A Critical Review on the Properties and Applications of Sulfur-Based Concrete
Abstract
:1. Introduction
2. Source of Sulfur
2.1. Natural Sources of Sulfur
2.2. Man-Made Sources of Sulfur
2.2.1. Natural Gas
2.2.2. Petroleum
2.2.3. Oil Sands
2.2.4. Sulfide Smelting
3. Production of Sulfur
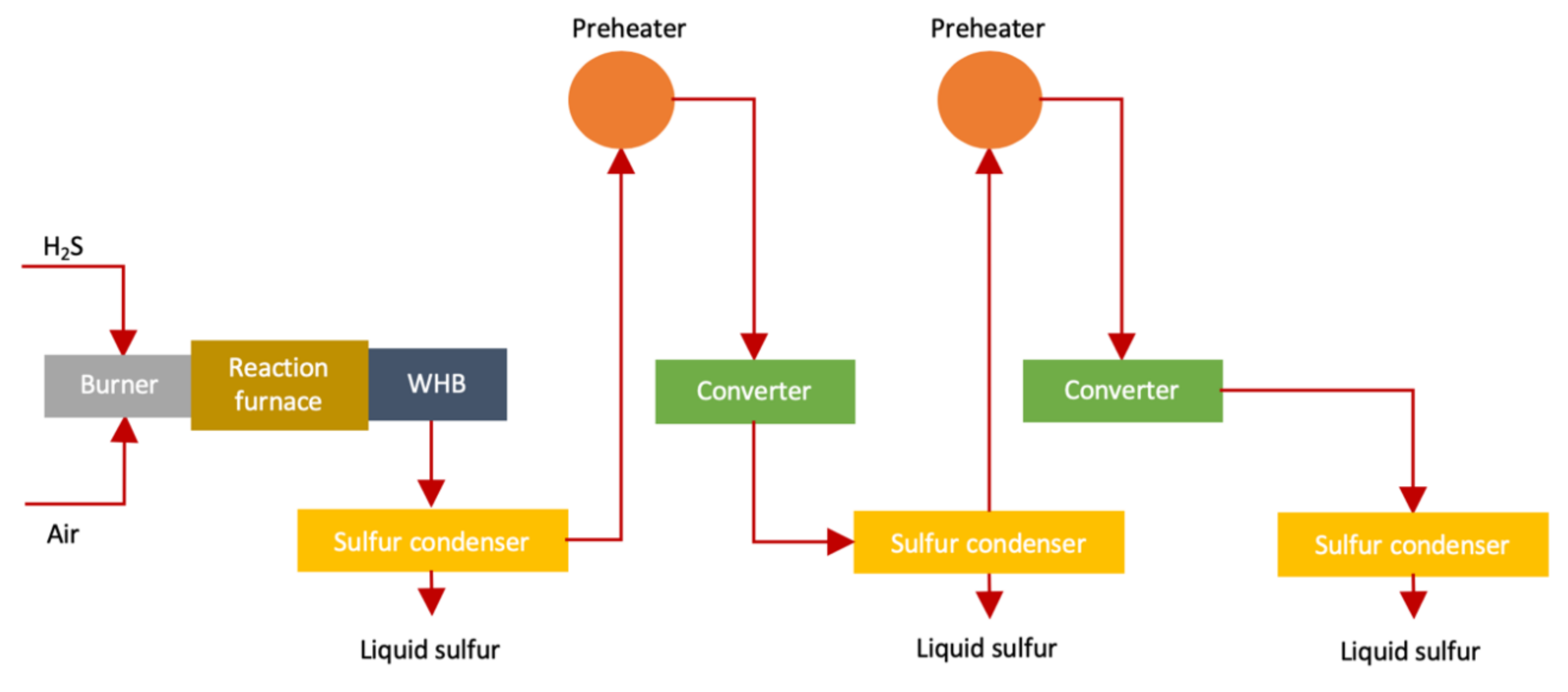
3.1. Claus Process
3.2. Frasch Mining
- Huge, porous and rich sulfur deposits.
- Impermeable covering rock above the deposit.
- Reliable and sufficient water supply.
- Cost effective source of fuel required to heat large water quantities needed to melt the sulfur deposit and to provide enough power required for proper functioning of energy-consuming machineries of the process.
4. Properties of Sulfur
4.1. Melting/Freezing Point
4.2. Viscosity
4.3. Density
4.4. Color
4.5. Thermal Conductivity
4.6. Strength
5. Sulfur in the Concrete Industry
5.1. Composition and Mixing of Sulfur-based Concrete
- To enhance acid and salt resistance and reduce moisture absorption.
- To maintain (enhance) mechanical properties of sulfur-based concrete as per conventional concrete, maintain workability and minimize drying shrinkage after hardening.
5.2. Sulfur Emissions from Construction Enterprises
- The use of vertical grinding units and the passage of waste gases through the mill for heat recovery and to reduce the sulfur content in the gas. In a mill, a gas containing SO2 is mixed with calcium carbonate from raw materials and forms calcium sulfate (gypsum) [97].
- The use of wet or dry scrubbers. Dry gas cleaning is more expensive, so this method is used less frequently than wet gas cleaning and is usually used when sulfur dioxide emissions can exceed 1500 mg/m3 [100].
- Emissions of sulfur dioxide in the production of lime are usually lower than in the production of cement, due to the lower sulfur content in raw materials. The followings are recommended ways to reduce emissions of sulfur dioxide:
- Selection of low volatile quarry materials [101].
