Preliminary Interpretation of the Induction Period in Hydration of Sodium Hydroxide/Silicate Activated Slag
Abstract
:1. Introduction
2. Materials and Methods
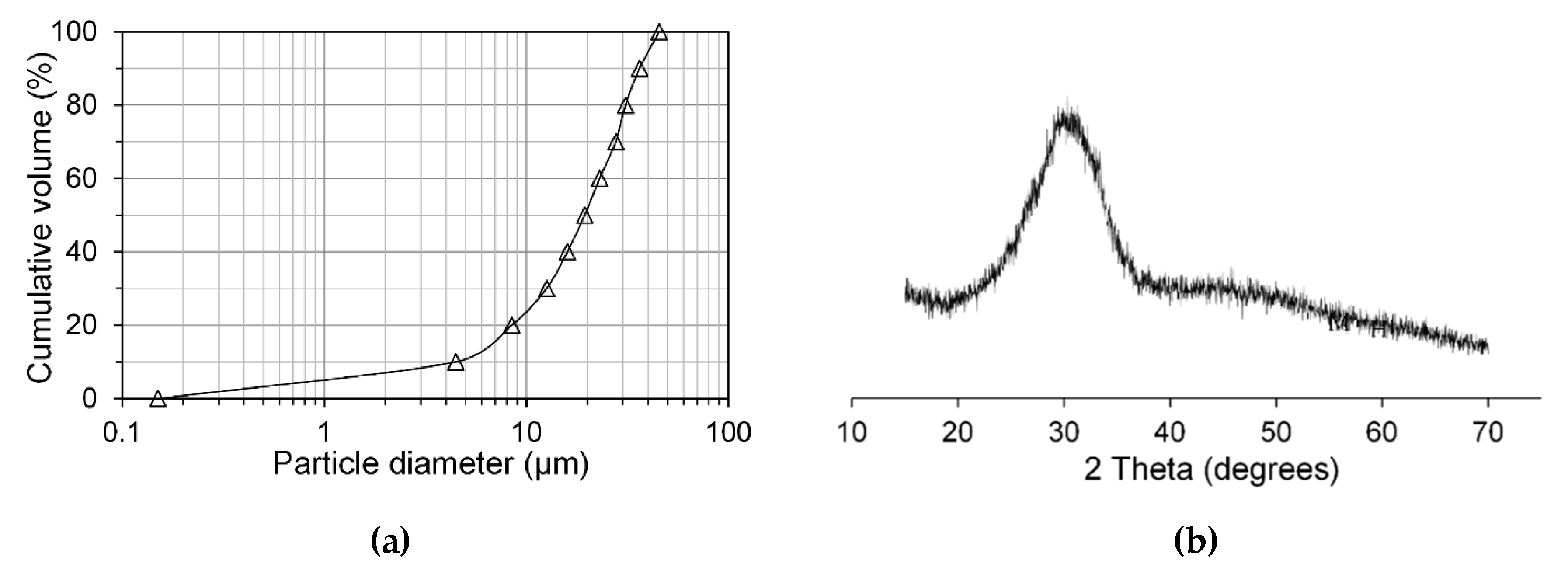
2.1. Materials and Mixtures
2.2. Isothermal Calorimtry Test
2.3. Dissolution Test
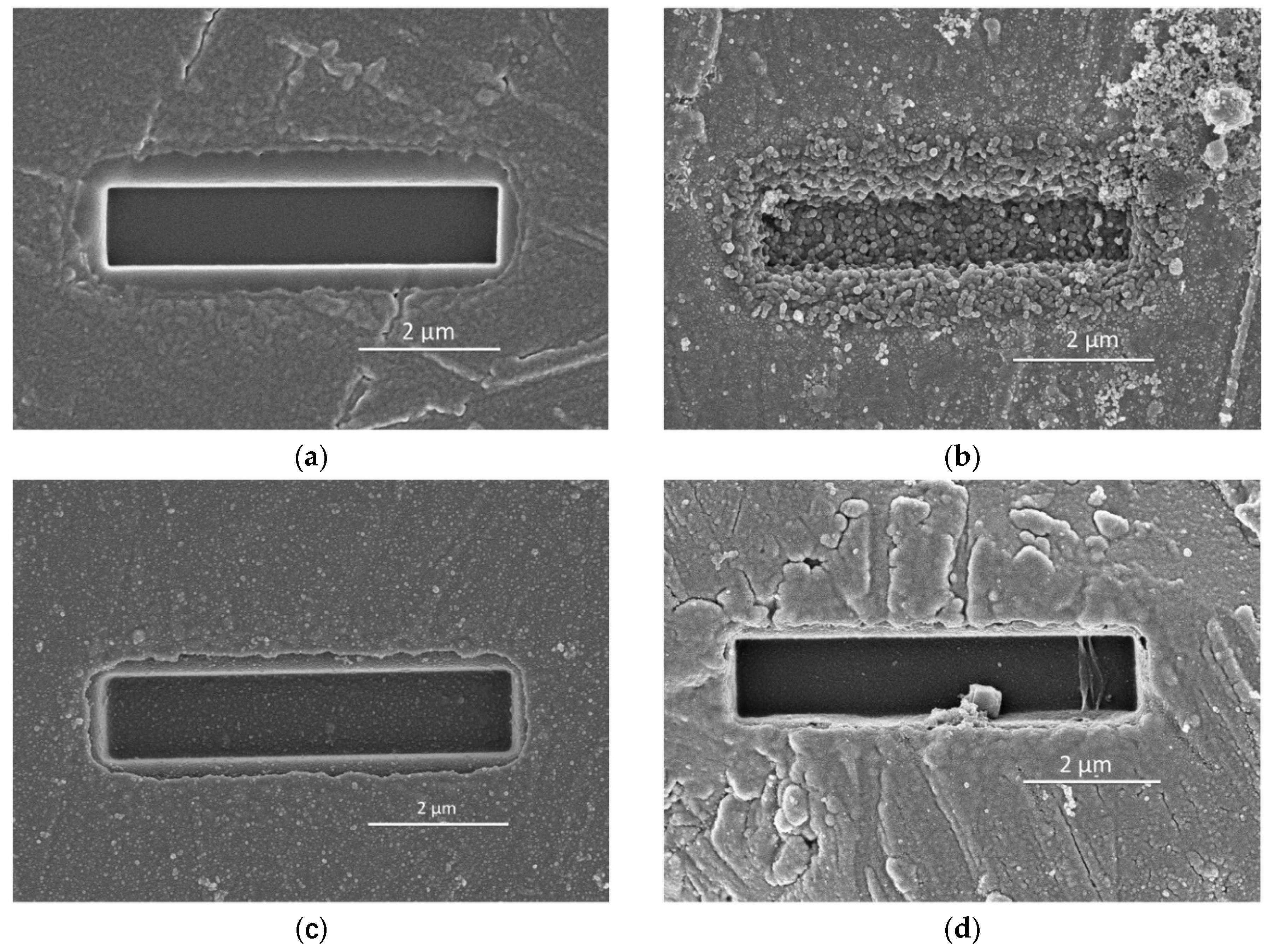
2.4. Scanning Electron Microscopy (SEM)
2.5. Thermodynamic Analysis of Solution
