CO2 Uptake and Physicochemical Properties of Carbonation-Cured Ternary Blend Portland Cement–Metakaolin–Limestone Pastes
Abstract
1. Introduction
2. Experimental Program
2.1. Materials and Sample Preparation
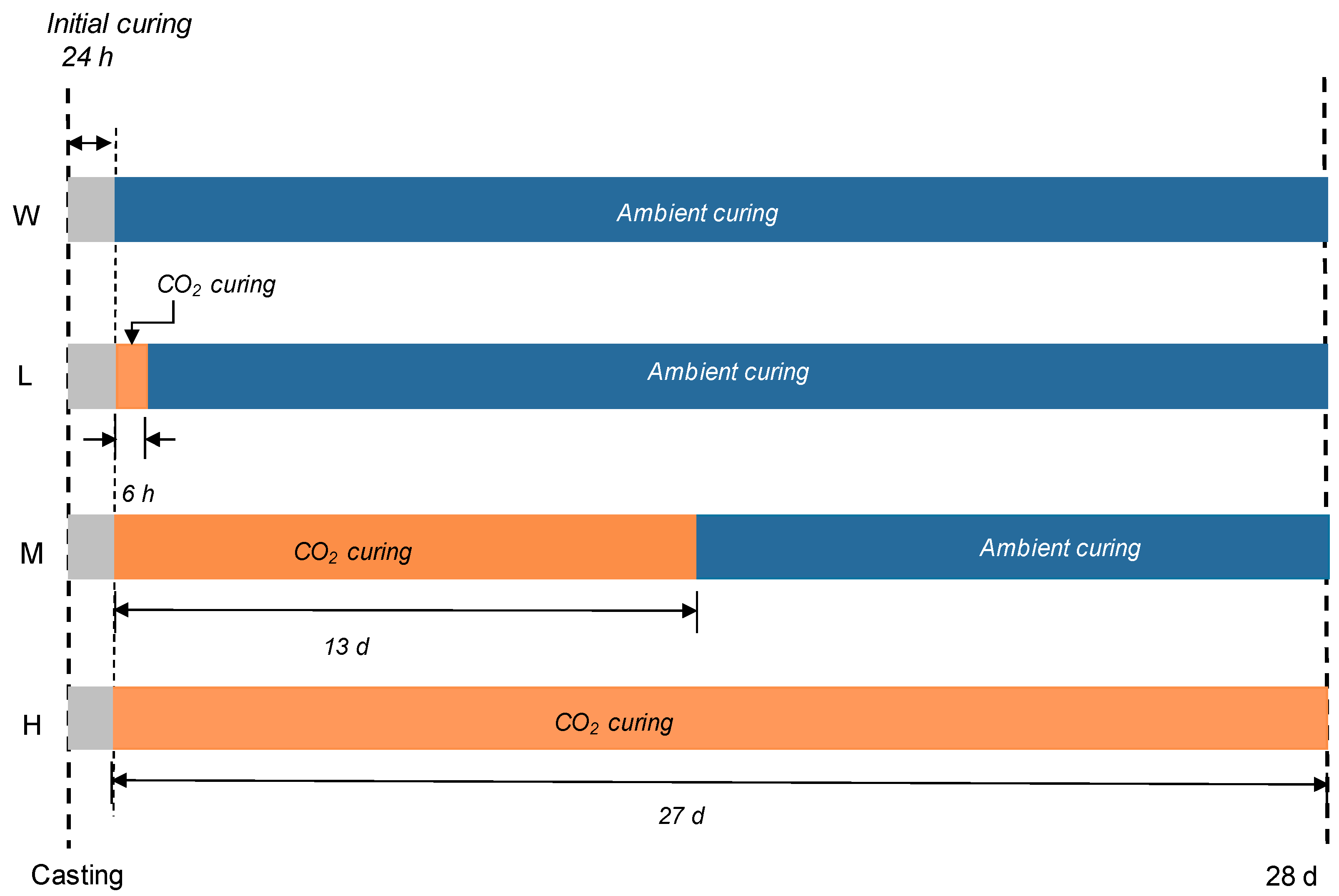
2.2. Curing Conditions and Test Methods
3. Results and Discussion
3.1. Compressive Strength
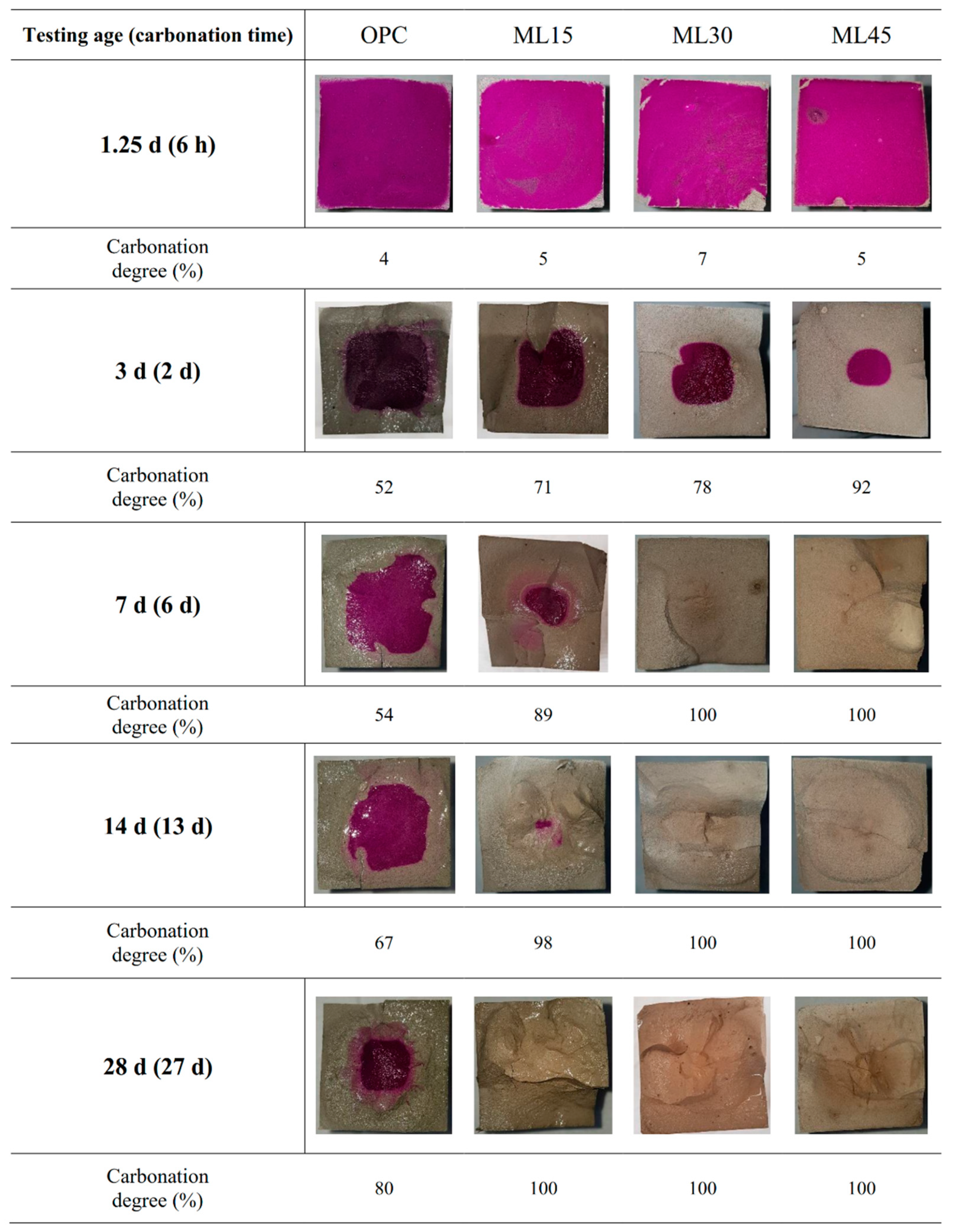
