Analysis and Prediction of Corrosion of Refractory Materials by Sodium Salts during Waste Liquid Incineration—Thermodynamic Study
Abstract
:1. Introduction
2. Thermodynamic Calculations
3. Thermodynamic Experimental Procedure
4. Results and Discussion
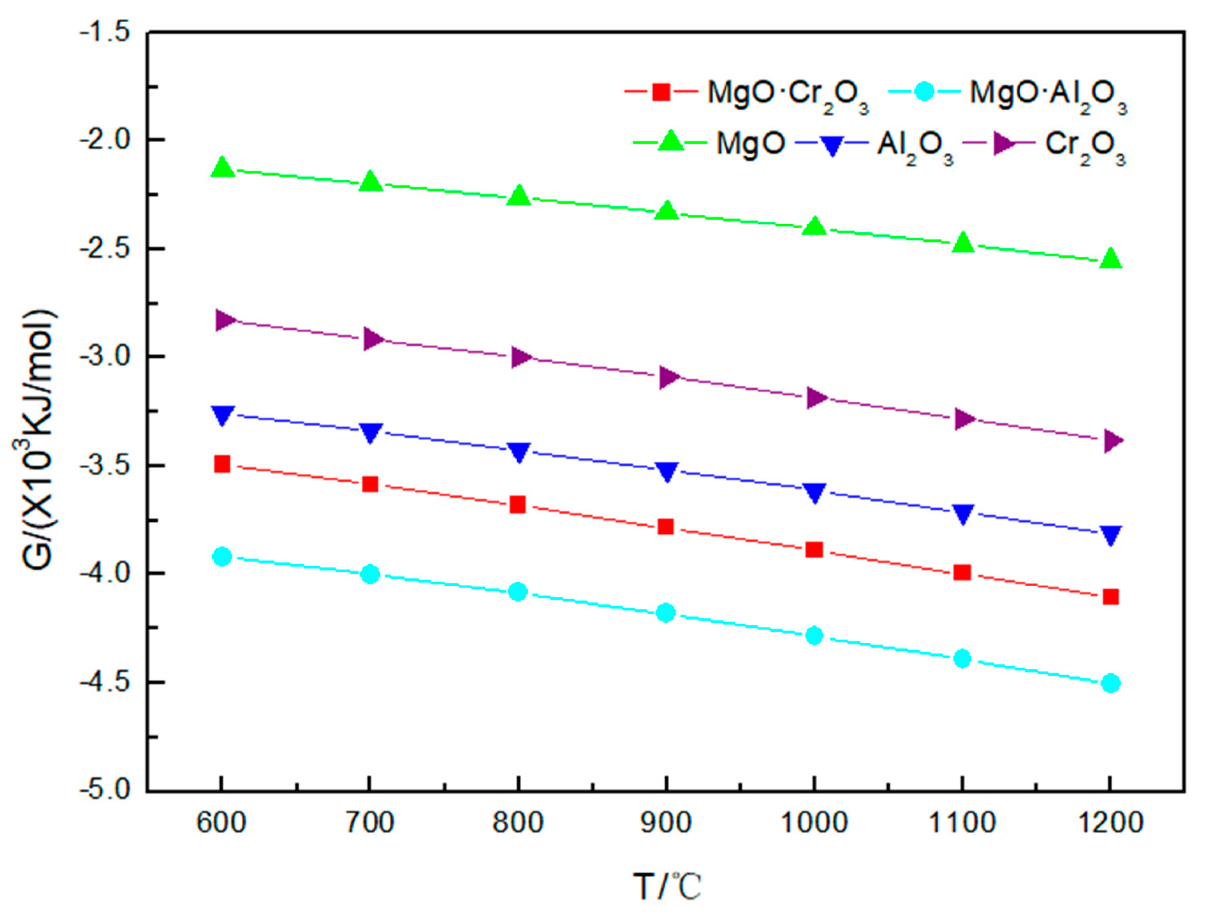
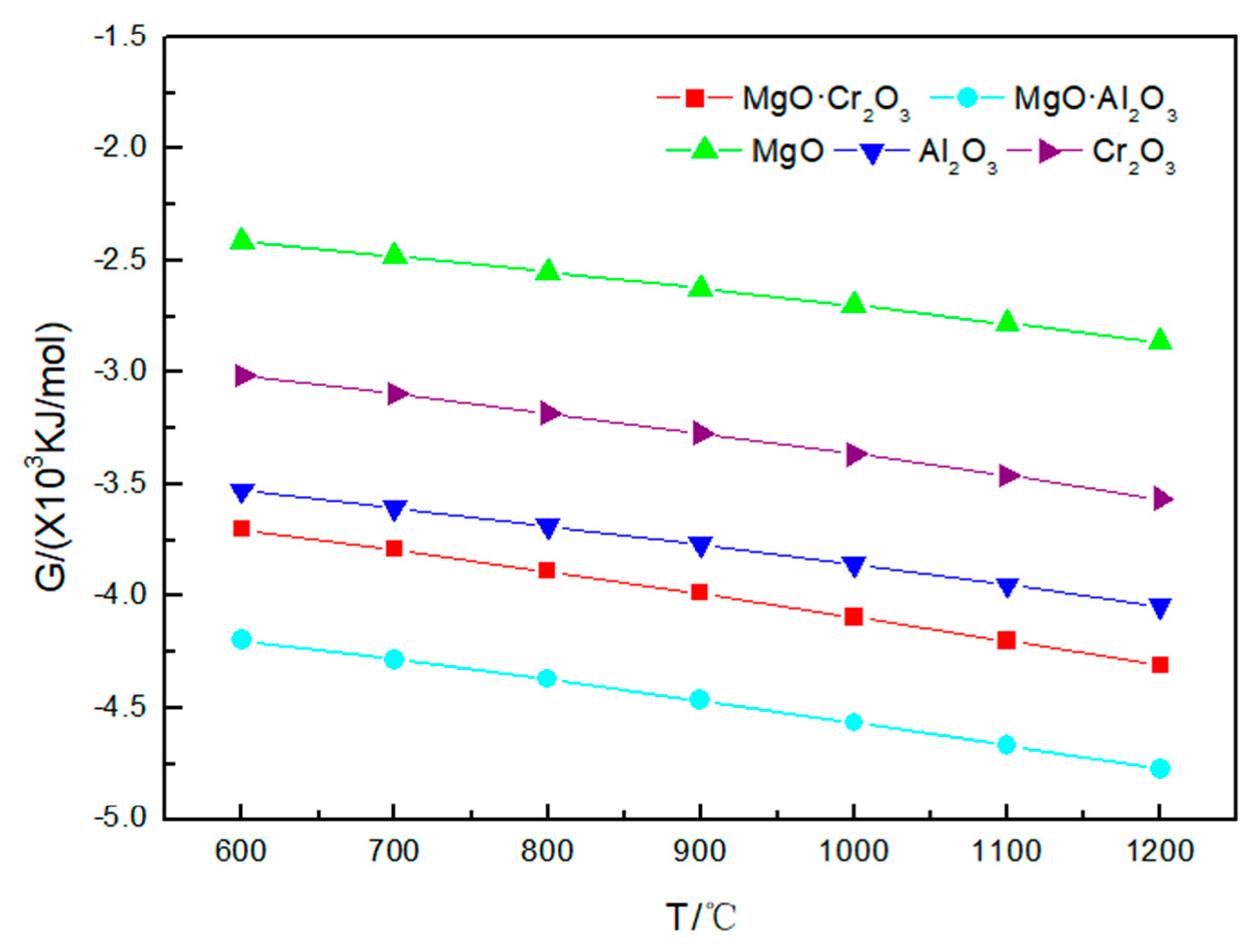
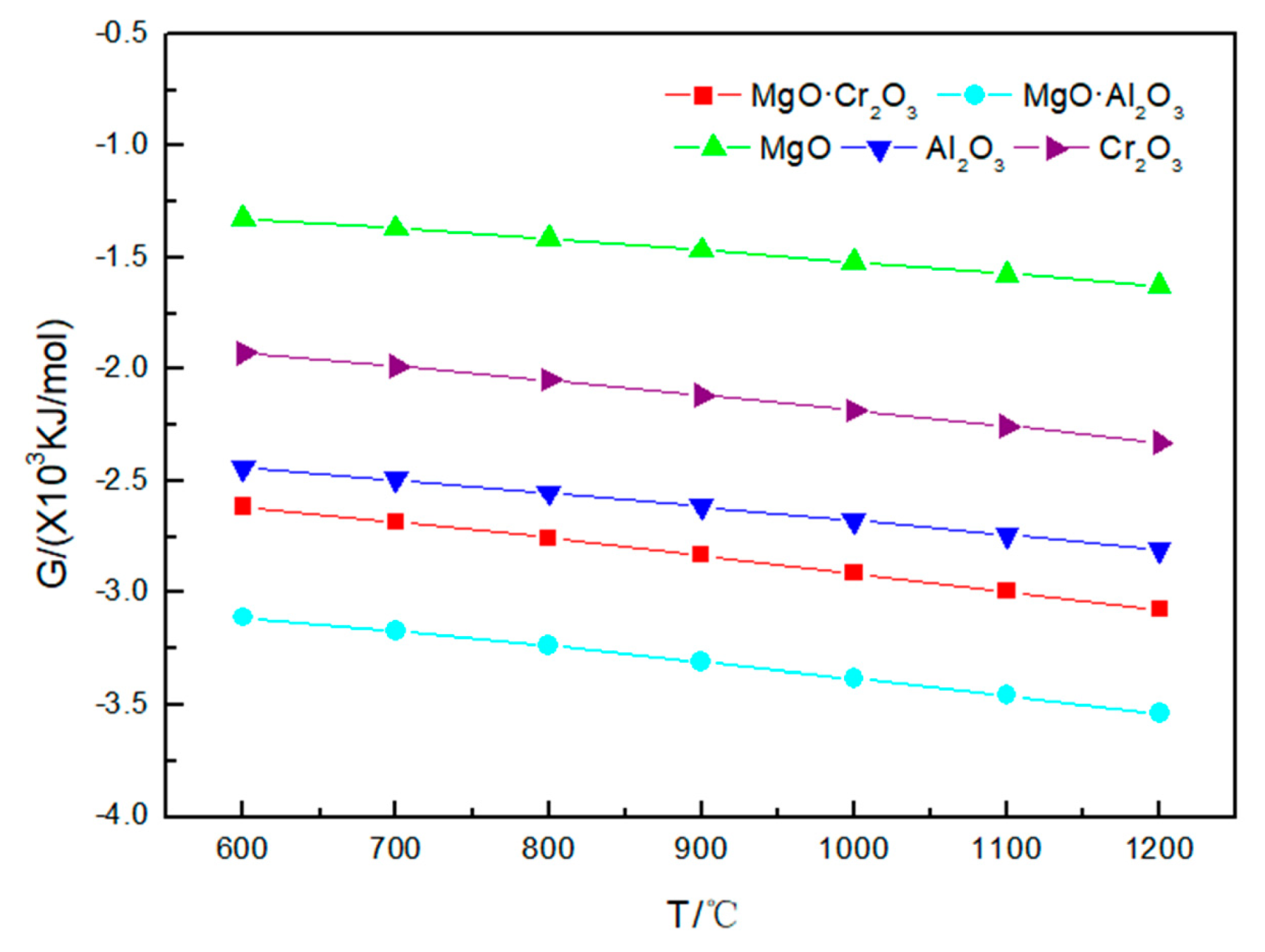
4.1. Corrosion Effect of Na2CO3 on Refractory Materials
4.2. Corrosion Reaction of Na2SO4 and Refractory Materials
4.3. Corrosion Reaction of NaCl and Refractory Materials
4.4. Thermodynamic Experiment of NaCl Corrosion of MgO
5. Conclusions
- The temperature has a great influence on the corrosion of refractory materials by sodium salt at 600–1200 °C. Besides MgO and MgO·Al2O3, the higher the temperature, the stronger the corrosion of refractory materials by sodium sulfate and sodium chloride.
- Among the five refractory materials, MgO has the best resistance to sodium salt corrosion, followed by MgO·Cr2O3 and MgO·Al2O3; Cr2O3 and Al2O3 have the worst resistance to sodium salt corrosion. Due to the presence of O2, Cr3+ is oxidized to Cr6+ during corrosion.
- The accuracy of the thermodynamic calculation of FactSageTM software was verified for the MgO-NaCl system by analyzing the results of thermodynamic experiments. Combined with the author’s previous research work, high-content MgO refractories can solve the corrosion problem of refractories caused by NaCl and KCl.
