The Influence of the Continuous Casting Conditions on the Properties of High-Strength Two-Phase CuMg Alloys
Abstract
:1. Introduction
2. Experimental Procedures
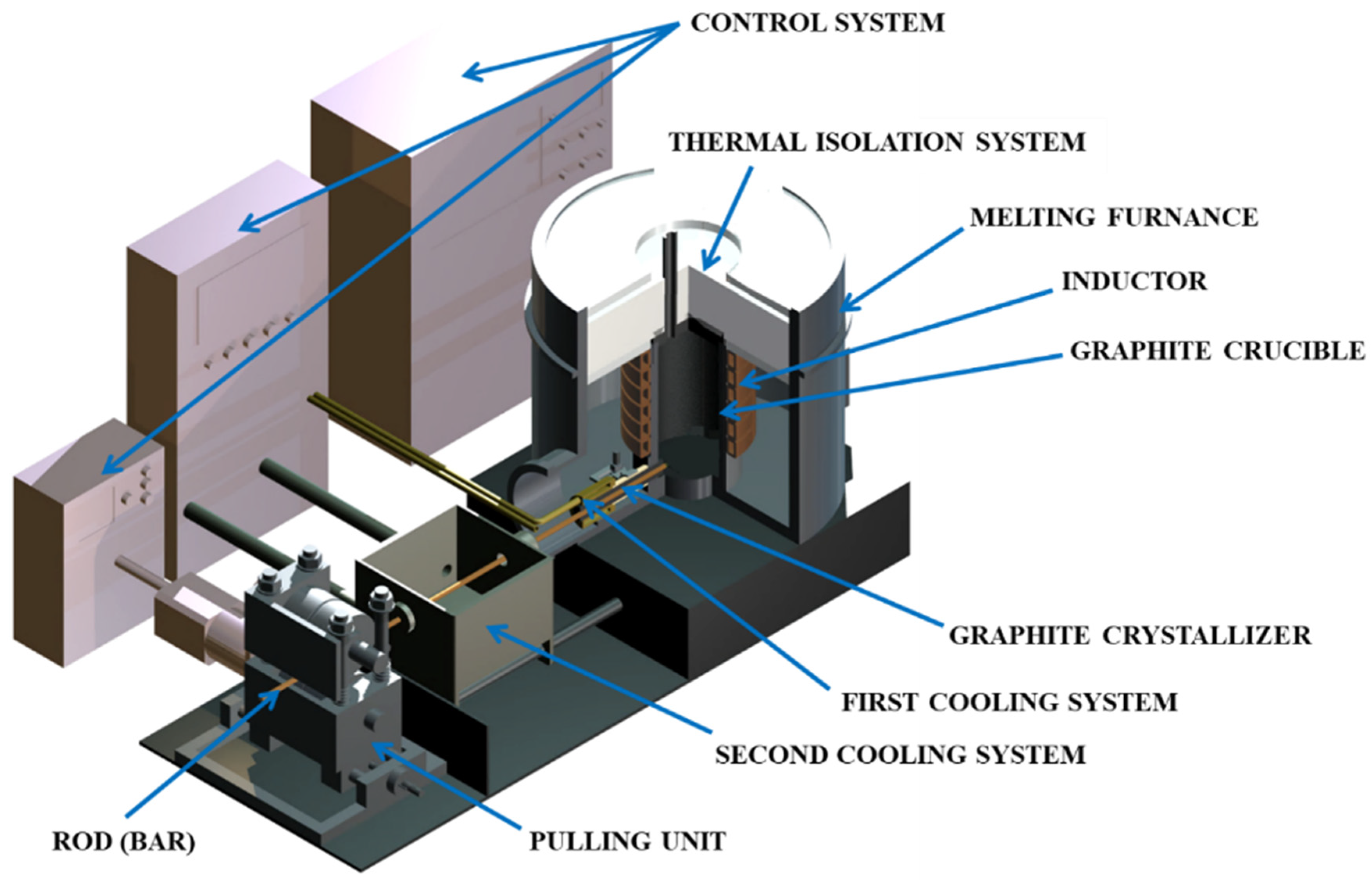
2.1. Metallurgical Synthesis
2.2. Electrical Conductivity and Hardness Measurements
2.3. SEM and XRD Observations
2.4. Compression Tests
3. Results and Discussion
3.1. Electrical Conductivity and Hardness Measurements
3.2. SEM and XRD Observations
3.3. Compression Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, P.; Tong, L.; Wang, J.; Shi, L.; Tang, H. Challenges and developments of copper wire bonding technology. Microelectron. Reliab. 2012, 52, 1092–1098. [Google Scholar] [CrossRef]
- Guo, N.; Li, D.; Yu, H.; Xin, R.; Zhang, Z.; Li, X.; Liu, C.; Song, B.; Chai, L. Annealing behavior of gradient structured copper and its effect on mechanical properties. Mater. Sci. Eng. A 2017, 702, 331–342. [Google Scholar] [CrossRef]
- Zasadzińska, M.; Knych, T.; Strzępek, P.; Jurkiewicz, B.; Franczak, K. Analysis of the strengthening and recrystallization of electrolytic copper (Cu-ETP) and oxygen free copper (Cu-OF). Arch. Civ. Mech. Eng. 2019, 19, 186–193. [Google Scholar] [CrossRef]
- Nishikawa, K.I.; Semboshi, S.; Konno, T.J. Transmission Electron Microscopy Observations on Cu-Mg Alloy Systems. SSP 2007, 127, 103–108. [Google Scholar] [CrossRef]
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A. Review of corrosive environments for copper and its corrosion inhibitors. Arab. J. Chem. 2020, 13, 481–544. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Lu, Z. In situ investigation of atmospheric corrosion behavior of copper under thin electrolyte layer and static magnetic field. Microelectron. Reliab. 2020, 108, 113630. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Lu, Z. Atmospheric corrosion behavior of copper under static magnetic field environment. Mater. Lett. 2020, 266, 127472. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, Q.; Qiang, L.; Zhang, B.; Zhang, J. Comparison study of gold coatings prepared by traditional and modified galvanic replacement deposition for corrosion prevention of copper. Microelectron. Reliab. 2020, 110, 113695. [Google Scholar] [CrossRef]
- Mashtalyar, D.; Gnedenkov, S.; Sinebryukhov, S.; Imshinetskiy, I.; Gnedenkov, A.; Bouznik, V. Composite coatings formed using plasma electrolytic oxidation and fluoroparaffin materials. J. Alloy Compd. 2018, 767, 353–360. [Google Scholar] [CrossRef]
- Sun, M.; Yerokhin, A.; Bychkova, M.; Shtansky, D.V.; Levashov, E.; Matthews, A. Self-healing plasma electrolytic oxidation coatings doped with benzotriazole loaded halloysite nanotubes on AM50 magnesium alloy. Corros. Sci. 2016, 111, 753–769. [Google Scholar] [CrossRef]