3.2. Carbonation Degree and pH Variation
3.3. Phase Identification by X-Ray Diffractometry
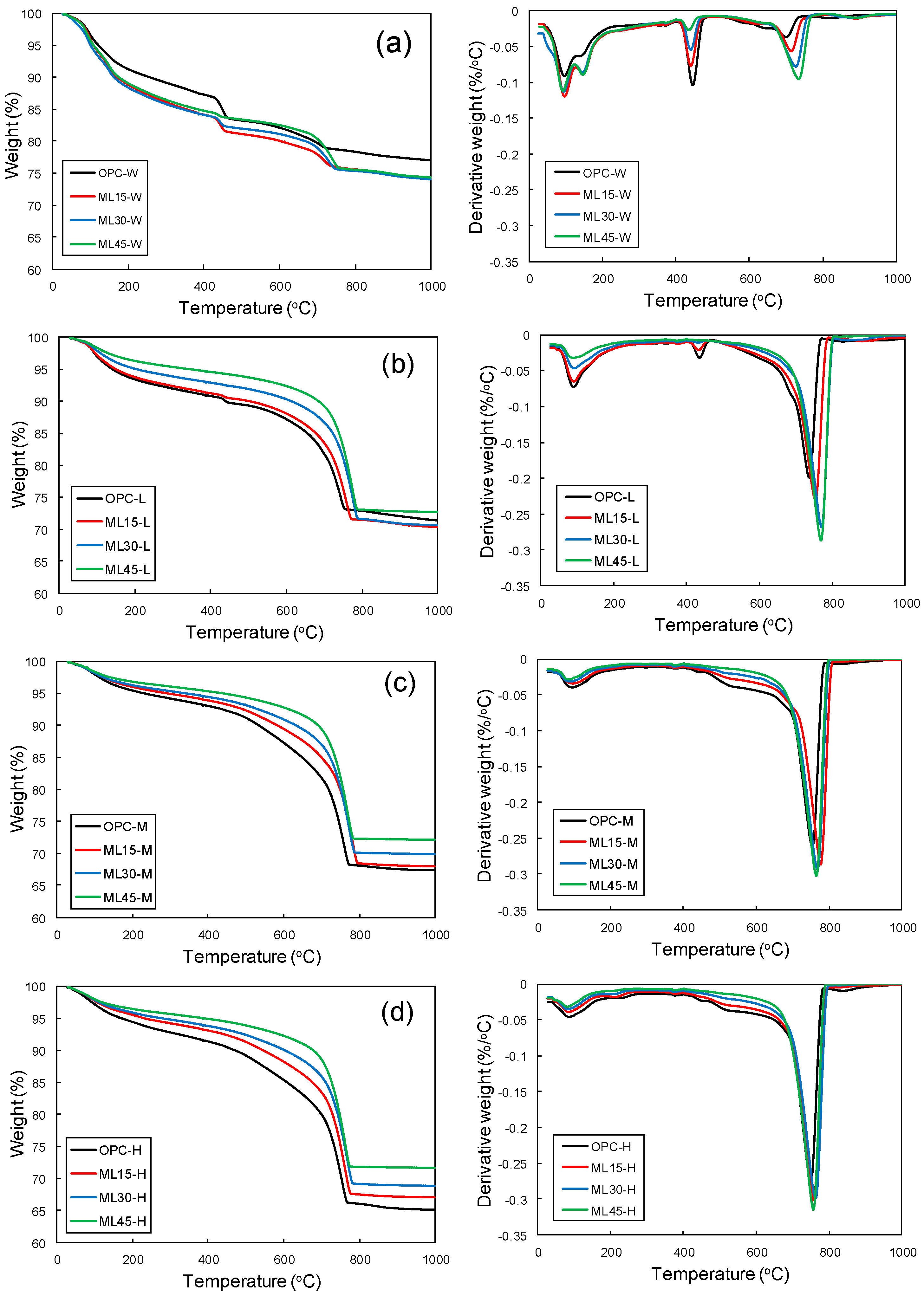
3.4. CO2 Uptake by Thermogravimetric Analysis
3.5. Volume of Permeable Voids and Sorptivity
4. Conclusions
- (1)
- The compressive strength of the blended samples exhibited a reduction in the strength as compared with that of the OPC sample. The loss of the compressive strength was increased as the substitution level increased from 15% to 45%. In addition, an increase in the duration of carbonation from 6 h to 27 d resulted in the significant loss of strength levels. The blends with a high substitution and longer exposure duration to CO2 experienced significant changes in mechanical strength.