5.3. Modified Sulfur-based Concrete
5.3.1. The Microstructure of Various Sulfur Modified Composites
5.3.2. Melt Viscosity and Mixture Mobility
5.3.3. Hardened Properties
5.3.4. Durability Properties
5.3.5. Deformative properties
6. Applications of Sulfur in Concrete
7. Future Works
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ober, J.A. Materials Flow of Sulfur; U.S. Geological Surevey: Reston, VA, USA, 2002; pp. 1258–2331. [Google Scholar]
- Fediuk, R.; Yevdokimova, Y.G.; Smoliakov, A.; Stoyushko, N.Y.; Lesovik, V. Use of geonics scientific positions for designing of building composites for protective (fortification) structures. In IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2017; p. 012011. [Google Scholar]
- Vlahovic, M.M.; Martinovic, S.P.; Boljanac, T.D.; Jovanic, P.B.; Volkov-Husovic, T.D. Durability of sulfur concrete in various aggressive environments. Constr. Build. Mater. 2011, 25, 3926–3934. [Google Scholar] [CrossRef]
- Fontana, J.J.; Farrell, L.J.; Alexanderson, J.; Ball, H.P., Jr.; Bartholomew, J.J.; Biswas, M.; Bolton, D.J.; Carter, P.D.; Chrysogelos, J., Jr.; Clapp, T.R.; et al. Guide for Mixing and Placing Sulfur Concrete in Construction; ACI: Farmington Hills, MI, USA, 1988. [Google Scholar]
- Mohamed, A.-M.O.; El Gamal, M. Hydro-mechanical behavior of a newly developed sulfur polymer concrete. Cem. Concr. Compos. 2009, 31, 186–194. [Google Scholar] [CrossRef]
- Dehestani, M.; Teimortashlu, E.; Molaei, M.; Ghomian, M.; Firoozi, S.; Aghili, S. Experimental data on compressive strength and durability of sulfur concrete modified by styrene and bitumen. Data Brief 2017, 13, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Al-Otaibi, S.; Al-Aibani, A.; Al-Bahar, S.; Abdulsalam, M.; Al-Fadala, S. Potential for producing concrete blocks using sulphur polymeric concrete in Kuwait. J. King Saud Univ. Eng. Sci. 2019, 31, 327–331. [Google Scholar] [CrossRef]
- Yang, C.; Lv, X.; Tian, X.; Wang, Y.; Komarneni, S. An investigation on the use of electrolytic manganese residue as filler in sulfur concrete. Constr. Build. Mater. 2014, 73, 305–310. [Google Scholar] [CrossRef]
- El Gamal, M.M.; El-Dieb, A.S.; Mohamed, A.-M.O.; El Sawy, K.M. Performance of modified sulfur concrete exposed to actual sewerage environment with variable temperature, humidity and gases. J. Build. Eng. 2017, 11, 1–8. [Google Scholar] [CrossRef]
- Toutanji, H.A.; Evans, S.; Grugel, R.N. Performance of lunar sulfur concrete in lunar environments. Constr. Build. Mater. 2012, 29, 444–448. [Google Scholar] [CrossRef]
- Szajerski, P.; Bogobowicz, A.; Bem, H.; Gasiorowski, A. Quantitative evaluation and leaching behavior of cobalt immobilized in sulfur polymer concrete composites based on lignite fly ash, slag and phosphogypsum. J. Clean. Prod. 2019, 222, 90–102. [Google Scholar] [CrossRef]
- Shin, M.; Kim, K.; Gwon, S.-W.; Cha, S. Durability of sustainable sulfur concrete with fly ash and recycled aggregate against chemical and weathering environments. Constr. Build. Mater. 2014, 69, 167–176. [Google Scholar] [CrossRef]
- Thackray, M. Melting point intervals of sulfur allotropes. J. Chem. Eng. Data 1970, 15, 495–497. [Google Scholar] [CrossRef]
- Khademi, A.G.; Sar, H.I.K. Comparison of sulfur concrete, cement concrete and cement-sulfur concrete and their properties and application. Curr. World Environ. 2015, 10, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Burwell, J.Τ. The Unit Cell and Space Group of Monoclinic Sulphur. Z. Krist. Cryst. Mater. 1937, 97, 123–124. [Google Scholar] [CrossRef]
- Loov, R.E.; Vroom, A.H.; Ward, M.A. Sulfur concrete-a new construction material. PCI J. 1974, 5, 86–95. [Google Scholar] [CrossRef]
- Leutner, B.; Diehl, L. Manufacture of Sulfur Concrete. U.S. Patent No. 4,025,352, 24 May 1977. [Google Scholar]
- Gregor, R.; Hackl, A. A New Approach to Sulfur Concrete; ACS Publications: Washington, DC, USA, 1978. [Google Scholar]
- Sullivan, T.A.; McBee, W.C. Development and Testing of Superior Sulfur Concretes; US Department of the Interior, Bureau of Mines: Washington, DC, USA, 1976; Volume 8160. [Google Scholar]
- Beaudoin, J.J.; Sereda, P.J. Use of compacts to study the mechanical properties of sulfur. Powder Technol. 1975, 13, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Mehta, H.C.; Chen, W.-F. Structural Use of Sulfur for Impregnation of Building Materials; Fritz Engineering Laboratory, Lehigh University: Bethlehem, PA, USA, 1974. [Google Scholar]
- Kesicki, F. The third oil price surge–What’s different this time? Energy Policy 2010, 38, 1596–1606. [Google Scholar] [CrossRef]