The Considered Reaction Products in the Thermodynamic Analysis
3. Results
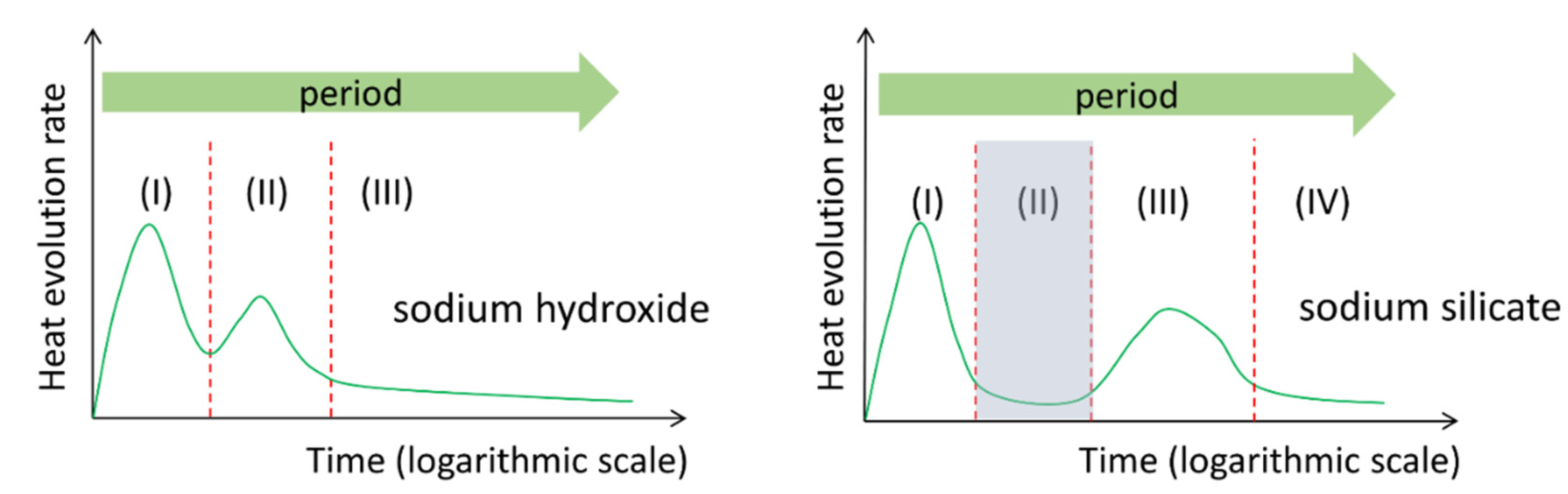
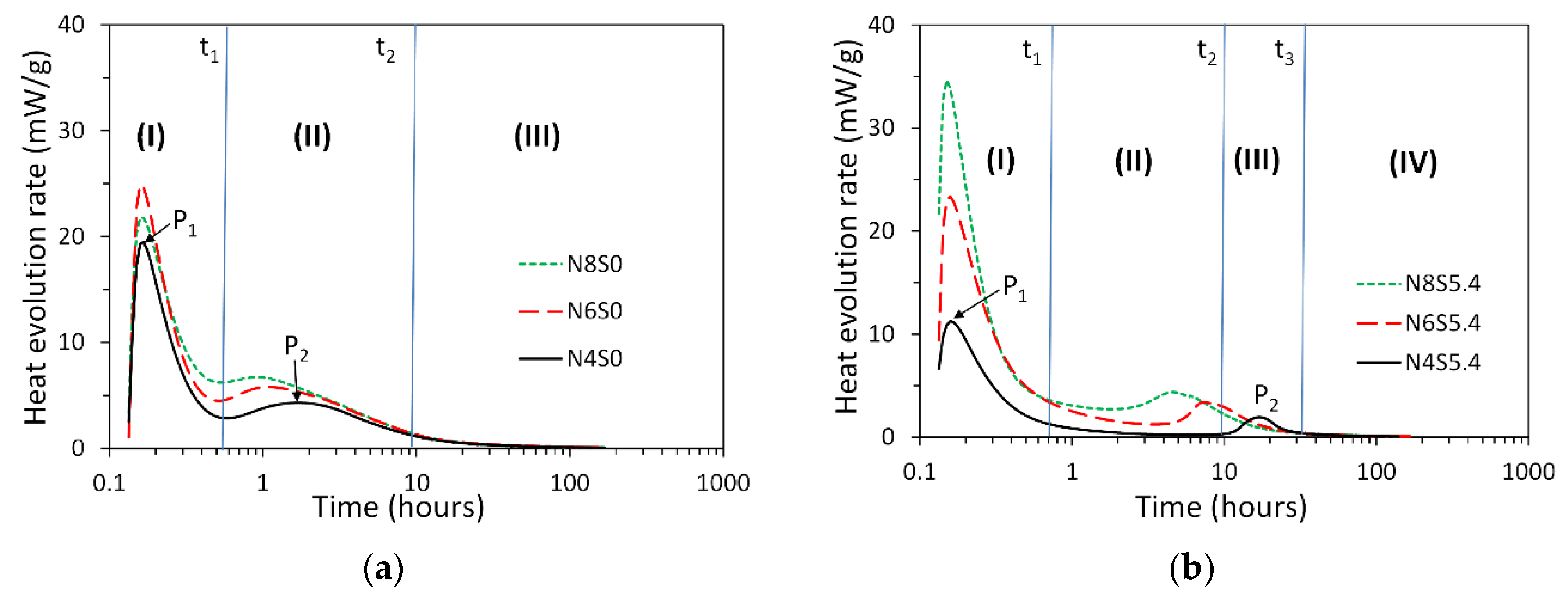
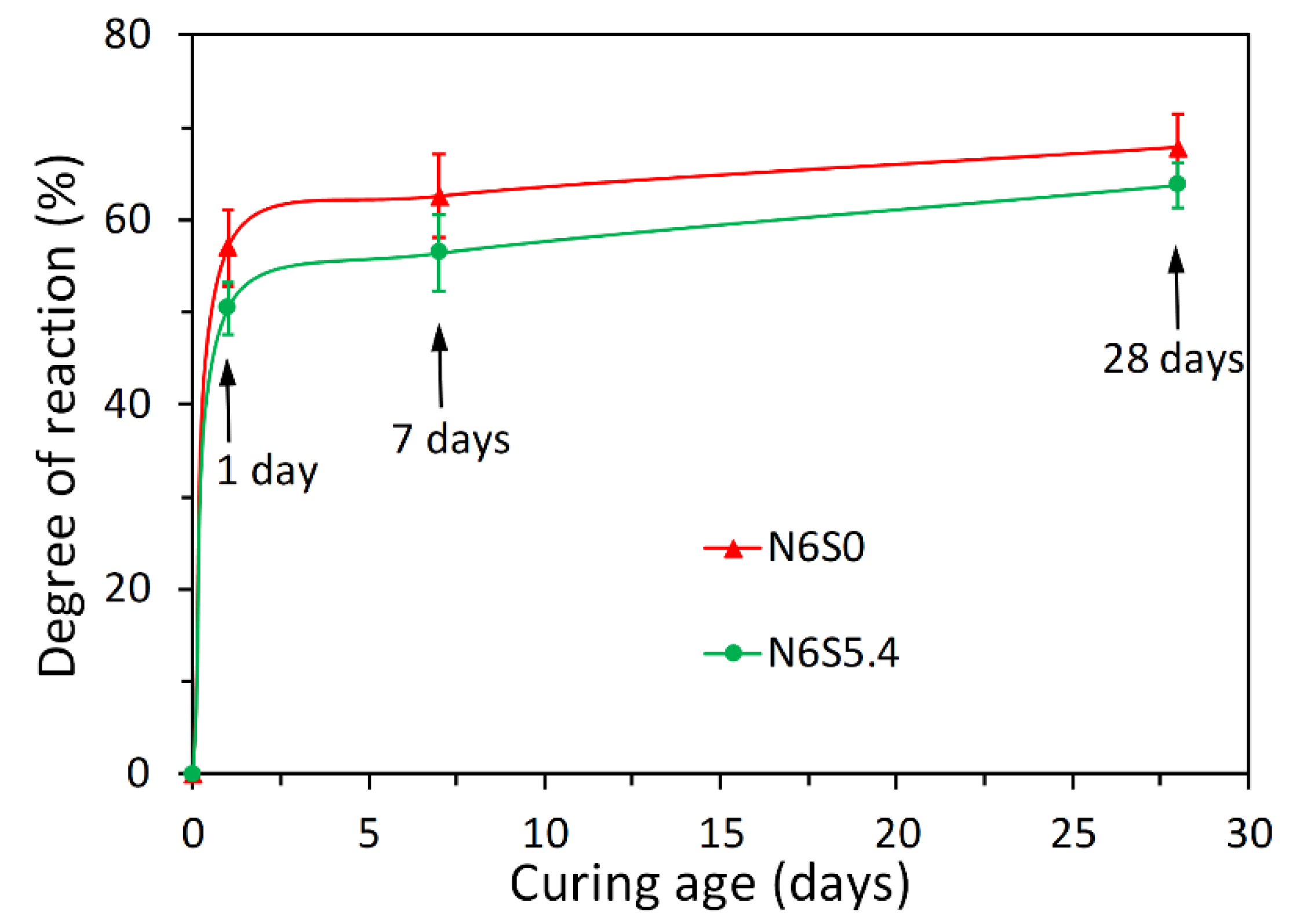
3.1. Reaction Kinetics of Alkali-Activated Slag Paste
3.2. Dissolution Results
3.2.1. Dissolution Results
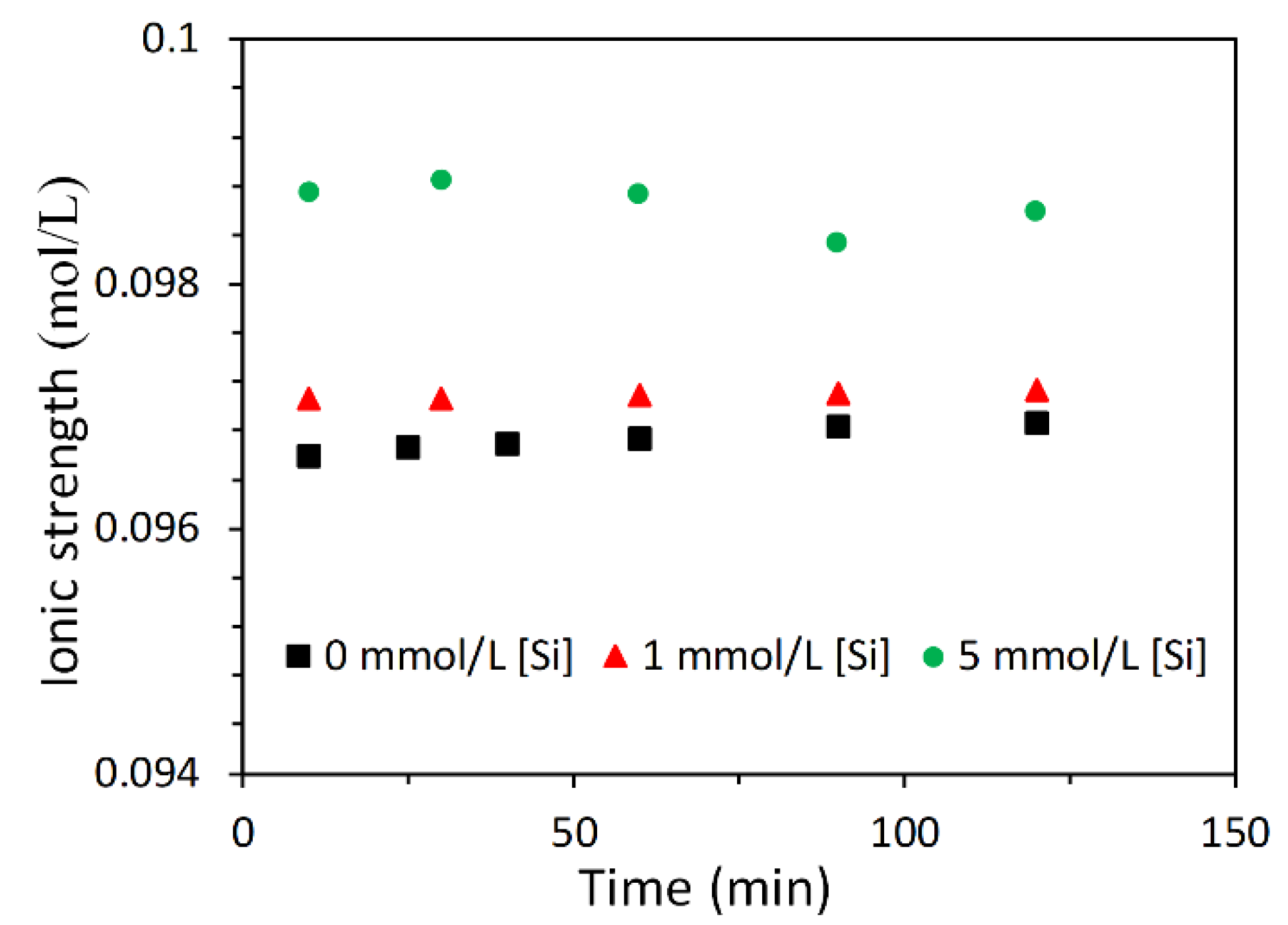
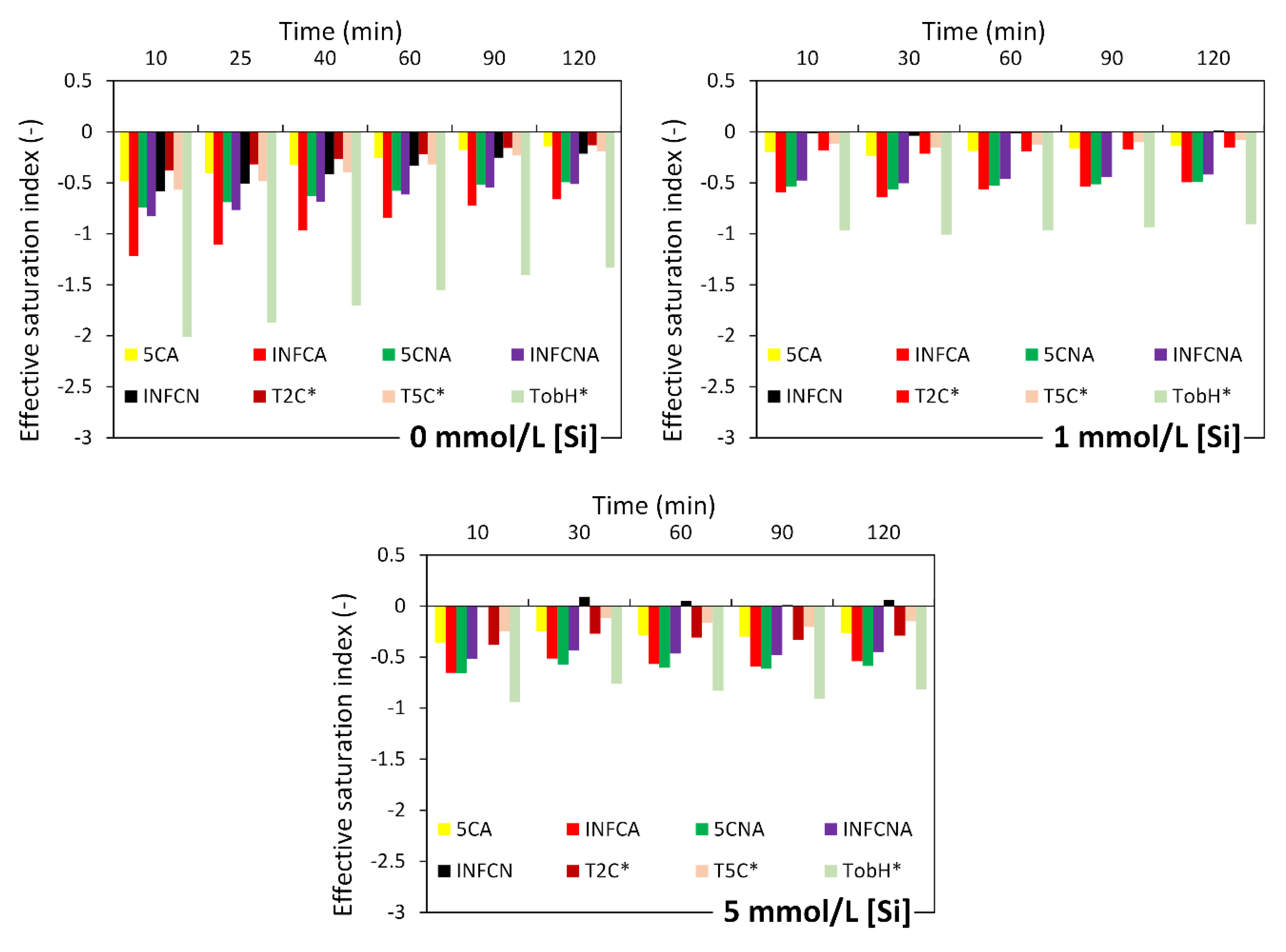