- (2)
- Blended samples showed higher rates of carbonation than the OPC samples at all carbonation curing ages. At 27 d of carbonation curing, OPC sample showed carbonation degree of 80%, while the ML30 and ML45 samples exhibited complete carbonation even at 6 d of carbonation. Carbonation degree was governed both by carbonation duration and by cement replacement level.
- (3)
- The XRD and TGA analyses showed the consumption of portlandite upon carbonation, which was proportional with the carbonation-curing duration. Upon carbonation, the main phases observed were CaCO3 polymorphs.
- (4)
- The replacement of the PC by metakaolin and limestone vastly improved the CO2 uptake capacity, showing environmental benefits. The increase in the CO2 uptake of the ML45 samples with respect to the OPC samples was 54%, 42%, and 50% for L-, M-, and H-series, respectively.
- (5)
- An increase in the volume of permeable voids was observed upon exposure to CO2 for the blended samples due to reduced portlandite amount which promoted carbonation of C-S-H which ultimately coarsens the porosity. The ML45-H sample showed volume of permeable voids of 38.6% which is 18.4% higher than that of the OPC-W sample.
Author Contributions
Funding
Conflicts of Interest
References
- Shanks, W.; Dunant, C.F.; Drewniok, M.P.; Lupton, R.C.; Serrenho, A.; Allwood, J.M. How much cement can we do without? Lessons from cement material flows in the UK. Resour. Conserv. Recycl. 2019, 141, 441–454. [Google Scholar] [CrossRef]
- Kajaste, R.; Hurme, M. Cement industry greenhouse gas emissions—Management options and abatement cost. J. Clean. Prod. 2016, 112, 4041–4052. [Google Scholar] [CrossRef]
- Park, S.M.; Seo, J.H.; Lee, H.K. Thermal evolution of hydrates in carbonation-cured Portland cement. Mater. Struct. 2018, 51, 7. [Google Scholar] [CrossRef]
- Kaliyavaradhan, S.K.; Ling, T.-C. Potential of CO2 sequestration through construction and demolition (C & D) waste—An overview. J. CO2 Util. 2017, 20, 234–242. [Google Scholar]
- Earth’s CO2 Homepage. Available online: https://www.co2.earth/ (accessed on 20 April 2020).