- Jones, D.S.; Pujadó, P.P. Handbook of Petroleum Processing; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Epstein, P.R.; Selber, J.; Borasin, S.; Foster, S.; Jobarteh, K.; Link, N.; Miranda, J.; Pomeranse, E.; Rabke-Verani, J.; Reyes, D. A Life Cycle Analysis of Its Health and Environmental Impacts; The Center for Health and the Global Environment, Harvard Medical School, EUA, Marzo: Bostona, MA, USA, 2002. [Google Scholar]
- Skalny, J. Concrete Durability, A Multibillion-Dollar Opportunity: Report of the Committee on the Concrete Durability: Needs and Opportunities; National Materials Advisory Board: Washington, DC, USA, 1987. [Google Scholar]
- Al-Tayyib, A.-H.J.; Tewfik, M.F.; Khan, M.S. Strength and durability of sulfur mortar. J. Mater. Civ. Eng. 1991, 3, 154–164. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, C.; Li, B.; Wang, J.; Ravat, R.; Chen, X.; Wei, J.; Yu, Q. Linking the SO2 emission of cement plants to the sulfur characteristics of their limestones: A study of 80 NSP cement lines in China. J. Clean. Prod. 2019, 220, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Gaysin, V.V.; Porfiryeva, R.T.; Akhmetov, T.G. Surface modification of silica-containing materials. In Proceedings of the 15th International Congress of Chemical Engineering CHISA, Praha, Czech Republic, 25–29 August 2002. [Google Scholar]
- Paparozzi, E.T.; Darrow, P.O.; McCallister, D.E.; Stroup, W.W. Effect of varying the nitrogen and sulfur supply on the flowering of poinsettia. J. Plant Nutr. 1994, 17, 593–606. [Google Scholar] [CrossRef]
- Goar, B. Sulfur Recovery Technology; American Institute of Chemical Engineers: New York, NY, USA, 1986. [Google Scholar]
- Howarth, R.W.; Stewart, J.; Ivanov, M.V. Sulphur Cycling on the Continents: Wetlands, Terrestrial Ecosystems and Associated Water Bodies; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1992. [Google Scholar]
- Soleimani, M.; Bassi, A.; Margaritis, A. Biodesulfurization of refractory organic sulfur compounds in fossil fuels. Biotechnol. Adv. 2007, 25, 570–596. [Google Scholar] [CrossRef]
- Rappold, T.; Lackner, K. Large scale disposal of waste sulfur: From sulfide fuels to sulfate sequestration. Energy 2010, 35, 1368–1380. [Google Scholar] [CrossRef]
- Arndt, R.L.; Carmichael, G.R.; Streets, D.G.; Bhatti, N. Sulfur dioxide emissions and sectorial contributions to sulfur deposition in Asia. Atmos. Environ. 1997, 31, 1553–1572. [Google Scholar] [CrossRef]
- Darnell, G. Sulfur Polymer Cement, a New Stabilization Agent for Mixed and Low-Level Radioactive Waste; EG and G Idaho, Inc.: Idaho Falls, ID, USA, 1991. [Google Scholar]
- Mohamed, A.-M.O.; El-Gamal, M. Sulfur Concrete for the Construction Industry: A Sustainable Development Approach; J. Ross Publishing: New York, NY, USA, 2010. [Google Scholar]
- Fediuk, R.S. Mechanical Activation of Construction Binder Materials by Various Mills. In IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2016; Volume 125, p. 012019. [Google Scholar]
- Lu, J.-G.; Zheng, Y.-F.; He, D.-L. Selective absorption of H2S from gas mixtures into aqueous solutions of blended amines of methyldiethanolamine and 2-tertiarybutylamino-2-ethoxyethanol in a packed column. Sep. Purif. Technol. 2006, 52, 209–217. [Google Scholar] [CrossRef]
- Speight, J.G.; Ozum, B. Petroleum Refining Processes; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Demirbas, A.; Alidrisi, H.; Balubaid, M. API gravity, sulfur content, and desulfurization of crude oil. Pet. Sci. Technol. 2015, 33, 93–101. [Google Scholar] [CrossRef]
- Taylor, G. Oil Sands Development and Acid Rain in Alberta. Alternatives 1981, 9, 3–10. [Google Scholar]
- Clark, P.D.; Hyne, J.B.; Tyrer, J.D. Some chemistry of organosulphur compound types occurring in heavy oil sands: 2. Influence of pH on the high temperature hydrolysis of tetrahydrothiophene and thiophene. Fuel 1984, 63, 125–128. [Google Scholar] [CrossRef]
- Korea, N. 2006 Minerals Yearbook; US Geological Survey: Reston, VA, USA, 2007. [Google Scholar]
- Le, K. A Digital Home for Athabasca’s Images: Reading Illegible Oil Sands Territories. Master’s Thesis, Carleton University, Ottawa, ON, Canada, 2019. [Google Scholar]
- Taylor, L.; Brown, T.; Lusty, P.; Hitchen, K.; Colman, T.; Highley, D. United Kingdom Minerals Yearbook 2005: Statistical Data to 2004; British Geological Survey: Keyworth, UK, 2006. [Google Scholar]
- Sohn, H.; Kang, S.; Chang, J. Sulfide smelting fundamentals, technologies and innovations. Min. Metall. Explor. 2005, 22, 65–76. [Google Scholar] [CrossRef]