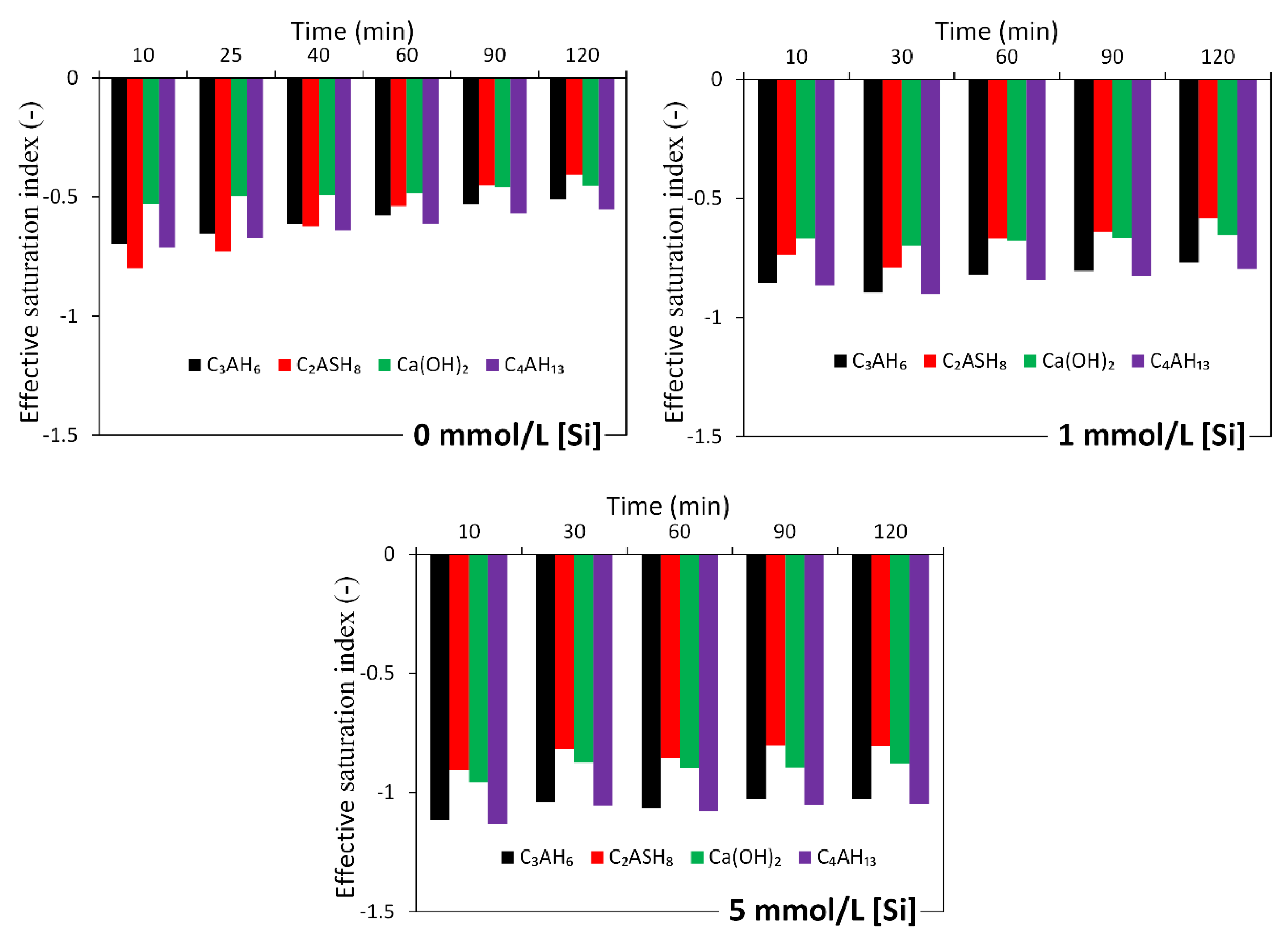
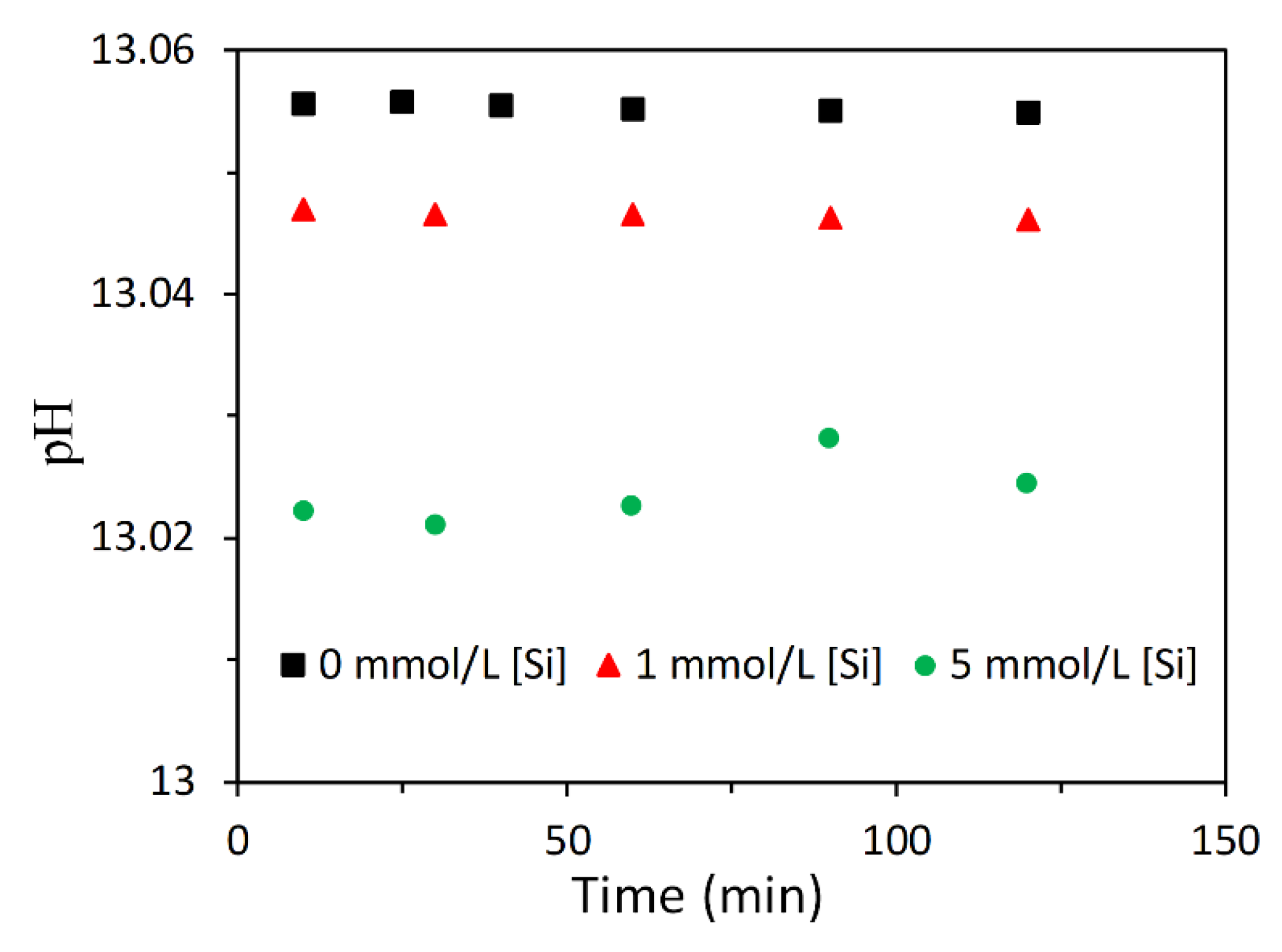
3.2.2. Effect of Soluble Si on the Dissolution of Slag
4. Discussion
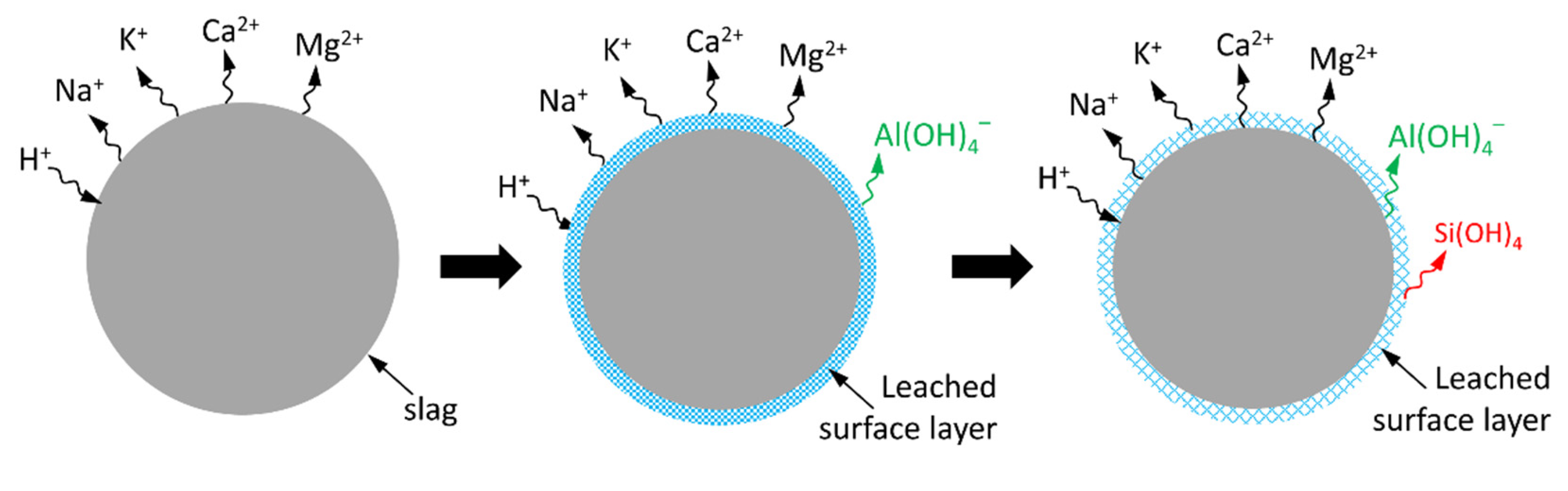
4.1. Dissolution of Slag
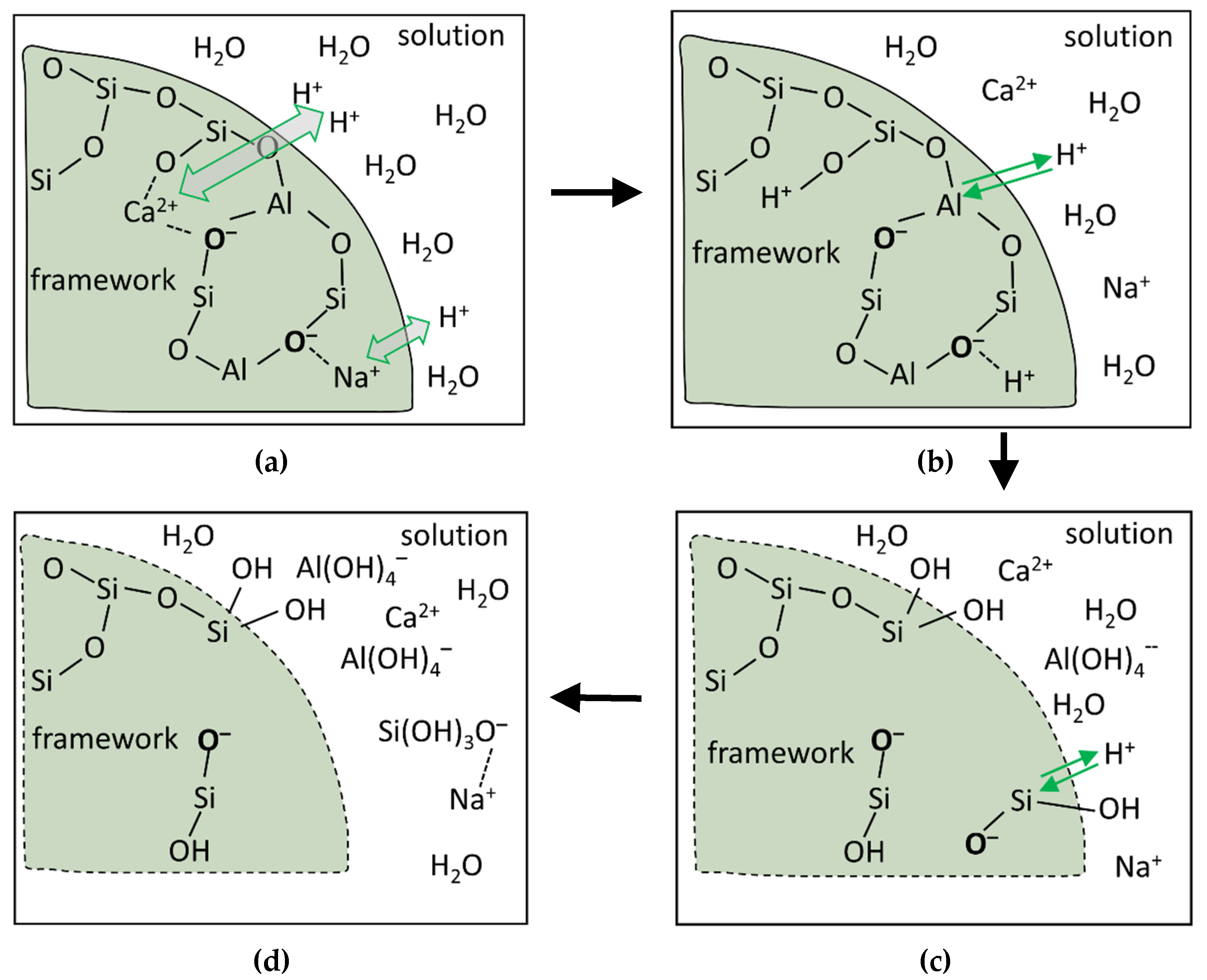
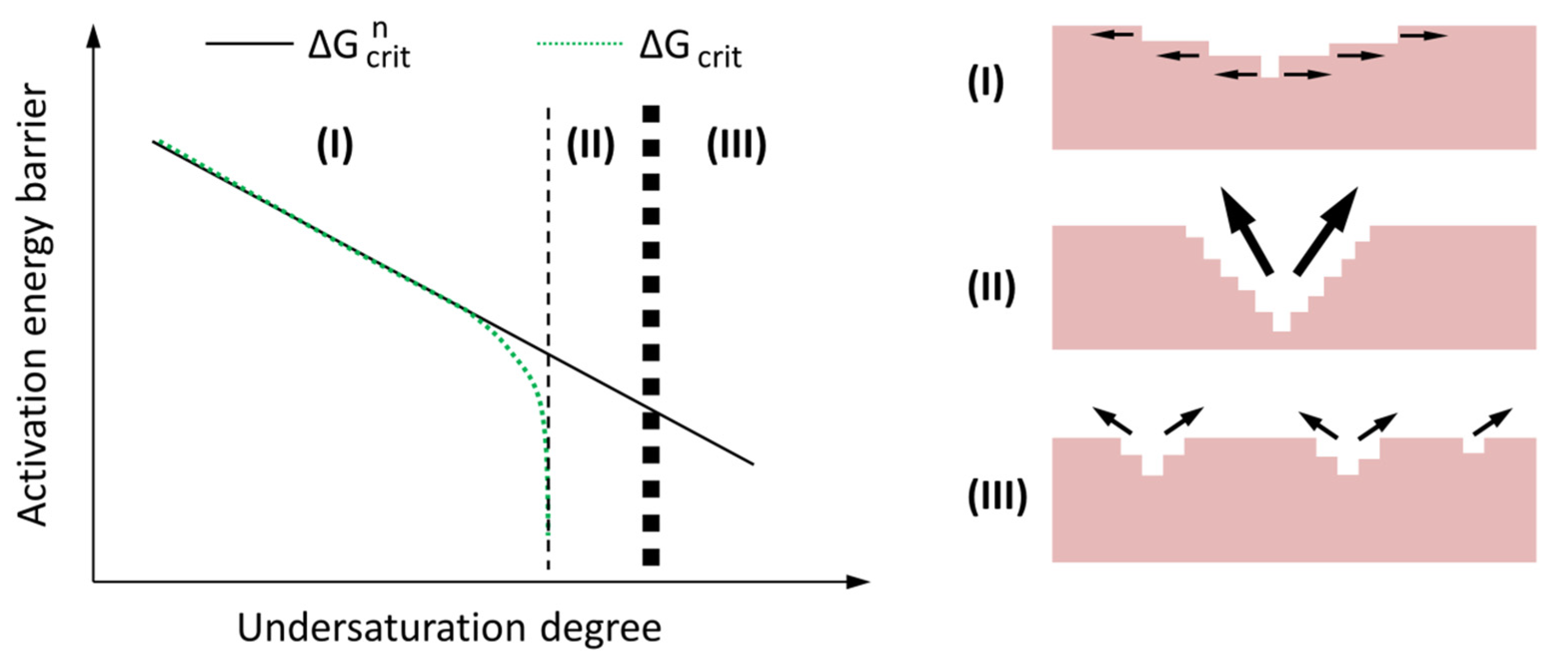
4.2. A Dissolution Theory Based Mechanism Applied to the Dissolution of Slag