Author Contributions
Funding
Conflicts of Interest
References
- Prigent, P.; Bouchetou, M.L.; Poirier, J. Andalusite: An amazing refractory raw material with excellent corrosion resistance to sodium vapours. Ceram. Int. 2011, 37, 2287–2296. [Google Scholar] [CrossRef]
- Xu, T.T.; Xu, Y.B.; Li, Y.W.; Sang, S.B.; Wang, Q.H.; Zhu, T.B.; Nath, M.; Zhang, B. Corrosion mechanisms of magnesia-chrome refractories in copper slag and concurrent formation of hexavalent chromium. J. Alloy. Compd. 2019, 786, 306–313. [Google Scholar] [CrossRef]
- Hirata, T.; Morimoto, T.; Ohta, S.; Uchida, N. Improvement of the corrosion resistance of alumina-chromia ceramic materials in molten slag. J. Eur Ceram. Soc. 2003, 23, 2089–2096. [Google Scholar] [CrossRef]
- Bouchetou, M.L.; Poirier, J.; Arbelaez Morales, L.; Chotard, T.; Joubert, O.; Weissenbacher, M. Synthesis of an innovative zirconia-mullite raw material sintered from andalusite and zircon precursors and an evaluation of its corrosion and thermal shock performance. Ceram. Int. 2019, 45, 12832–12844. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhao, P.D.; Chen, J.W.; Zhao, H.Z.; He, K. Corrosion resistance of Al–Cr-slag containing chromium–corundum refractories to slags with different basicity. Ceram. Int. 2018, 44, 12162–12168. [Google Scholar] [CrossRef]
- Hirata, T.; Akiyama, K.; Yamamoto, H. Corrosion resistance of Cr2O3-Al2O3 ceramics by molten sodium sulphate-vanadium pentoxide. J. Mater. Sci. 2001, 36, 5927–5934. [Google Scholar] [CrossRef]
- Mahnicka-Goremikina, L.; Svinka, R.; Svinka, V. Influence of ZrO2 and WO3 doping additives on the thermal properties of porous mullite ceramics. Ceram. Int. 2018, 44, 16873–16879. [Google Scholar] [CrossRef]
- Kujur, M.K.; Roy, I.; Kumar, K.; Chintaiah, P.; Ghosh, S.; Ghosh, N.K. Influence of ZrO2 and WO3 doping additives on the thermal properties of porous mullite ceramics. Mater. Today: Proc. 2018, 5, 2359–2366. [Google Scholar]
- Wiedemeier, H.; Singh, M. Thermal stability of refractory materials for high-temperature composite applications. J. Mater. Sci. 1991, 26, 2421–2430. [Google Scholar] [CrossRef]
- He, L.P.; Chen, D.C.; Shang, S.P. Fabrication and wear properties of Al2O3-SiC ceramic coatings using aluminum phosphate as binder. J. Mater. Sci. 2004, 39, 4887–4892. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Iida, E.; Shikama, H.; Inoue, K. Applicaton of chrome-free bricks for incinerated-ash melting furnaces. J. Tech. Assoc Refract. 2005, 25, 232. [Google Scholar] [CrossRef]
- Moda, J.; Tanaka, K.; Kitamura, S. Chrome-free castables for waste melting furnances. J. Tech. Assoc Refract. 2008, 28, 204–209. [Google Scholar]
- Fan, R.D.; Zhao, H.Z.; Zhang, H.; Zhao, P.D.; Chen, J.W.; Wang, X.H. Effect of partial substitution of alumina-chromium slag for Al2O3 on microstructures and properties of Al2O3-SiC-C trough castables. Ceram. Int. 2019, 45, 11204–11215. [Google Scholar] [CrossRef]
- Igabo, K.; Sakida, S.; Benino, Y. Development of Cr-free refractories for high temperature municipal waste incinerators. J. Tech. Assoc Refract. 2008, 28, 125. [Google Scholar]
- Gehre, P.; Aneziris, C.G.; Veres, D.; Parr, C.; Fryda, H.; Neuroth, M. Improved spinel-containing refractory castables for slagging gasifiers. J. Eur Ceram. Soc. 2013, 33, 1077–1086. [Google Scholar] [CrossRef]
- Ren, B.; Li, Y.W.; Nath, M.; Wang, Q.H.; Xu, Y.B. Enhanced alkali vapor attack resistance of bauxite-SiC refractories for the working lining of cement rotary kilns via incorporation of andalusite. Ceram. Int. 2018, 44, 22113–22120. [Google Scholar] [CrossRef]
- Bianco, R.; Jacobson, N. Corrosion of cordierite ceramics by sodium sulphate at 1000 °C. J. Mater. Sci. 1989, 24, 2903–2910. [Google Scholar] [CrossRef]
- Stjernberg, J.; Olivas-Ogaz, M.A.; Antti, M.L.; Ion, J.C.; Lindblom, B. Laboratory scale study of the degradation of mullite /corundum refractories by reaction with alkali-doped deposit materials. Ceram. Int. 2013, 39, 791–800. [Google Scholar] [CrossRef]