- Moskalyk, R.; Alfantazi, A. Review of copper pyrometallurgical practice: Today and tomorrow. Miner. Eng. 2003, 16, 893–919. [Google Scholar] [CrossRef]
- Hakimi, M.H.; Najaf, A.A.; Abdula, R.A.; Mohialdeen, I.M. Generation and expulsion history of oil-source rock (Middle Jurassic Sargelu Formation) in the Kurdistan of north Iraq, Zagros folded belt: Implications from 1D basin modeling study. J. Pet. Sci. Eng. 2018, 162, 852–872. [Google Scholar] [CrossRef]
- Mango, H.; Ryan, P. Source of arsenic-bearing pyrite in southwestern Vermont, USA: Sulfur isotope evidence. Sci. Total Environ. 2015, 505, 1331–1339. [Google Scholar] [CrossRef]
- Gracia, V.; Vàzquez, E.; Carmona, S. Utilization of by-produced sulfur for the manufacture of unmodified sulfur concrete. In Proceedings of the International RILEM Conference on the Use of Recycled Materials in Building and Structures, Barcelona, Spain, 8–11 November 2004; pp. 1054–1063. [Google Scholar]
- Szynkiewicz, A.; Goff, F.; Vaniman, D.; Pribil, M.J. Sulfur cycle in the Valles Caldera volcanic complex, New Mexico–Letter 1: Sulfate sources in aqueous system, and implications for S isotope record in Gale Crater on Mars. Earth Planet. Sci. Lett. 2019, 506, 540–551. [Google Scholar] [CrossRef]
- Sołek-Podwika, K.; Ciarkowska, K.; Kaleta, D. Assessment of the risk of pollution by sulfur compounds and heavy metals in soils located in the proximity of a disused for 20 years sulfur mine (SE Poland). J. Environ. Manag. 2016, 180, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Petlovanyi, M.V.; Lozynskyi, V.H.; Saik, P.B.; Sai, K.S. Modern experience of low-coal seams underground mining in Ukraine. Int. J. Min. Sci. Technol. 2018, 28, 917–923. [Google Scholar] [CrossRef]
- Gladkikh, V.; Korolev, E.; Smirnov, V.; Sukhachev, I. Modeling the rutting kinetics of the sulfur-extended asphalt. Procedia Eng. 2016, 165, 1417–1423. [Google Scholar] [CrossRef]
- Brookfield, M.E.; Hashmat, A. The geology and petroleum potential of the North Afghan platform and adjacent areas (northern Afghanistan, with parts of southern Turkmenistan, Uzbekistan and Tajikistan). Earth Sci. Rev. 2001, 55, 41–71. [Google Scholar] [CrossRef]
- Ikehata, K.; Maruoka, T. Sulfur isotopic systematics during the October 2017 eruption of the Shinmoe-dake volcano, Japan. Appl. Geochem. 2019, 102, 102–107. [Google Scholar] [CrossRef]
- Kurek, M.R.; Gilhooly, W.P., III; Druschel, G.K.; O’Beirne, M.D.; Werne, J.P. The use of dithiothreitol for the quantitative analysis of elemental sulfur concentrations and isotopes in environmental samples. Chem. Geol. 2018, 481, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Fike, D.; Li, A.; Dong, J.; Xu, F.; Zhuang, G.; Rendle-Bühring, R.; Wan, S. Pyrite sulfur isotopes constrained by sedimentation rates: Evidence from sediments on the East China Sea inner shelf since the late Pleistocene. Chem. Geol. 2019, 505, 66–75. [Google Scholar] [CrossRef]
- Sim, M.S.; Sessions, A.L.; Orphan, V.J.; Adkins, J.F. Precise determination of equilibrium sulfur isotope effects during volatilization and deprotonation of dissolved H2S. Geochim. Cosmochim. Acta 2019, 248, 242–251. [Google Scholar] [CrossRef] [Green Version]
- Searcy, D.G. Elemental sulfur reduction to H2S by Tetrahymena thermophila. Eur. J. Protistol. 2018, 62, 56–68. [Google Scholar] [CrossRef]
- Velasco, A.; Morgan-Sagastume, J.M.; González-Sánchez, A. Evaluation of a hybrid physicochemical/biological technology to remove toxic H2S from air with elemental sulfur recovery. Chemosphere 2019, 222, 732–741. [Google Scholar] [CrossRef]
- Fischer, H. Burner/fire box design improves sulfur recovery. Hydrocarb. Process. 1974, 53, 125–130. [Google Scholar]
- Nobles, J.E.; Palm, J.W.; Knudtson, D.K. Plant performance proves process. Hydrocarb. Process. 1977, 56, 143–145. [Google Scholar]
- El-Bishtawi, R.; Haimour, N. Claus recycle with double combustion process. Fuel Process. Technol. 2004, 86, 245–260. [Google Scholar] [CrossRef]
- Haynes, W. Brimstone: The Stone That Burns: The Story of the Frasch Sulphur Industry; Van Nostrand: New York, NY, USA, 1959. [Google Scholar]
- Toon, S. Sulphur—A sweet or sour future? Ind. Miner. 1986, 221, 16–37. [Google Scholar]
- Morse, D.E. Sulfur, in Metals and minerals. In U.S. Bureau of Mines Minerals Yearbook; United States Geological Survey: Washington, DC, USA, 1983; pp. 799–818. [Google Scholar]
- Sander, U.; Fischer, H.; Rothe, U.; Kola, R. Sulphur, sulphur dioxide and sulphuric acid. In Sulphursulphur Dioxide Sulphuric Acid; British Sulphur Corporation Ltd.: London, UK, 1984. [Google Scholar]