4.3. Interpreting the Action Mechanism of Soluble Si in the Dissolution of Slag in Alkaline Solution
4.4. Interpreting the Effects of Soluble Ca and Al in Solution on the Reaction of Slag
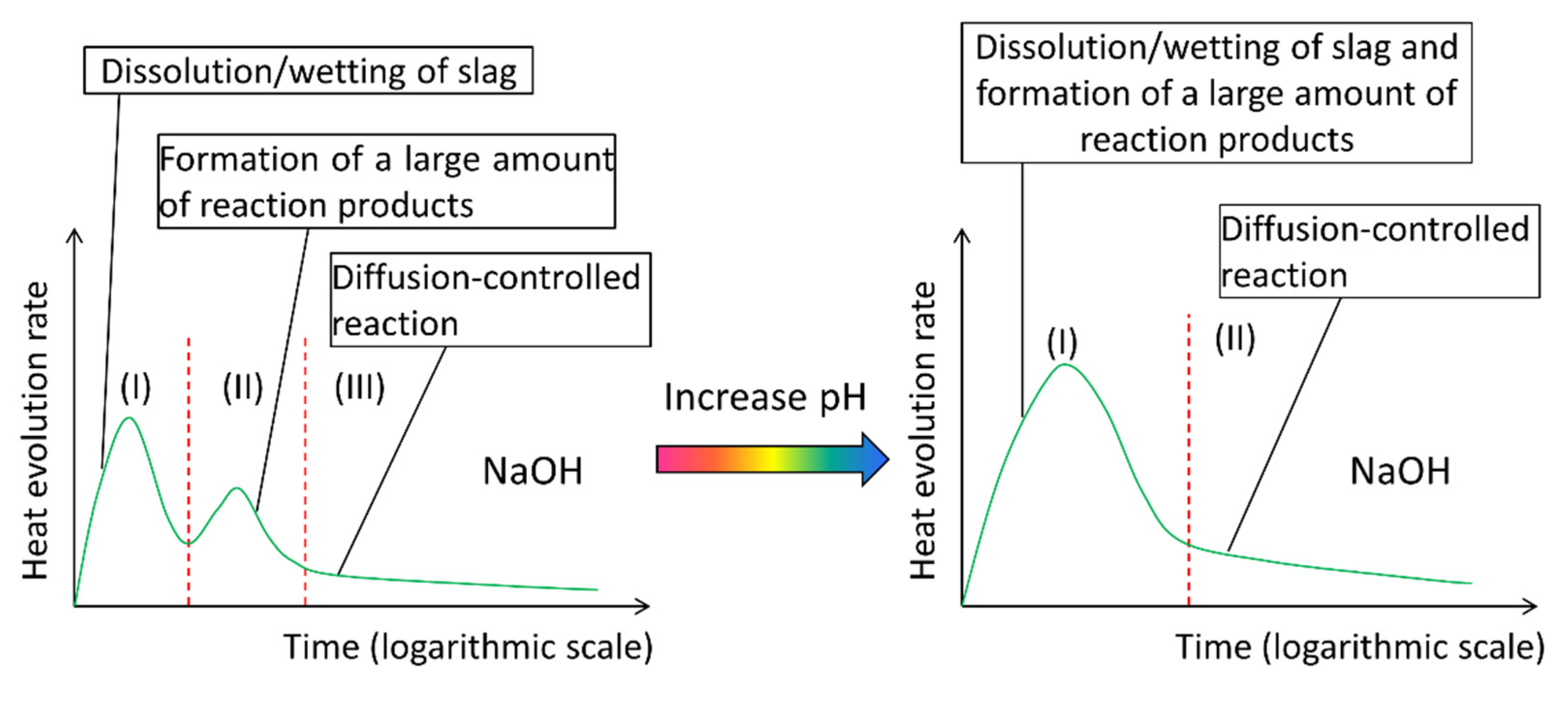
4.5. Interpreting the Reaction Process and Origin of the Induction Period of Alkali-Activated Slag
5. Conclusions
- The heat release rate curves of alkali-activated slag paste depended on the presence of soluble Si in alkaline activator. For NaOH activated slag paste, three reaction periods were identified according to P1 and P2. For sodium silicate activated slag paste, one more reaction period was found between P1 and P2, i.e., the induction period.
- The dissolution test revealed that the soluble Si in alkaline solution slowed down the dissolution of slag. The action mechanism of soluble Si, Ca and Al in the dissolution or reaction of slag was well interpreted by the dissolution theory-based mechanism. This demonstrates that the dissolution theory-based mechanism is applicable for describing the reaction process of alkali-activated slag paste, particularly for understanding the induction period.