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Snellings, R.; Bernal, S.A. Supplementary cementitious materials: New sources, characterization, and performance insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Gartner, E.; Sui, T. Alternative cement clinkers. Cem. Concr. Res. 2018, 114, 27–39. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S.J. Alkali Activated Materials: State-of-the-Art Reports, RILEM TC 224-AAM; Springer/RILEM: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Zhang, D.; Ghouleh, Z.; Shao, Y. Review on carbonation curing of cement-based materials. J. CO2 Util. 2017, 21, 119–131. [Google Scholar] [CrossRef]
- Jang, J.G.; Kim, G.M.; Kim, H.J.; Lee, H.K. Review on recent advances in CO2 utilization and sequestration technologies in cement-based materials. Constr. Build. Mater. 2016, 127, 762–773. [Google Scholar] [CrossRef]
- Seo, J.H.; Park, S.M.; Lee, H.K. Evolution of the binder gel in carbonation-cured Portland cement in an acidic medium. Cem. Concr. Res. 2018, 109, 81–89. [Google Scholar] [CrossRef]
- Berger, R.L.; Young, J.F.; Leung, K. Acceleration of hydration of calcium silicates by carbon dioxide treatment. Nat. Phys. Sci. 1972, 240, 16–18. [Google Scholar] [CrossRef]
- Young, J.F.; Berger, R.L.; Breese, J. Accelerated Curing of Compacted Calcium Silicate Mortars on Exposure to CO2. J. Am. Ceram. Soc. 1974, 394–397. [Google Scholar] [CrossRef]
- Jang, J.G.; Lee, H.K. Microstructural densification and CO2 uptake promoted by the carbonation curing of belite-rich Portland cement. Cem. Concr. Res. 2016, 82, 50–57. [Google Scholar] [CrossRef]
- Zhang, D.; Asce, S.M.; Cai, X.; Shao, Y. Carbonation Curing of Precast Fly Ash Concrete. J. Mater. Civ. Eng. 2016, 28, 1–9. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Early age carbonation curing for precast reinforced concretes. Constr. Build. Mater. 2016, 113, 134–143. [Google Scholar] [CrossRef]
- Boumaaza, M.; Huet, B.; Turcry, P.; Aït-Mokhtar, A. The CO2-binding capacity of synthetic anhydrous and hydrates: Validation of a test method based on the instantaneous reaction rate. Cem. Concr. Res. 2020, 135, 106113. [Google Scholar] [CrossRef]
- Snellings, R. Assessing, Understanding and Unlocking Supplementary Cementitious Materials. RILEM Tech. Lett. 2016, 1, 50. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Ramezanianpour, A.A.; Bahrami Jovein, H. Influence of metakaolin as supplementary cementing material on strength and durability of concretes. Constr. Build. Mater. 2012, 30, 470–479. [Google Scholar] [CrossRef]
- Sabir, B.; Wild, S.; Bai, J. Metakaolin and calcined clays as pozzolans for concrete: A review. Cem. Concr. Compos. 2001, 23, 441–454. [Google Scholar] [CrossRef]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K. Cement substitution by a combination of metakaolin and limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Lothenbach, B.; Le Saout, G.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Mohamed, A.R.; Elsalamawy, M.; Ragab, M. Modeling the influence of limestone addition on cement hydration. Alexandria Eng. J. 2015, 54, 1–5. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Khan, M.S.H.; Castel, A. Engineering Properties of Limestone Calcined Clay Concrete. J. Adv. Concr. Technol. 2018, 16, 343–357. [Google Scholar] [CrossRef]
- Ferreiro, S.; Herfort, D.; Damtoft, J.S. Effect of raw clay type, fineness, water-to-cement ratio and fly ash addition on workability and strength performance of calcined clay – Limestone Portland cements. Cem. Concr. Res. 2017, 101, 1–12. [Google Scholar] [CrossRef]