- Meyer, B. Sulfur, Energy, and Environment; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Meyer, B. Elemental sulfur. Chem. Rev. 1976, 76, 367–388. [Google Scholar] [CrossRef]
- Cunningham, W.A. Sulfur. III. J. Chem. Educ. 1935, 12, 120. [Google Scholar] [CrossRef]
- Pacor, P. Applicability of the du pont 900 DTA apparatus in quantitative differential thermal analysis. Anal. Chim. Acta 1967, 37, 200–208. [Google Scholar] [CrossRef]
- Schmumb, W.C. Gmelin’s Handbuch der Anorganischen Chemie. System 9: Schwefel. Parts A2 and B1; ACS Publications: Washington, DC, USA, 1954. [Google Scholar]
- Miller, G.W. Thermal analysis of polymers. VIII. Dilatometric and thermal optical behavior of sulfur. J. Appl. Polym. Sci. 1971, 15, 1985–1994. [Google Scholar] [CrossRef]
- Schmidt, M. Schwefel—Was ist das eigentlich? Chem. Unserer Zeit 1973, 7, 11–18. [Google Scholar] [CrossRef]
- Köpf, H.; Block, B.; Schmidt, M. Di-π-cyclopentadienyl-titan (IV)-pentaselenid und-pentasulfid, zwei Hetero-cyclohexachalkogene in fixierter Konformation. Chem. Ber. 1968, 101, 272–276. [Google Scholar] [CrossRef]
- Fediuk, R.; Pak, A.; Kuzmin, D. Fine-Grained Concrete of Composite Binder. In IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2017; Volume 262. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Greer, S. The density of liquid sulfur near the polymerization temperature. J. Chem. Phys. 1992, 96, 2175–2182. [Google Scholar] [CrossRef]
- Steudel, R.; Eckert, B. Solid sulfur allotropes. In Elemental Sulfur and Sulfur-Rich Compounds I; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1–80. [Google Scholar]
- Debaerdemaeker, T.; Hellner, E.; Kutoglu, A.; Schmidt, M.; Wilhelm, E. Crystalline and Molecular Structure of Cycloicosasulfur S20 Synthesis; Springer: Berlin/Heidelberg, Germany, 1973; Volume 60, p. 300. [Google Scholar]
- Caron, A.; Donohue, J. The x-ray powder pattern of rhombohedral sulfur. J. Phys. Chem. 1960, 64, 1767–1768. [Google Scholar] [CrossRef]
- Kawada, I.; Hellner, E. Zur Struktur von Cycloheptaschwefel. Angew. Chem. 1970, 82, 390. [Google Scholar] [CrossRef]
- Pawley, G.; Rinaldi, R. Constrained refinement of orthorhombic sulphur. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1972, 28, 3605–3609. [Google Scholar] [CrossRef]
- Watanabe, Y. The crystal structure of monoclinic γ-sulphur. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1974, 30, 1396–1401. [Google Scholar] [CrossRef] [Green Version]
- Kutoglu, A.; Hellner, E. Kristallstruktur von Cyclododecaschwefel, S12. Angew. Chem. 1966, 78, 1021. [Google Scholar] [CrossRef]
- Schmidt, M.; Wilhelm, E.; Debaerdemaeker, T.; Hellner, E.; Kutoglu, A. Darstellung und Kristallstruktur von Cyclooktadekaschwefel, S18, und Cycloikosaschwefel, S20. Z. Anorg. Allg. Chem. 1974, 405, 153–162. [Google Scholar] [CrossRef]
- Lind, M.; Geller, S. Structure of Pressure-Induced Fibrous Sulfur. J. Chem. Phys. 1969, 51, 348–353. [Google Scholar] [CrossRef]
- Santos, M.C.; Nunes, C.; Saraiva, J.A.; Coimbra, M.A. Chemical and physical methodologies for the replacement/reduction of sulfur dioxide use during winemaking: Review of their potentialities and limitations. Eur. Food Res. Technol. 2012, 234, 1–12. [Google Scholar] [CrossRef]
- Bae, S.G.; Gwon, S.W.; Kim, S.W.; Cha, S.W. Physical properties of sulfur concrete with modified sulfur binder. J. Korean Soc. Civ. Eng. 2014, 34, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Frolova, I.; Tikhonov, V.V.; Poltoranina, A.P.; Usoltseva, N.; Fu, S.C.; Knyazev, A.S. Sulfur-containing composite material for the concrete production. Key Eng. Mater. 2016, 712, 171–175. [Google Scholar] [CrossRef]
- Sugawara, A. Thermal conductivity of sulfur accompanying crystal transition and phase change. J. Appl. Phys. 1965, 36, 2375–2377. [Google Scholar] [CrossRef]
- Tuller, W.N. The Sulphur Data Book; Freeport Sulphur Company: New York, NY, USA, 1954; p. 143. [Google Scholar]
- Choura, M.; Keskes, M.; Chaari, D.; Ayadi, H. Study of the mechanical strength and leaching behavior of phosphogypsum in a sulfur concrete matrix. IOSR J. Environ. Sci. Toxicol. Food Technol. 2015, 9, 8–13. [Google Scholar] [CrossRef]
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and developments in green cement and concrete technology. Int. J. Sustain. Built Environ. 2012, 1, 194–216. [Google Scholar] [CrossRef] [Green Version]
- Orlowski, J.; Leszczewski, M.; Margal, I. Stability of polymer sulfur concrete with steel reinforcement. Tech. Sci. 2004, 7, 101–108. [Google Scholar]
- STARcrete. STARcrete™ is a Sulfur-Based Concrete with Unique Properties. Available online: http://starcrete.com/durability.html (accessed on 22 March 2020).