- In NaOH activated slag paste, a large undersaturation degree resulted in rapid dissolution of slag, leading to no noticeable induction period. In sodium silicate activated slag paste, the undersaturation with respect to Si was very low that it could not supply sufficient energy to remove the activation energy barriers for the formation of vacancy islands and etch pits on the surface of slag. This retarded the dissolution of slag, as a result of which, an induction period occurred.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, S.-D.; Pu, X.-C.; Scrivener, K.; Pratt, P. Alkali-activated slag cement and concrete: A review of properties and problems. Adv. Cem. Res. 1995, 7, 93–102. [Google Scholar] [CrossRef]
- Roy, D.M. Alkali-activated cements opportunities and challenges. Cem. Concr. Res. 1999, 29, 249–254. [Google Scholar] [CrossRef]
- Van Deventer, J.S.J.; Provis, J.L.; Duxson, P. Technical and commercial progress in the adoption of geopolymer cement. Miner. Eng. 2012, 29, 89–104. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review: Part 1. Historical background, terminology, reaction mechanisms and hydration products. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef] [Green Version]
- Awoyera, P.; Adesina, A. A critical review on application of alkali activated slag as a sustainable composite binder. Case Stud. Constr. Mater. 2019, 11, e00268. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Yang, K.-H.; Song, J.-K.; Song, K.-I. Assessment of CO2 reduction of alkali-activated concrete. J. Clean. Prod. 2013, 39, 265–272. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, J.; Puertas, F. Alkali-activated slag mortars: Mechanical strength behaviour. Cem. Concr. Res. 1999, 29, 1313–1321. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Abdollahnejad, Z.; Camões, A.F.; Jamshidi, M.; Ding, Y. Durability of alkali-activated binders: A clear advantage over Portland cement or an unproven issue? Constr. Build. Mater. 2012, 30, 400–405. [Google Scholar] [CrossRef] [Green Version]
- Bernal, S.A.; Provis, J.L. Durability of alkali-activated materials: Progress and perspectives. J. Am. Ceram. Soc. 2014, 97, 997–1008. [Google Scholar] [CrossRef]
- Deir, E.; Gebregziabiher, B.S.; Peethamparan, S. Influence of starting material on the early age hydration kinetics, microstructure and composition of binding gel in alkali activated binder systems. Cem. Concr. Compos. 2014, 48, 108–117. [Google Scholar] [CrossRef]
- Altan, E.; Erdoğan, S.T. Alkali activation of a slag at ambient and elevated temperatures. Cem. Concr. Compos. 2012, 34, 131–139. [Google Scholar] [CrossRef]
- Haha, M.B.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag–Part II: Effect of Al2O3. Cem. Concr. Res. 2012, 42, 74–83. [Google Scholar] [CrossRef]
- Gerstig, M.; Wadsö, L. A method based on isothermal calorimetry to quantify the influence of moisture on the hydration rate of young cement pastes. Cem. Concr. Res. 2010, 40, 867–874. [Google Scholar] [CrossRef]
- Xu, Q.; Ruiz, J.M.; Hu, J.; Wang, K.; Rasmussen, R.O. Modeling hydration properties and temperature developments of early-age concrete pavement using calorimetry tests. Thermochim. Acta 2011, 512, 76–85. [Google Scholar] [CrossRef]
- Bentz, D.P.; Sato, T.; De la Varga, I.; Weiss, W.J. Fine limestone additions to regulate setting in high volume fly ash mixtures. Cem. Concr. Compos. 2012, 34, 11–17. [Google Scholar] [CrossRef]
- Siler, P.; Kratky, J.; De Belie, N. Isothermal and solution calorimetry to assess the effect of superplasticizers and mineral admixtures on cement hydration. J. Therm. Anal. Calorim. 2012, 107, 313–320. [Google Scholar] [CrossRef]
- Gruyaert, E.; Robeyst, N.; De Belie, N. Study of the hydration of Portland cement blended with blast-furnace slag by calorimetry and thermogravimetry. J. Therm. Anal. Calorim. 2010, 102, 941–951. [Google Scholar] [CrossRef]
- Han, F.; Liu, R.; Wang, D.; Yan, P. Characteristics of the hydration heat evolution of composite binder at different hydrating temperature. Thermochim. Acta 2014, 586, 52–57. [Google Scholar] [CrossRef]
- Ravikumar, D.; Neithalath, N. Reaction kinetics in sodium silicate powder and liquid activated slag binders evaluated using isothermal calorimetry. Thermochim. Acta 2012, 546, 32–43. [Google Scholar] [CrossRef]
- Gebregziabiher, B.S.; Thomas, R.; Peethamparan, S. Very early-age reaction kinetics and microstructural development in alkali-activated slag. Cem. Concr. Compos. 2015, 55, 91–102. [Google Scholar] [CrossRef]
- Shi, C.J.; Day, R.L. A calorimetric study of early hydration of alkali-slag cements. Cem. Concr. Res. 1995, 25, 1333–1346. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.; Brouwers, H. Reaction kinetics, gel character and strength of ambient temperature cured alkali activated slag–fly ash blends. Constr. Build. Mater. 2015, 80, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Bernal, S.A.; Provis, J.L.; Rose, V.; De Gutierrez, R.M. Evolution of binder structure in sodium silicate-activated slag-metakaolin blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Jin, Y.; Feng, W.; Zheng, D.; Dong, Z.; Cui, H.; Li, M.; Sang, G.; Tang, W. Study on the interaction mechanism between slags and alkali silicate activators: A hydration kinetics approach. Constr. Build. Mater. 2020, 250, 118900. [Google Scholar] [CrossRef]
- Ouyang, X.; Ma, Y.; Liu, Z.; Liang, J.; Ye, G. Effect of the sodium silicate modulus and slag content on fresh and hardened properties of alkali-activated fly ash/slag. Minerals 2019, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Liu, Z.; Zhang, D.; Wang, J.; Huang, T.; Wang, D. Reaction kinetics and kinetics models of alkali activated phosphorus slag. Constr. Build. Mater. 2020, 237, 117728. [Google Scholar] [CrossRef]
- Gebregziabiher, B.S.; Thomas, R.J.; Peethamparan, S. Temperature and activator effect on early-age reaction kinetics of alkali-activated slag binders. Constr. Build. Mater. 2016, 113, 783–793. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Brouwers, H. The hydration of slag, Part 1: Reaction models for alkali-activated slag. J. Mater. Sci. 2007, 42, 428–443. [Google Scholar] [CrossRef]
- ASTM. Standard Practice for Measuring Hydration Kinetics of Hydraulic Cementitious Mixtures Using Isothermal Calorimetry; C1679-14; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Ye, G. Experimental Study and Numerical Simulation of the Development of the Microstructure and Permeability of Cementitious Materials. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2003. [Google Scholar]
- Zuo, Y.; Ye, G. Pore Structure Characterization of Sodium Hydroxide Activated Slag Using Mercury Intrusion Porosimetry, Nitrogen Adsorption, and Image Analysis. Materials 2018, 11, 1035. [Google Scholar] [CrossRef] [Green Version]
- Lothenbach, B. Thermodynamic equilibrium calculations in cementitious systems. Mater. Struct. 2010, 43, 1413–1433. [Google Scholar] [CrossRef] [Green Version]
- Kulik, D.A.; Wagner, T.; Dmytrieva, S.V.; Kosakowski, G.; Hingerl, F.F.; Chudnenko, K.V.; Berner, U.R. GEM-Selektor geochemical modeling package: Revised algorithm and GEMS3K numerical kernel for coupled simulation codes. Comput. Geosci. 2013, 17, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Wagner, T.; Kulik, D.A.; Hingerl, F.F.; Dmytrieva, S.V. GEM-Selektor geochemical modeling package: TSolMod library and data interface for multicomponent phase models. Can. Mineral. 2012, 50, 1173–1195. [Google Scholar] [CrossRef]