- Dhandapani, Y.; Santhanam, M. Assessment of pore structure evolution in the limestone calcined clay cementitious system and its implications for performance. Cem. Concr. Compos. 2017, 84, 36–47. [Google Scholar] [CrossRef]
- Dhandapani, Y.; Sakthivel, T.; Santhanam, M.; Gettu, R.; Pillai, R.G. Mechanical properties and durability performance of concretes with Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 136–151. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Castel, A. Reinforcement corrosion in limestone flash calcined clay cement-based concrete. Cem. Concr. Res. 2020, 132, 106051. [Google Scholar] [CrossRef]
- Shi, Z.; Lothenbach, B.; Geiker, M.R.; Kaufmann, J.; Leemann, A.; Ferreiro, S.; Skibsted, J. Experimental studies and thermodynamic modeling of the carbonation of Portland cement, metakaolin and limestone mortars. Cem. Concr. Res. 2016, 88, 60–72. [Google Scholar] [CrossRef]
- Vizcaíno-Andrés, L.M.; Sánchez-Berriel, S.; Damas-Carrera, S.; Pérez-Hernández, A.; Scrivener, K.L.; Martirena-Hernández, J.F. Industrial trial to produce a low clinker, low carbon cement. Mater. Construcción 2015, 65, e045. [Google Scholar]
- Bishnoi, S.; Maity, S.; Mallik, A.; Joseph, S.; Krishnan, S. Pilot scale manufacture of limestone calcined clay cement: The Indian experience. Indian Concr. J. 2014, 88, 22–28. [Google Scholar]
- Emmanuel, A.C.; Haldar, P.; Maity, S.; Bishnoi, S. Second pilot production of limestone calcined clay cement in India: The experience. Indian Concr. J. 2016, 90, 57–63. [Google Scholar]
- Huang, H.; Guo, R.; Wang, T.; Hu, X.; Garcia, S.; Fang, M.; Luo, Z.; Maroto-Valer, M.M. Carbonation curing for wollastonite-Portland cementitious materials: CO2 sequestration potential and feasibility assessment. J. Clean. Prod. 2019, 211, 830–841. [Google Scholar] [CrossRef]
- Sharma, D.; Goyal, S. Accelerated carbonation curing of cement mortars containing cement kiln dust: An effective way of CO2 sequestration and carbon footprint reduction. J. Clean. Prod. 2018, 192, 844–854. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X.; Chen, T. Influence of mineral admixtures on carbonation curing of cement paste. Constr. Build. Mater. 2019, 212, 653–662. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. ASTM C109/109M-20a: Standard Test Method for Compressive strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens); ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- Siddique, S.; Naqi, A.; Jang, J.G. Influence of water to cement ratio on CO2 uptake capacity of belite-rich cement upon exposure to carbonation curing. Cem. Concr. Compos. 2020, 111, 103616. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials, 4th ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- American Society for Testing and Materials. ASTM C1585-13, Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic-Cement Concretes; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- American Society for Testing and Materials. ASTM C642-13, Standard Test Method for Density, Absorption, and Voids in Hardened Concrete; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Chen, T.; Gao, X. Effect of carbonation curing regime on strength and microstructure of Portland cement paste. J. CO2 Util. 2019, 34, 74–86. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials, 4th ed.; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Ben Haha, M.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Silva, D.A.; Roman, H.R.; Gleize, P.J.P. Evidences of chemical interaction between EVA and hydrating Portland cement. Cem. Concr. Res. 2002, 32, 1383–1390. [Google Scholar] [CrossRef]