- Fediuk, R. Reducing permeability of fiber concrete using composite binders. Spec. Top. Rev. Porous Media 2018, 9, 79–89. [Google Scholar] [CrossRef]
- Fedkin, N.M.; Li, C.; Dickerson, R.R.; Canty, T.; Krotkov, N.A. Linking improvements in sulfur dioxide emissions to decreasing sulfate wet deposition by combining satellite and surface observations with trajectory analysis. Atmos. Environ. 2019, 199, 210–223. [Google Scholar] [CrossRef]
- Hu, B.; Li, Z.; Zhang, L. Long-run dynamics of sulphur dioxide emissions, economic growth, and energy efficiency in China. J. Clean. Prod. 2019, 227, 942–949. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Li, Y.; Yao, Q. The health costs of the industrial leap forward in China: Evidence from the sulfur dioxide emissions of coal-fired power stations. China Econ. Rev. 2018, 49, 68–83. [Google Scholar] [CrossRef]
- Brown, M.A.; Li, Y.; Massetti, E.; Lapsa, M. US sulfur dioxide emission reductions: Shifting factors and a carbon dioxide penalty. Electr. J. 2017, 30, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Lin, B.; Zhang, Y. Sulfur dioxide emission reduction of power plants in China: Current policies and implications. J. Clean. Prod. 2016, 113, 133–143. [Google Scholar] [CrossRef]
- Peters, B.; Smuła-Ostaszewska, J. Simultaneous prediction of potassium chloride and sulphur dioxide emissions during combustion of switchgrass. Fuel 2012, 96, 29–42. [Google Scholar] [CrossRef]
- Huang, J.-T. Sulfur dioxide (SO2) emissions and government spending on environmental protection in China-Evidence from spatial econometric analysis. J. Clean. Prod. 2018, 175, 431–441. [Google Scholar] [CrossRef]
- Smith, S.J.; Pitcher, H.; Wigley, T.M. Global and regional anthropogenic sulfur dioxide emissions. Glob. Planet. Chang. 2001, 29, 99–119. [Google Scholar] [CrossRef]
- Sullivan, T.; McBee, W.; Blue, D. Sulfur in Coatings and Structural Materials; ACS Publications: Washington, DC, USA, 1975. [Google Scholar]
- Lin, S.-L.; Lai, J.S.; Chian, E.S. Modifications of sulfur polymer cement (SPC) stabilization and solidification (S/S) process. Waste Manag. 1995, 15, 441–447. [Google Scholar] [CrossRef]
- Yang, Z.; Cui, W.; Wang, K.; Song, Y.; Zhao, F.; Wang, N.; Long, Y.; Wang, H.; Huang, C. Chemical Modification of the sp-Hybridized Carbon Atoms of Graphdiyne by Using Organic Sulfur. Chem. Eur. J. 2019, 25, 5643–5647. [Google Scholar] [CrossRef]
- Grugel, R.N. Integrity of sulfur concrete subjected to simulated lunar temperature cycles. Adv. Space Res. 2012, 50, 1294–1299. [Google Scholar] [CrossRef]
- Fediuk, R.; Smoliakov, A.; Stoyushko, N. Increase in composite binder activity. In IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2016; Volume 156, p. 012042. [Google Scholar]
- Rassokhin, A.; Ponomarev, A.; Figovsky, O. Silica fumes of different types for high-performance fine-grained concrete. Mag. Civ. Eng. 2018, 2, 151–160. [Google Scholar] [CrossRef]
- Volodchenko, A.; Lesovik, V.; Volodchenko, A.; Glagolev, E.; Zagorodnjuk, L.; Pukharenko, Y. Composite performance improvement based on non-conventional natural and technogenic raw materials. Int. J. Pharm. Technol. 2016, 8, 18856–18867. [Google Scholar]
- Svatovskaya, L.; Kabanov, A.; Sychov, M. Soling, aerating and phosphating for soil strengthening and detoxication. Procedia Eng. 2017, 189, 398–403. [Google Scholar] [CrossRef]
- López, C.M.; Bueno, J.P.; López, M.M.; Araiza, J.R.; Manzano-Ramírez, A. Fly ash lightweight material of the cellular concrete type using sol-gel and thermal treatment. Constr. Build. Mater. 2019, 206, 512–518. [Google Scholar] [CrossRef]
- Prasad, R.; Mahmoud, A.E.-R.; Parashar, S. Enhancement of electromagnetic shielding and piezoelectric properties of White Portland cement by hydration time. Constr. Build. Mater. 2019, 204, 20–27. [Google Scholar] [CrossRef]
- McBee, W.C.; Sulliven, T.A.; Jong, B.W. Modified sulfur concrete technology. In Proceedings of the SULFUR-81 International Conference On Sulfur, Calgary, AB, Canada, 25–28 May 1981; pp. 367–388. [Google Scholar]
- Blight, L.; Currell, B.; Nash, B.; Scott, R.; Stillo, C. Preparation and Properties of Modified Sulfur Systems; ACS Publications: Washington, DC, USA, 1978. [Google Scholar]
- Gwon, S.; Ahn, E.; Shin, M. Self-healing of modified sulfur composites with calcium sulfoaluminate cement and superabsorbent polymer. Compos. Part B Eng. 2019, 162, 469–483. [Google Scholar] [CrossRef]
