation and growth of silver nanoparticles. RSC Adv. 2016, 6, 60056–60067. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- PDF 4+ ICDD (International Centre for Diffraction Data) XRD Database References and Notes No. 00-021-1272.
- Ahn, Y.U.; Kim, E.J.; Kim, H.T.; Hahn, S.H. Variation of structural and optical properties of sol-gel TiO2 thin films with catalyst concentration and calcination temperature. Mater. Lett. 2003, 57, 4660–4666. [Google Scholar] [CrossRef]
- Hidaka, H.; Honjo, H.; Horikoshi, S.; Serpone, N. Photoinduced Agn0 cluster deposition Photoreduction of Ag+ ions on a TiO2-coated quartz crystal microbalance monitored in real time. Sens. Actuators B Chem. 2007, 123, 822–828. [Google Scholar] [CrossRef]
- Tran, H.; Scott, J.; Chiang, K.; Amal, R. Clarifying the role of silver deposits on titania for the photocatalytic mineralisation of organic compounds. J. Photochem. Photobiol. A 2006, 183, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Lightcap, I.V.; Murphy, S.; Schumer, T.; Kamat, P.V. Electron hopping through single-to-few-layer graphene oxide films. Side-selective photocatalytic deposition of metal nanoparticles. J. Phys. Chem. Lett. 2012, 3, 1453–1458. [Google Scholar] [CrossRef]
- Chen, X.; Wu, G.; Chen, J.; Chen, X.; Xie, Z.; Wang, X. Synthesis of “Clean” and Well-Dispersive Pd Nanoparticles with Excellent Electrocatalytic Property on Graphene Oxide. J. Am. Chem. Soc. 2011, 133, 3693–3695. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, X.; Qi, X.; Wu, S.; Xue, C.; Boey, F.Y.C.; Yan, Q.; Chen, P.; Zhang, H. In Situ Synthesis of Metal Nanoparticles on Single-Layer Graphene Oxide and Reduced Graphene Oxide Surfaces. J. Phys. Chem. C 2009, 113, 10842–10846. [Google Scholar] [CrossRef]
- Pacakova, B.; Vejpravova, J.; Repko, A.; Mantlikova, A.; Kalbac, M. Formation of wrinkles on graphene induced by nanoparticles: Atomic force microscopy study. Carbon 2015, 95, 573–579. [Google Scholar] [CrossRef] [Green Version]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV−Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Nidheesh, P.V.; Rajan, R. Removal of rhodamine B from a water medium using hydroxyl and sulphate radicals generated by iron loaded activated carbon. RSC Adv. 2016, 6, 5330–5340. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Thomas, M.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Study on UV-LED/TiO2 process for degradation of Rhodamine B dye. Chem. Eng. J. 2011, 169, 126–134. [Google Scholar] [CrossRef]
- Kazemifard, S.; Naji, L.; Taromi, F.A. Enhancing the photovoltaic performance of bulk heterojunction polymer solar cells by adding Rhodamine B laser dye as co-sensitizer. J. Colloid Interface Sci. 2018, 515, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, J.; Eda, T.; Hanaya, M. Comparative study of conduction-band and valence-band edges of TiO2, SrTiO3, and BaTiO3 by ionization potential measurements. Chem. Phys. Lett. 2017, 685, 23–26. [Google Scholar] [CrossRef]
- Tyona, M.D.; Jambure, S.B.; Lokhande, C.D.; Banpurkar, A.G.; Osuji, R.U.; Ezema, F.I. Dye-sensitized solar cells based on Al-doped ZnO photoelectrodes sensitized with rhodamine. Mater. Lett. 2018, 220, 281–284. [Google Scholar] [CrossRef]
- Hsiao, Y.-C.; Wu, T.-F.; Wang, Y.-S.; Hu, C.-C.; Huang, C. Evaluating the sensitizing effect on the photocatalytic decoloration of dyes using anatase-TiO2. Appl. Catal. B 2014, 148–149, 250–257. [Google Scholar] [CrossRef]
- Zaleska-Medyńska, A. Metal Oxide-Based Photocatalysis: Fundamentals and Prospects for Application; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Zhang, L.; Ni, C.; Jiu, H.; Xie, C.; Yan, J.; Qi, G. One-pot synthesis of Ag-TiO2/reduced graphene oxide nanocomposite for high performance of adsorption and photocatalysis. Ceram. Int. 2017, 43, 5450–5456. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Jiu, H.; NI, C.; Zhang, X.; Xu, M. Graphene-based hollow TiO2 composites with enhanced photocatalytic activity for removal of pollutants. J. Phys. Chem. Solids 2015, 86, 82–89. [Google Scholar] [CrossRef]
- Fang, H.; Pan, Y.; Yin, M.; Pan, C. Enhanced photocatalytic activity and mechanism of Ti3C2−OH/Bi2WO6:Yb3+, Tm3+ towards degradation of RhB under visible and near infrared light irradiation. Mater. Res. Bull. 2020, 121, 110618. [Google Scholar] [CrossRef]
- Neelgund, G.G.M.; Oki, A. ZnO conjugated graphene: An efficient sunlight driven photocatalyst for degradation of organic dyes. Mater. Res. Bull. 2020, 129, 110911. [Google Scholar] [CrossRef]
- Kusior, A.; Michalec, K.; Jelen, P.; Radecka, M. Shaped Fe2O3 nanoparticles—Synthesis and enhanced photocatalytic degradation towards RhB. Appl. Surf. Sci. 2019, 476, 342–352. [Google Scholar] [CrossRef]