- Gnedenkov, S.; Sinebryukhov, S.; Egorkin, V.; Mashtalyar, D.; Vyaliy, I.; Nadaraia, K.; Imshinetskiy, I.; Nikitin, A.; Subbotin, E.; Gnedenkov, A. Magnesium fabricated using additive technology: Specificity of corrosion and protection. J. Alloy Compd. 2019, 808, 151629. [Google Scholar] [CrossRef]
- Karavai, O.; Bastos, A.; Zheludkevich, M.; Taryba, M.; Lamaka, S.V.; Ferreira, M.G.S. Localized electrochemical study of corrosion inhibition in microdefects on coated AZ31 magnesium alloy. Electrochim. Acta 2010, 55, 5401–5406. [Google Scholar] [CrossRef]
- Liu, G.; Jia, S.; Hong, S.; Lim, J.; Zhu, Y.; Mimura, K.; Isshiki, M. Corrosion resistance of dilute CuMg alloys at elevated temperature. Corros. Sci. 2009, 51, 463–468. [Google Scholar] [CrossRef]
- Schubert, K.; Anderko, K. Crystal Structure of the NiMg2, CuMg2, and AuMg3. Z. Metallkde 1951, 42 Pt 11, 321–325. [Google Scholar]
- Lieser, K.H.; Witte, H. The Ternary Systems Mg-Cu-Zn; Mg-Ni-Zn; Mg-Cu-Ni. Z. Metallkde 1952, 43, 396–401. [Google Scholar]
- Miroshnichenko, I.S.; Brekharya, G.P.; Bereza, E.Y. Formation of a Metastable Cubic Phase in the System Cu-Cu2Mg. Russ. Metall. 1980, 6, 226–229. [Google Scholar]
- Chen, W.; Sun, J. The electronic structure and mechanical properties of MgCu2 Laves phase compound. Phys. B Condens. Matter 2006, 382, 279–284. [Google Scholar] [CrossRef]
- Mao, P.; Yu, B.; Liu, Z.; Wang, F.; Ju, Y. Mechanical properties and electronic structures of MgCu2, Mg2Ca and MgZn2 Laves phases by first principles calculations. Trans. Nonferrous Met. Soc. China 2014, 24, 2920–2929. [Google Scholar] [CrossRef]
- Coughanowr, C.A.; Ansara, I.; Luoma, R.; Hämäläinen, M.; Lukas, H.L. Assessment of the Cu-Mg System. Z. Metallkde 1991, 82 Pt 7, 574–581. [Google Scholar]
- Rodriguez-Calvillo, P.; Ferrer, N.; Cabrera-Marrero, J.-M. Analysis of microstructure and strengthening in CuMg alloys deformed by equal channel angular pressing. J. Alloy Compd. 2015, 626, 340–348. [Google Scholar] [CrossRef]
- Ito, Y.; Matsunaga, H.; Mori, H.; Maki, K. Effect of Plastic Deformation on the Proof Strength and Electrical Conductivity of Copper-Magnesium Supersaturated Solid-Solution Alloys. Mater. Trans. 2014, 55, 1738–1741. [Google Scholar] [CrossRef] [Green Version]
- Gorsse, S.; Ouvrard, B.; Gouné, M.; Poulon-Quintin, A. Microstructural design of new high conductivity—High strength Cu-based alloy. J. Alloys Compd. 2015, 633 Pt 422015, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, C.; Ortega, I.; Jacobo, V.H.; Ortíz, A.; Bravo, A.E.; Schouwenaars, R. Microstructures of tribologically modified surface layers in two-phase alloys. IOP Conf. Series Mater. Sci. Eng. 2014, 63, 012018. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Tian, B.; Yakubov, V.; An, J.; Volinsky, A.A.; Liu, Y.; Song, K.; Li, L.; Fu, M. Effects of Ce and Y addition on microstructure evolution and precipitation of Cu-Mg alloy hot deformation. J. Alloy Compd. 2019, 781, 118–130. [Google Scholar] [CrossRef]