- Vroom, A.H. Sulfur concrete goes global. Concr. Int. 1998, 20, 68–71. [Google Scholar]
- Anyszka, R.; Bieliński, D.M.; Siciński, M.; Imiela, M.; Szajerski, P.; Pawlica, J.; Walendziak, R. Sulfur Concrete–Promising Material for Space-Structures Building. In Proceedings of the European Conference on Spacecraft Structures Materials and Environmental Testing, Toulouse, France, 27–30 September 2016. [Google Scholar]
- López Gómez, F.A.; Román, C.; Padilla, I.; López-Delgado, A.; Alguacil, F.J. The application of sulfur concrete to the stabilization of Hg-contaminated soil. In Proceedings of the 1st Spanish National Conference on Advances in Materials Recycling and Eco-Energy, Madrid, Spain, 12–13 November 2009. [Google Scholar]
- Vasiliev, Y.E.; Motin, N.V.; Pekar, S.S.; Shibin, A.N.; Yakobi, V.V. Method for producing modified sulfur. Russian Patent No. 2554585, 30 August 2013. [Google Scholar]
- Mohamed, A.-M.O.; El Gamal, M. Sulfur based hazardous waste solidification. Environ. Geol. 2007, 53, 159–175. [Google Scholar] [CrossRef]
- Dugarte, M.; Martinez-Arguelles, G.; Torres, J. Experimental Evaluation of Modified Sulfur Concrete for Achieving Sustainability in Industry Applications. Sustainability 2019, 11, 70. [Google Scholar] [CrossRef] [Green Version]
- Sabour, M.; Dezvareh, G.; Bazzazzadeh, R. Corrosion prediction using the weight loss model in the sewer pipes made from sulfur and cement concretes and Response Surface Methodology (RSM). Constr. Build. Mater. 2019, 199, 40–49. [Google Scholar] [CrossRef]
- Yeoh, D.; Boon, K.H.; Jamaluddin, N. Exploratory Study on the mechanical and physical properties of concrete containing sulfur. J. Teknol. 2015, 77, 77. [Google Scholar] [CrossRef] [Green Version]
- Gladkikh, V.; Korolev, E.V.; Poddaeva, O.I.; Smirnov, V.A. Sulfur-extended high-performance green paving materials. Adv. Mater. Res. 2015, 1079, 58–61. [Google Scholar] [CrossRef]
- Diehl, L. Dicyclopentadiene-modified sulfur and its use as a binder, quoting sulfur concrete as an example. In New Uses for Sulfur and Pyrites; The Sulfur Institute: Madrid, Spain, 1976; pp. 202–214. [Google Scholar]
- Grugel, R.N.; Toutanji, H. Sulfur “concrete” for lunar applications–Sublimation concerns. Adv. Space Res. 2008, 41, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, N.; Singh, R.S.; Hills, C.D. Microbial removal of sulphur from petroleum coke (petcoke). Fuel 2019, 235, 1501–1505. [Google Scholar] [CrossRef]
- Cieszynska-Semenowicz, M.; Rogowska, J.; Ratajczyk, W.; Ratajczyk, J.; Wolska, L. Toxicity studies of elemental sulfur in marine sediments. Int. J. Sediment Res. 2018, 33, 191–197. [Google Scholar] [CrossRef]









| Property | Unit | OPC-Based Concrete | Sulfur-Based Concrete | Refs |
|---|---|---|---|---|
| Time of strength gain | Time | 28 days | 3 h | [7] |
| Compressive strength | MPa | 15–25 | 55–65 | [14] |
| Tensile strength | MPa | 3–4 | 5–7 | [25] |
| Wearing capacity | % | 17 | 3 | [26] |
| Flexural strength | MPa | 6–9 | 10–15 | |
| Freezing resistance | % | 50 | 300 | [4] |
| Acids resistance | at 100/cent humidity | 23 | 84 | [27] |
| Water resistance | % | 0.8 | 1.0 | [28] |
| Allotrope of Sulfur | Melting Point (°C) | Refs. |
|---|---|---|
| α-sulfur | 110.06 | [36] |
| 115.1 | [13] | |
| 112.8 | [36,69] | |
| β-sulfur | 114.6 | [69] |
| 119.6 | [71] | |
| 120.4 | [13] | |
| 133 | [69] | |
| γ-sulfur | 106.8 | [72] |
| 108 | [73] | |
| 108.6 | [13] | |
| δ-sulfur | 160 | [13] |
| ω-sulfur | 77 | [73] |
| 90 | ||
| 160 | ||
| 104 | [69] | |
| Fibrous | 75 | [73] |
| 104 | [72] | |
| Hexasulfur | 50 | [74] |
| Heptasulfur | 39 | [75] |
| Cyclo-S12 | 148 | [74] |
| Cyclo-S18 | 128 | [76] |
| Cyclo-S20 | 124 |
| Allotrope of Sulfur | Color | Refs |
|---|---|---|
| Octasulfur alpha | Bright yellow | [15,76,80,81,82,83,84,85,86] |
| Octasulfur beta | Yellow | |
| Octasulfur gamma | Light yellow | |
| Hexasulfur | Orange to red | |
| Heptasulfur | Light yellow | |
| Anneasulfur | Deep yellow | |
| Decasulfur | Yellow to green | |
| Octadecasulfur | Lemon to yellow |
| Properties of Concrete | Sulfur-Based Concrete Compared to Cement Concrete |
|---|---|
| Wear resistance | ↑ |
| Permeability | ↓ |
| Bond strength to concrete/reinforcing steel | ↑ |
| Thermal conductivity | ↓ |
| Elastic modulus | ↑ |