- Liang, H.; Ji, Z.; Zhang, H.; Wang, X.; Wang, J. Photocatalysis oxidation activity regulation of Ag/TiO2 composites evaluated by the selective oxidation of Rhodamine, B. Appl. Surf. Sci. 2017, 422, 1–10. [Google Scholar] [CrossRef]
- Wu, T.; Liu, G.; Zhao, J.; Hidaka, H.; Serpone, N. Photoassisted degradation of dye pollutants. V. Self-photosensitized oxidative transformation of rhodamine B under visible light Irradiation in aqueous TiO2 dispersions. J. Phys. Chem. B 1998, 102, 5845–5851. [Google Scholar] [CrossRef]
- Cheng, F.; Zhao, J.; Hidaka, H. Highly selective deethylation of rhodamine B: Adsorption and photooxidation pathways of the dye on the TiO2/SiO2 composite photocatalyst. Int. J. Photoenergy 2003, 5, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Takizawa, T.; Honda, K. Photocatalysis through excitation of adsorbates. 1. Highly efficient N-deethylation of rhodamine B adsorbed to cadmium sulfide. J. Phys. Chem. 1977, 81, 1845–1851. [Google Scholar] [CrossRef]
- Merka, O.; Yarovyi, V.; Bahnemann, D.W.; Wark, M. pH-Control of the photocatalytic degradation mechanism of rhodamine B over Pb3Nb4O13. J. Phys. Chem. C 2011, 115, 8014–8023. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Dhodamani, A.G.; Suzuki, N.; Terashima, C.; Fujishima, A.; Mathe, V.L. Enhanced photocatalytic performance of ultrasound treated GO/TiO2 composite for photocatalytic degradation of salicylic acid under sunlight illumination. Ultrason Sonochem 2020, 61, 104849. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2—graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ASC Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef]
- Chen, W.; Yuan, J.; Jiang, Z.; Hu, G.; Shangguan, W.; Sun, Y.; Su, J. Controllable O2•− oxidization graphene in TiO2/graphene composite and its effect on photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2013, 38, 13110–13116. [Google Scholar]
- Yang, J.; Zhu, H.; Peng, Y.; Li, P.; Chen, S.; Yang, B.; Zhang, J. Photocatalytic Performance and Degradation Pathway of Rhodamine B with TS-1/C3N4 Composite under Visible Light. Nanomaterials 2020, 10, 756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, M.; Yu, H.; Zuo, Y.; Gao, J.; He, G.; Sun, Z. Facile fabrication of Ag/graphene oxide/TiO2 nanorod array as a powerful substrate for photocatalytic degradation and surface-enhanced Raman scattering detection. Appl. Catal. B 2019, 252, 174–186. [Google Scholar] [CrossRef]











| Type of Coating | Synthesis Conditions | Area | Average Size [nm] | Maximal Diameter [nm] | Surface Density [AgNSs/µm2] |
|---|---|---|---|---|---|
| TiO2-AgNSs | under UV | on TiO2 | 18 ± 3 | 37 | 55 |
| TiO2-AgNSs-GO | under UV | on TiO2 | 18 ± 3 | 37 | 55 |
| under GO | 18 ± 3 | 37 | 55 | ||
| TiO2-GO-AgNSs | under UV | on TiO2 | 19 ± 3 | 39 | 20 |
| on GO | 26 ± 4 | 66 | 35 | ||
| TiO2-(GO-AgNSs) | darkroom | on TiO2 | - | - | - |
| on GO | 19 ± 3 | 53 | 3 |
| Type of Coating | UV | Vis | |
|---|---|---|---|
| k’ [min−1] * | k’ [min−1] * | Wc [%] | |
| TiO2 | 0.0204 | 0.0088 | 65 |
| TiO2-GO | 0.0256 | 0.0095 | 53 |
| TiO2-AgNSs | 0.0352 | 0.0047 | 67 |
| TiO2-AgNSs-GO | 0.0405 | 0.0082 | 60 |
| TiO2-GO-AgNSs | 0.0309 | 0.0053 | 55 |
| TiO2-(GO-AgNSs) | 0.0331 | 0.0102 | 52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spilarewicz-Stanek, K.; Jakimińska, A.; Kisielewska, A.; Batory, D.; Piwoński, I. Understanding the Role of Silver Nanostructures and Graphene Oxide Applied as Surface Modification of TiO2 in Photocatalytic Transformations of Rhodamine B under UV and Vis Irradiation. Materials 2020, 13, 4653. https://doi.org/10.3390/ma13204653
Spilarewicz-Stanek K, Jakimińska A, Kisielewska A, Batory D, Piwoński I. Understanding the Role of Silver Nanostructures and Graphene Oxide Applied as Surface Modification of TiO2 in Photocatalytic Transformations of Rhodamine B under UV and Vis Irradiation. Materials. 2020; 13(20):4653. https://doi.org/10.3390/ma13204653
Chicago/Turabian StyleSpilarewicz-Stanek, Kaja, Anna Jakimińska, Aneta Kisielewska, Damian Batory, and Ireneusz Piwoński. 2020. "Understanding the Role of Silver Nanostructures and Graphene Oxide Applied as Surface Modification of TiO2 in Photocatalytic Transformations of Rhodamine B under UV and Vis Irradiation" Materials 13, no. 20: 4653. https://doi.org/10.3390/ma13204653
APA StyleSpilarewicz-Stanek, K., Jakimińska, A., Kisielewska, A., Batory, D., & Piwoński, I. (2020). Understanding the Role of Silver Nanostructures and Graphene Oxide Applied as Surface Modification of TiO2 in Photocatalytic Transformations of Rhodamine B under UV and Vis Irradiation. Materials, 13(20), 4653. https://doi.org/10.3390/ma13204653