- Myers, R.J.; Bernal, S.A.; Provis, J.L. A thermodynamic model for C-(N-)A-S-H gel: CNASH_ss. Derivation and validation. Cem. Concr. Res. 2014, 66, 27–47. [Google Scholar] [CrossRef]
- Myers, R.J.; Lothenbach, B.; Bernal, S.A.; Provis, J.L. Thermodynamic modelling of alkali-activated slag cements. Appl. Geochem. 2015, 61, 233–247. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Lothenbach, B.; Gruskovnjak, A. Hydration of alkali-activated slag: Thermodynamic modelling. Adv. Cem. Res. 2007, 19, 81–92. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Richardson, I.G.; Brough, A.R.; Groves, G.W.; Dobson, C.M. The characterization of hardened alkali-activated blast-furnace slag pastes and the nature of the calcium silicate hydrate (C-S-H) phase. Cem. Concr. Res. 1994, 24, 813–829. [Google Scholar] [CrossRef]
- Zuo, Y.; Nedeljković, M.; Ye, G. Coupled thermodynamic modelling and experimental study of sodium hydroxide activated slag. Constr. Build. Mater. 2018, 188, 262–279. [Google Scholar] [CrossRef]
- Huanhai, Z.; Xuequan, W.; Zhongzi, X.; Mingshu, T. Kinetic study on hydration of alkali-activated slag. Cem. Concr. Res. 1993, 23, 1253–1258. [Google Scholar] [CrossRef]
- Helgeson, H.C.; Kirkham, D.H.; Flowers, G.C. Theoretical prediction of the thermodynamic behavior of aqueous electrolytes by high pressures and temperatures; IV, Calculation of activity coefficients, osmotic coefficients, and apparent molal and standard and relative partial molal properties to 600 degrees C and 5 kb. Am. J. Sci. 1981, 281, 1249–1516. [Google Scholar]
- Ben Haha, M.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Quantitative analysis of phase assemblage and chemical shrinkage of alkali-activated slag. J. Adv. Concr. Technol. 2016, 14, 245–260. [Google Scholar] [CrossRef] [Green Version]
- Oelkers, E.H.; Gislason, S.R. The mechanism, rates and consequences of basaltic glass dissolution: I. An experimental study of the dissolution rates of basaltic glass as a function of aqueous Al, Si and oxalic acid concentration at 25 C and pH = 3 and 11. Geochim. Cosmochim. Acta 2001, 65, 3671–3681. [Google Scholar] [CrossRef]
- Oelkers, E.H. General kinetic description of multioxide silicate mineral and glass dissolution. Geochim. Cosmochim. Acta 2001, 65, 3703–3719. [Google Scholar] [CrossRef]
- Gislason, S.R.; Oelkers, E.H. Mechanism, rates, and consequences of basaltic glass dissolution: II. An experimental study of the dissolution rates of basaltic glass as a function of pH and temperature. Geochim. Cosmochim. Acta 2003, 67, 3817–3832. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Schott, J.; Devidal, J.-L. The effect of aluminum, pH, and chemical affinity on the rates of aluminosilicate dissolution reactions. Geochim. Cosmochim. Acta 1994, 58, 2011–2024. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L. Designing precursors for geopolymer cements. J. Am. Ceram. Soc. 2008, 91, 3864–3869. [Google Scholar] [CrossRef]
- Lasaga, A.C.; Luttge, A. Variation of Crystal Dissolution Rate Based on a Dissolution Stepwave Model. Science 2001, 291, 2400–2404. [Google Scholar] [CrossRef]
- Dove, P.M.; Han, N.; De Yoreo, J.J. Mechanisms of classical crystal growth theory explain quartz and silicate dissolution behavior. Proc. Natl. Acad. Sci. USA 2005, 102, 15357–15362. [Google Scholar] [CrossRef] [Green Version]
- Juilland, P.; Gallucci, E.; Flatt, R.; Scrivener, K. Dissolution theory applied to the induction period in alite hydration. Cem. Concr. Res. 2010, 40, 831–844. [Google Scholar] [CrossRef]
- Suraneni, P.; Palacios, M.; Flatt, R.J. New insights into the hydration of slag in alkaline media using a micro-reactor approach. Cem. Concr. Res. 2016, 79, 209–216. [Google Scholar] [CrossRef]
- Nicoleau, L.; Schreiner, E.; Nonat, A. Ion-specific effects influencing the dissolution of tricalcium silicate. Cem. Concr. Res. 2014, 59, 118–138. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; van Deventer, J.S.J. Microscopy and microanalysis of inorganic polymer cements. 2: The gel binder. J. Mater. Sci. 2009, 44, 620–631. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S. The mechanism of geopolymer gel formation investigated through seeded nucleation. Colloids Surf. A Physicochem. Eng. Asp. 2008, 318, 97–105. [Google Scholar] [CrossRef]

| Oxide | SiO2 | CaO | Al2O3 | MgO | Fe2O3 | SO3 | K2O | TiO2 | LOI * |
|---|---|---|---|---|---|---|---|---|---|
| Weight (%) | 32.91 | 40.96 | 11.85 | 9.23 | 0.46 | 1.61 | 0.33 | 1.00 | 1.15 |
| Mixture | Slag (g) | Na2O (g) | SiO2 (g) | Ms† | Water (g) |
|---|---|---|---|---|---|
| N4S0 | 100 | 4 | 0 | 0 | 40 |
| N6S0 | 100 | 6 | 0 | 0 | 40 |
| N8S0 | 100 | 8 | 0 | 0 | 40 |
| N4S5.4 | 100 | 4 | 5.4 | 1.395 | 40 |
| N6S5.4 | 100 | 6 | 5.4 | 0.93 | 40 |
| N8S5.4 | 100 | 8 | 5.4 | 0.6975 | 40 |
| End-Member | Chemical Reactions | Log Ks0 |
|---|---|---|
| CNASH gel ideal solid solution eight end-members, ‘CNASH_ss’ model [36] | ||
| 5CA | (CaO)1.25·(Al2O3)0.125·(SiO2)·(H2O)1.625 | −10.75 |
| INFCA | (CaO)·(Al2O3)0.15625·(SiO2)1.1875·(H2O)1.65625 | −8.90 |
| 5CNA | (CaO)1.25·(Na2O)0.25·(Al2O3)0.125·(SiO2)·(H2O)1.25 | −10.40 |
| INFCNA | (CaO)·(Na2O)0.34375·(Al2O3)0.15625·(SiO2)1.1875·(H2O)1.3 | −10.00 |
| INFCN | (CaO)·(Na2O)0.3125·(SiO2)1.5·(H2O)1.1875 | −10.70 |
| T2C* | (CaO)1.5·(SiO2)·(H2O)2.5 | −11.60 |
| T5C* | (CaO)1.25·(SiO2)1.25·(H2O)2. | −10.50 |
| TobH* | (CaO)·(SiO2)1.5·(H2O)2.5 | −7.90 |
| Crystalline reaction products in alkali-activated slag [33,39] | ||
| C2ASH8 | (CaO)2·(Al2O3)·(SiO2)·(H2O)8 | −19.10 |
| C3AH6 | (CaO)3·(Al2O3)·(H2O)6 | −20.85 |
| Ca(OH)2 | Ca(OH)2 | −5.20 |
| C4AH13 | (CaO)4·(Al2O3)·(H2O)13 | −25.41 |
| [Si] | Element | 10 min | 25 min | 40 min | 60 min | 90 min | 120 min |
|---|---|---|---|---|---|---|---|
| 0 mmol/L | (Si) | 0.0786 | 0.0961 | 0.1400 | 0.1929 | 0.2464 | 0.2857 |
| (Al) | 0.0415 | 0.0459 | 0.0681 | 0.0900 | 0.1111 | 0.1270 | |
| (Ca) | 0.1300 | 0.1650 | 0.1775 | 0.1975 | 0.2500 | 0.2700 | |
| [Si] | element | 10 min | 30 min | 60 min | 90 min | 120 min | |
| 1 mmol/L | (Si) | 1.4500 | 1.4821 | 1.5000 | 1.5429 | 1.5679 | |
| (Al) | 0.0333 | 0.0296 | 0.0519 | 0.0556 | 0.0704 | ||
| (Ca) | 0.1400 | 0.1175 | 0.1350 | 0.1475 | 0.1625 | ||
| 5 mmol/L | (Si) | 5.1071 | 5.3214 | 5.0714 | 4.2143 | 4.7857 | |
| (Al) | 0.0444 | 0.0407 | 0.0407 | 0.0593 | 0.0481 | ||
| (Ca) | 0.0575 | 0.1050 | 0.0850 | 0.0725 | 0.0925 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, Y.; Ye, G. Preliminary Interpretation of the Induction Period in Hydration of Sodium Hydroxide/Silicate Activated Slag. Materials 2020, 13, 4796. https://doi.org/10.3390/ma13214796
Zuo Y, Ye G. Preliminary Interpretation of the Induction Period in Hydration of Sodium Hydroxide/Silicate Activated Slag. Materials. 2020; 13(21):4796. https://doi.org/10.3390/ma13214796
Chicago/Turabian StyleZuo, Yibing, and Guang Ye. 2020. "Preliminary Interpretation of the Induction Period in Hydration of Sodium Hydroxide/Silicate Activated Slag" Materials 13, no. 21: 4796. https://doi.org/10.3390/ma13214796
APA StyleZuo, Y., & Ye, G. (2020). Preliminary Interpretation of the Induction Period in Hydration of Sodium Hydroxide/Silicate Activated Slag. Materials, 13(21), 4796. https://doi.org/10.3390/ma13214796