- Boris, R.; Antonovič, V.; Keriene, J.; Stonys, R.; Kudžma, A.; Zdanevičius, P. Study of alkali resistance of refractory materials used in boilers operating on wood fuel. Refract. Ind Ceram. 2017, 57, 651–654. [Google Scholar] [CrossRef]
- Gregurek, D.; Schmidl, J.; Reinharter, K.; Reiter, V.; Spanring, A. Copper anode furnace: Chemical, mineralogical and thermo-chemical considerations of refractory wear mechanisms. J. Miner. Metals Mater. Soc. 2018, 70, 2428–2434. [Google Scholar] [CrossRef]
- Stjernberg, J.; Lindblom, B.; Wikström, J.; Antti, M.L.; Odénc, M. Microstructural characterization of alkali metal mediated high temperature reactions in mullite based refractories. Ceram. Int. 2010, 36, 733–740. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Qian, X.; Lv, X.D.; Liu, P.S.; Zhang, J.Z. Slag–refractory interactions during ilmenite smelting: Thermodynamic simulation and experimental data. Refract. Ind Ceram. 2018, 59, 134–139. [Google Scholar]
- Zhao, Y.; Cheng, G.S.; Xiang, Y.; Long, F.; Dong, C.Q. Thermodynamic study of the corrosion of refractories by sodium carbonate. Materials 2018, 11, 2197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.J. Physical Chemistry of Materials; Chemical Industry Press: Beijing, China, 2006. [Google Scholar]


| Species | Temperature (°C) | |||
|---|---|---|---|---|
| 600 | 700 | 800 | 900–1200 | |
| Al2O3 | 0.16667 mol Na2Al12O19 | 2 mol NaAlO2 | ||
| Equation (1) | Equation (2) | |||
| MgO | - | |||
| Cr2O3 | 1 mol Na2CrO4 | |||
| Equation (4) | ||||
| MgO·Cr2O3 | 1 mol Na2CrO4, 0.5mol MgO | |||
| Equation (5) | ||||
| MgO·Al2O3 | 0.00962 mol NaAlO2, 0.00481 mol MgO | 0.08292 mol NaAlO2, 0.04146 mol MgO | 0.53843 mol NaAlO2, 0.26922 mol MgO | 2 mol NaAlO2, 1 mol MgO |
| Equation (3) | Equation (3) | Equation (3) | Equation (3) | |
| Species | Temperature (°C) | |||
|---|---|---|---|---|
| 600–900 | 1000 | 1100 | 1200 | |
| Al2O3 | - | 0.0003 mol NaAl9O14 | 0.0033mol NaAl9O14 | 0.031672 mol NaAl9O14 |
| Equation (6) | Equation (6) | Equation (6) | ||
| MgO | - | |||
| Cr2O3 | - | 0.0002 mol Na2CrO4 | 0.0013 mol Na 2CrO4 | 0.0073 mol Na 2CrO4 |
| Equation (7) | Equation (7) | Equation (7) | ||
| MgO·Cr2O3 | - | 0.0001 mol Na2CrO4 | 0.0008 mol Na2CrO4, 0.0004mol MgO | |
| Equation (8) | Equation (8) | |||
| MgO·Al2O3 | - | |||
| Species | Temperature (°C) | ||||
|---|---|---|---|---|---|
| 600–800 | 900 | 1000 | 1100 | 1200 | |
| Al2O3 | - | 0.0001 mol NaAl9O14 | 0.0003 mol NaAl9O14 | 0.0008 mol NaAl9O14 | 0.0019 mol NaAl9O14 |
| Equation (9) | Equation (9) | Equation (9) | Equation (9) | ||
| MgO | - | ||||
| Cr2O3 | - | 0.0001 mol Na2CrO4 | 0.0002 mol Na2CrO4 | 0.0003 mol Na2CrO4 | 0.0006 mol Na2CrO4 |
| Equation (10) | Equation (10) | Equation (10) | Equation (10) | ||
| MgO·Cr2O3 | - | 0.0001 mol Na2CrO4 | |||
| Equation (11) | |||||
| MgO·Al2O3 | - | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, G.; Zhao, Y.; Long, F.; Zhang, J.; Zhao, T.; Liu, L.; Wang, X.; Dong, C. Analysis and Prediction of Corrosion of Refractory Materials by Sodium Salts during Waste Liquid Incineration—Thermodynamic Study. Materials 2020, 13, 4729. https://doi.org/10.3390/ma13214729
Cheng G, Zhao Y, Long F, Zhang J, Zhao T, Liu L, Wang X, Dong C. Analysis and Prediction of Corrosion of Refractory Materials by Sodium Salts during Waste Liquid Incineration—Thermodynamic Study. Materials. 2020; 13(21):4729. https://doi.org/10.3390/ma13214729
Chicago/Turabian StyleCheng, Guishi, Ying Zhao, Fei Long, Jiahe Zhang, Tengfei Zhao, Lu Liu, Xiaoqiang Wang, and Changqing Dong. 2020. "Analysis and Prediction of Corrosion of Refractory Materials by Sodium Salts during Waste Liquid Incineration—Thermodynamic Study" Materials 13, no. 21: 4729. https://doi.org/10.3390/ma13214729