- Onuk, G.A. The use of copper and copper alloys in railway systems. In Proceedings of the Uluslar arası Raylı Sistemler Mühendisliği Sempozyumu (ISERSE’13), Karabük, Turkey, 9–11 October 2013. [Google Scholar]
- Yuan, X.; Li, G.; Zhang, X.; Pu, J.; Ren, P. An experimental investigation on fretting wear behavior of copper–magnesium alloy. Wear 2020, 203497. [Google Scholar] [CrossRef]
- Lin, H.; Hong, S. Effects of Mg content and annealing treatment on optical and electrical properties of CuMg and ITO/CuMg metallic glass films. J. Alloy Compd. 2018, 731, 248–252. [Google Scholar] [CrossRef]
- Tong, Y.-X.; Li, S.-Y.; Zhang, D.-T.; Li, L.; Zheng, Y.-F. High strength and high electrical conductivity CuMg alloy prepared by cryorolling. Trans. Nonferrous Met. Soc. China 2019, 29, 595–600. [Google Scholar] [CrossRef]
- Maki, K.; Ito, Y.; Matsunaga, H.; Mori, H. Solid-solution copper alloys with high strength and high electrical conductivity. Scr. Mater. 2013, 68, 777–780. [Google Scholar] [CrossRef] [Green Version]
- Tabor, D. The hardness of solids. Rev. Phys. Technol. 1970, 1 Pt 3, 145–179. [Google Scholar] [CrossRef]









| Selected Properties and Parameters | CuMg4.5 | CuMg5 | ||
|---|---|---|---|---|
| Density (g/cm3) | 7.93 | 7.80 | ||
| Continuous casting parameters | Cooling medium velocity | |||
| Primary; Secondary | ||||
| 0.4 L/min; 0.1 L/min | ||||
| Cooling medium temperature | ||||
| In; Out | ||||
| 10 °C; 35–40 °C | ||||
| Liquid metal temperature | ||||
| 1250 °C | ||||
| Cast rod temperature | ||||
| 190 °C–220 °C | ||||
| Standstill | ||||
| 2 s | ||||
| Feed | ||||
| 2 mm | 4 mm | 2 mm | 4 mm | |
| Element | wt.% | |||
| Mg | 4.4981 | 4.4831 | 5.0073 | 4.9922 |
| Cu | 95.4899 | 95.5018 | 94.9815 | 95.0008 |
| Ag | 0.0005 | 00.0008 | 0.0007 | 0.0005 |
| Zn | 0.00097 | 0.00149 | 0.00164 | 0.00073 |
| Pb | 0.00193 | 0.00185 | 0.00207 | 0.00123 |
| Fe | 0.00364 | 0.00379 | 0.00237 | 0.00223 |
| Ni | 0.00133 | 0.00116 | 0.00106 | 0.00101 |
| Sn | 0.00284 | 0.0047 | 0.00271 | 0.00071 |
| Si | 0.0003 | 0.0008 | 0.0002 | 0.0001 |
| Bi | 0.0005 | 0.0005 | 0.0005 | 0.0005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strzępek, P.; Mamala, A.; Zasadzińska, M.; Noga, P.; Sadzikowski, M. The Influence of the Continuous Casting Conditions on the Properties of High-Strength Two-Phase CuMg Alloys. Materials 2020, 13, 4805. https://doi.org/10.3390/ma13214805
Strzępek P, Mamala A, Zasadzińska M, Noga P, Sadzikowski M. The Influence of the Continuous Casting Conditions on the Properties of High-Strength Two-Phase CuMg Alloys. Materials. 2020; 13(21):4805. https://doi.org/10.3390/ma13214805
Chicago/Turabian StyleStrzępek, Paweł, Andrzej Mamala, Małgorzata Zasadzińska, Piotr Noga, and Michał Sadzikowski. 2020. "The Influence of the Continuous Casting Conditions on the Properties of High-Strength Two-Phase CuMg Alloys" Materials 13, no. 21: 4805. https://doi.org/10.3390/ma13214805