| Flexural strength | ↑ |
| Compressive/flexural/tensile strength | ↑ |
| Fire resistance | ↓ |
| Durability during thermal cycles | = or ↑ |
| Corrosion resistance | ↑ |
| Fatigue resistance | ↑ |
| Linear expansion coefficient | = |
| Compression creep | ↓ |
| Inorganic Additives | ||
|---|---|---|
| Modifier | Concentration % of the Mass of Sulfur | Result |
| Talk [3,110] | 26 | Acid resistance |
| Heavy metals and mercury [36] | 6 | Durability |
| Alumina [3] | 20–26 | Acid resistance |
| Fly ash [3] | 22–23 | |
| Silica [3] | 22–25 | |
| Organic additives | ||
| Dicyclopentadiene [5,9,12,117,118] | 0.1–50 | Increased strength in corrosive chemical environments, increased fire resistance |
| Dicyclopentadiene + cyclopentadiene + dipentene [5,9,12] | 1–30 | Rapid development of compressive strength |
| Olefin polysulfide additives [12,110] | 5–25 | Improving the strength characteristics |
| Epoxy resin [119] | 2–6 | Improving the strength characteristics |
| Polyolefin [7] | 2.5–5 | Plasticizer |
| Bitumen [6,12,110] | 1–4 | High corrosion resistance, high physical strength |
| Additive STX (Starcrete) [120] | 2–7 | High fatigue strength |
| Styrene [6,110] | 2–30 | Low water permeability |
| Ethylidene norbornene [121] | 1–5 | Provides, with smaller quantities of the modifier, an increase in the resistance of sulfur-based concrete in acidic and basic environments, has high strength, high frost resistance and also eliminates the toxicity of the material obtained |
| Authors | Content | Compressive Strength, MPa | Flexural Strength, MPa | Tensile Strength, MPa |
|---|---|---|---|---|
| Vlahovich et. al. [3] | Sulfur—30 wt. % Sand—63 wt. % Fillers—7 wt. % | 55 | 8 | 3 |
| Dehestani et. al. [6] | Sulfur—98 wt. % styrene—2 wt. % | 54 | 8 | 3 |
| Al-Otaibi et al. [7] | modified sulfur—1 wt. % granulated sulfur—11 wt. % sand—40 wt. % coarse aggregate, 42 wt. % fly ash—6 wt. % | 30 | 2 | 1 |
| Gracia et. al. [50] | Sulfur—25 wt. % Sand—70 wt. % slag—5 wt. % | 70 | 12 | 5 |
| Bae et. al. [88] | modified sulfur—15 wt. % fly ash—13 wt. % sand—32 wt. % coarse aggregate—40 wt. % | 83 | 13 | 6 |
| Choura et. al. [92] | Sulfur—50 wt. % Phosphogypsum—50 wt. % | 41 | 5 | 2 |
| Gwon et al. [118] | modified sulfur—40 vol. % sand—35 vol. % binary cement—25 vol. % | 62 | 9 | 4 |
| Anyszka et. al. [119] | modified sulfur—30 wt. % sand—70 wt. % | 115 | 16 | 7 |
| Lopez et. al. [120] | Sulfur—17 wt. % Polymer—2 wt. % Sand—49 wt. % coarse aggregate—24 wt. % soil—8 wt. % | 60 | 13 | 6 |
| Dugarte et. al. [123] | modified sulfur—30 vol. % sand—70 vol. % | 43 | 5 | 2 |
| Sabour et. al. [124] | Sulfur—25 wt. % Sand—70 wt. % slag—5 wt. % | 52 | 8 | 3 |
| Yeoh et. al. [125] | Sulfur—1 wt. % Cement—14 wt. %S and—30 wt. % coarse aggregate—40 wt. % water—15 wt. % | 42 | 5 | 2 |
| Author | Lost Weight, % | |||||
|---|---|---|---|---|---|---|
| H2SO4 | HCl | NaCl | SO4(NH4)2 | Kerosene | Thiobacillus Thiooxidans Bacterium | |
| Sabour et. al. [124] | 5 | 1 | - | - | - | 2.25 |
| Gwon et al. [117] | 0 | - | 1 | - | - | |
| Vlahovich et. al. [3] | 0 | −1 | 2 | - | - | - |
| Dehestani et. al. [6] | - | 0 | - | - | 3 | - |
| Dugarte et. al. [123] | 1 | −1 | 3 | 1 | - | - |
| Gracia et. al. [50] | - | - | - | - | 3 | - |
| Yeoh et. al. [125] | 4 | - | 2 | - | - | - |
| Choura et. al. [92] | 2 | - | - | - | - | - |
| Bae et. al. [88] | 3 | - | 3 | 1 | - | - |
| Anyszka et. al. [119] | 2 | - | - | - | - | - |
| Lopez et. al. [120] | 5 | - | 2 | - | 3 | - |
| Al-Otaibi et al. [7] | 4 | - | - | 1 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fediuk, R.; Mugahed Amran, Y.H.; Mosaberpanah, M.A.; Danish, A.; El-Zeadani, M.; Klyuev, S.V.; Vatin, N. A Critical Review on the Properties and Applications of Sulfur-Based Concrete. Materials 2020, 13, 4712. https://doi.org/10.3390/ma13214712
Fediuk R, Mugahed Amran YH, Mosaberpanah MA, Danish A, El-Zeadani M, Klyuev SV, Vatin N. A Critical Review on the Properties and Applications of Sulfur-Based Concrete. Materials. 2020; 13(21):4712. https://doi.org/10.3390/ma13214712
Chicago/Turabian StyleFediuk, Roman, Y. H. Mugahed Amran, Mohammad Ali Mosaberpanah, Aamar Danish, Mohamed El-Zeadani, Sergey V. Klyuev, and Nikolai Vatin. 2020. "A Critical Review on the Properties and Applications of Sulfur-Based Concrete" Materials 13, no. 21: 4712. https://doi.org/10.3390/ma13214712