- Jeong, Y.; Park, H.; Jun, Y.; Jeong, J.-H.; Oh, J.E. Microstructural verification of the strength performance of ternary blended cement systems with high volumes of fly ash and GGBFS. Constr. Build. Mater. 2015, 95, 96–107. [Google Scholar] [CrossRef]
- Borges, P.H.R.; Costa, J.O.; Milestone, N.B.; Lynsdale, C.J.; Streatfield, R.E. Carbonation of CH and C-S-H in composite cement pastes containing high amounts of BFS. Cem. Concr. Res. 2010, 40, 284–292. [Google Scholar] [CrossRef]
- Tu, Z.; Guo, M.Z.; Poon, C.S.; Shi, C. Effects of limestone powder on CaCO3 precipitation in CO2 cured cement pastes. Cem. Concr. Compos. 2016, 72, 9–16. [Google Scholar] [CrossRef]



| wt % | Portland Cement | Metakaolin | Limestone |
|---|---|---|---|
| CaO | 62.50 | 0.92 | 99.20 |
| SiO2 | 21.00 | 50.10 | 0.08 |
| Al2O3 | 5.90 | 38.40 | 0.01 |
| Fe2O3 | 3.20 | 5.69 | 0.03 |
| MgO | 0.11 | 0.11 | 0.28 |
| R2O | 0.80 | 0.62 | 0.01 |
| SO3 | 2.10 | 0.05 | 0.01 |
| TiO2 | 0.38 | 3.45 | - |
| P2O3 | 0.14 | 0.09 | 0.01 |
| Mn2O5 | 0.10 | 0.01 | - |
| SrO | 0.15 | 0.06 | 0.23 |
| Portland Cement | |
|---|---|
| Fineness | 3450 cm2/g |
| Initial setting time | 225 min |
| Final setting time | 345 min |
| Density | 3.14 g/cm2 |
| Standard compressive strength development | |
| 3 day | 15.6 MPa |
| 7 day | 25.2 MPa |
| 28 day | 51.2 MPa |
| Sample ID | Portland Cement | Metakaolin | Limestone | Water/Powder 1 Ratio |
|---|---|---|---|---|
| OPC | 1 | 0.00 | 0.00 | 0.5 |
| ML15 | 0.85 | 0.10 | 0.05 | 0.5 |
| ML30 | 0.70 | 0.20 | 0.10 | 0.5 |
| ML45 | 0.55 | 0.30 | 0.15 | 0.5 |
| Sample ID | CO2 Uptake (g/100g of Powder 1) | ||
|---|---|---|---|
| L-Series | M-Series | H-Series | |
| OPC | 11.3 | 13.7 | 13.8 |
| ML15 | 16.4 | 19.1 | 20.1 |
| ML30 | 16.6 | 19.3 | 20.1 |
| ML45 | 17.4 | 19.4 | 20.7 |
| Sample ID | Volume of Permeable Voids (%) | Initial Sorptivity Coefficient (×10−3 mm/s1/2) | Secondary Sorptivity Coefficient (×10−3 mm/s1/2) |
|---|---|---|---|
| OPC-W | 32.6 | 9.5 | 0.12 |
| ML15-W | 33.7 | 8.8 | 0.11 |
| ML30-W | 34.8 | 6.5 | 0.13 |
| ML45-W | 35.2 | 6.1 | 0.14 |
| OPC-L | 30.4 | 8.3 | 0.10 |
| ML15-L | 32.1 | 7.9 | 0.11 |
| ML30-L | 35.9 | 8.5 | 0.13 |
| ML45-L | 36.3 | 9.4 | 0.15 |
| OPC-M | 27.4 | 7.3 | 0.10 |
| ML15-M | 31.0 | 7.8 | 0.13 |
| ML30-M | 34.6 | 11.1 | 0.17 |
| ML45-M | 38.6 | 12.3 | 0.18 |
| OPC-H | 24.9 | 6.8 | 0.11 |
| ML15-H | 30.4 | 7.7 | 0.13 |
| ML30-H | 35.1 | 10.6 | 0.17 |
| ML45-H | 38.6 | 11.8 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hameed, R.; Seo, J.; Park, S.; Amr, I.T.; Lee, H.K. CO2 Uptake and Physicochemical Properties of Carbonation-Cured Ternary Blend Portland Cement–Metakaolin–Limestone Pastes. Materials 2020, 13, 4656. https://doi.org/10.3390/ma13204656
Hameed R, Seo J, Park S, Amr IT, Lee HK. CO2 Uptake and Physicochemical Properties of Carbonation-Cured Ternary Blend Portland Cement–Metakaolin–Limestone Pastes. Materials. 2020; 13(20):4656. https://doi.org/10.3390/ma13204656
Chicago/Turabian StyleHameed, Rizwan, Joonho Seo, Solmoi Park, Issam T. Amr, and H.K. Lee. 2020. "CO2 Uptake and Physicochemical Properties of Carbonation-Cured Ternary Blend Portland Cement–Metakaolin–Limestone Pastes" Materials 13, no. 20: 4656. https://doi.org/10.3390/ma13204656
APA StyleHameed, R., Seo, J., Park, S., Amr, I. T., & Lee, H. K. (2020). CO2 Uptake and Physicochemical Properties of Carbonation-Cured Ternary Blend Portland Cement–Metakaolin–Limestone Pastes. Materials, 13(20), 4656. https://doi.org/10.3390/ma13204656





