Influence of Dispersing Method on the Quality of Nano-Admixtures Homogenization in Cement Matrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanomaterials and Chemical Admixtures
- nano-SiO2, nano-silica—dispersion of colloidal silica with approximately 50% solids by weight, the particles were spheres with the size 40–140 nm, density 1.4 g/cm3, viscosity 8 cP, pH 9.5 (product of Levasil OF8);
- nano-Fe3O4, nano-magnetite—the particles have a cubic structure with the size of about 50–100 nm, purity 97% (product of Sigma Aldrich 637106, (Sigma Aldrich, Saint Louis, MO, USA);
- nano-Pb3O4—the particles were spheres with the size 1–2 µm, purity 99% (product of Sigma Aldrich 241547).
2.2. Cement and Fine Aggregate
2.3. Mixtures Proportioning and Preparation of Mortar Specimens
2.4. Test Methods
2.4.1. Dispersing the Nanomaterials in Water
2.4.2. Mechanical Properties of the Mortars
3. Results and discussion
3.1. Dispersing of the Nanomaterials in Water
3.2. Mechanical Properties of Mortars
4. Sum Up and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Han, B.; Ding, S.; Wang, J.; Ou, J. Nano-Engineered Cementitious Composites. Principles and Practices; Springer: Singapore, 2019. [Google Scholar]
- Raki, L.; Beaudoin, J.; Alizadeh, R.; Makar, J.; Sato, T. Cement and concrete nanoscience and nanotechnology. Materials 2010, 3, 918–942. [Google Scholar] [CrossRef]
- Chung, S.; Leonard, J.; Nettleship, I.; Lee, J.; Soong, Y.; Martello, D.; Chyu, M. Characterization of ZnO nanoparticle suspension in water: Effectiveness of ultrasonic dispersion. Powder Technol. 2009, 194, 75–80. [Google Scholar] [CrossRef]
- Kawashima, S.; Seo, J.-W.; Corr, D.; Hersam, M.; Shah, S. Dispersion of CaCO3 nanoparticles by sonication and surfactant treatment for application in fly ash–cement systems. Mater. Struct. 2014, 47, 1011–1023. [Google Scholar] [CrossRef]
- Horszczaruk, E.; Mijowska, E.; Cendrowski, K.; Mijowska, S.; Sikora, P. Effect of incorporation route on dispersion of mesoporous silica nanospheres in cement mortar. Constr. Build. Mater. 2014, 66, 418–421. [Google Scholar] [CrossRef]
- Horszczaruk, E.; Mijowska, E.; Cendrowski, K.; Sikora, P. Influence of the new method of nanosilica addition on the mechanical properties of cement mortars. Cem. Wapno Beton 2014, 5, 308–316. [Google Scholar]
- Parveen, S.; Rana, S.; Fangueiro, R. A review on nanomaterial dispersion, microstructure, and mechanical properties of carbon nanotube and nanofiber reinforced cementitious composites. J. Nanomater. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Chen, S.; Collins, F.; MacLeod, A.; Pan, Z.; Duan, W.; Wang, C. Carbon nanotube–cement composites: A retrospect. IES J. Part A Civ. Struct. Eng. 2011, 4, 254–265. [Google Scholar] [CrossRef]
- Chuah, S.; Pan, Z.; Sanjayan, J.; Wang, C.; Duan, W. Nano reinforced cement and concrete composites and new perspective from graphene oxide. Constr. Build. Mater. 2014, 73, 113–124. [Google Scholar] [CrossRef]
- Leemann, A.; Lothenbach, B.; Thalmann, C. Influence of superplasticizers on pore solution composition and on expansion of concrete due to alkali-silica reaction. Constr. Build. Mater. 2011, 25, 344–350. [Google Scholar] [CrossRef]
- Le Saoût, G.; Lothenbach, B.; Hori, A.; Higuchi, T.; Winnefeld, F. Hydration of Portland cement with additions of calcium sulfoaluminates. Cem. Concr. Res. 2013, 43, 81–94. [Google Scholar] [CrossRef]
- Madani, H.; Bagheri, A.; Parhizkar, T. The pozzolanic reactivity of monodispersed nanosilica hydrosols and their influence on the hydration characteristics of Portland cement. Cem. Concr. Res. 2012, 42, 1563–1570. [Google Scholar] [CrossRef]
- Kirby, G.; Lewis, J. Comb polymer architecture effects on the rheological property evolution of concentrated cement suspensions. J. Am. Ceram. Soc. 2004, 87, 1643–1652. [Google Scholar] [CrossRef]
- Nazari, A.; Riahi, S. The role of SiO2 nanoparticles and ground granulated blast furnace slag admixtures on physical, thermal and mechanical properties of self- compacting concrete. Mat. Sci. Eng. A Struct. 2011, 528, 2149–2157. [Google Scholar] [CrossRef]
- Mendoza, O.; Sierra, G.; Tobón, J. Influence of super plasticizer and Ca(OH)2 on the stability of functionalized multi-walled carbon nanotubes dispersions for cement composites applications. Constr. Build. Mater. 2013, 47, 771–778. [Google Scholar] [CrossRef]
- Najigivi, A.; Khaloo, A.; Iraji, A.; Rashid, S. Investigating the effects of using different types of SiO2 nanoparticles on the mechanical properties of binary blended concrete. Compos. Part B Eng. 2013, 54, 52–58. [Google Scholar] [CrossRef]
- Yousefi, A.; Allahverdi, A.; Hejazi, P. Effective dispersion of nano-TiO2 powder for enhancement of photocatalytic properties in cement mixes. Constr. Build. Mater. 2013, 41, 224–230. [Google Scholar] [CrossRef]
- Nochaiya, T.; Chaipanich, A. Behavior of multi-walled carbon nanotubes on the porosity and microstructure of cement-based materials. Appl. Surf. Sci. 2011, 257, 1941–1945. [Google Scholar] [CrossRef]
- Fakhim, B.; Hassani, A.; Rashidi, A.; Ghodousi, P. Preparation and microstructural properties study on cement composites reinforced with multi-walled carbon nanotubes. J. Compos. Mater. 2015, 49, 85–98. [Google Scholar] [CrossRef]
- Kim, H.-K.; Nam, I.; Lee, H. Enhanced effect of carbon nanotube on mechanical and electrical properties of cement composites by incorporation of silica fume. Compos. Struct. 2014, 107, 60–69. [Google Scholar] [CrossRef]
- Sáez de Ibarra, Y.; Gaitero, J.; Erkizia, E.; Campillo, I. Atomic force microscopy and nanoindentation of cement pastes with nanotube dispersions. Phys. Status Solidi A 2006, 203, 1076–1081. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, W.; Jiang, H.; Wang, C. Effects of carbon nanotubes on mechanical and 2D-3D microstructure properties of cement mortar. J. Wuhan Univ. Technol. 2014, 29, 513–517. [Google Scholar] [CrossRef]
- Zou, B.; Chen, S.; Korayem, A.; Collins, F.; Wang, C.; Duan, W. Effect of ultrasonication energy on engineering properties of carbon nanotube reinforced cement pastes. Carbon 2015, 85, 212–220. [Google Scholar] [CrossRef]
- Mendoza, O.; Sierra, G.; Tobón, J. Effect of the reagglomeration process of multi-walled carbon nanotubes dispersions on the early activity of nanosilica in cement composites. Constr. Build. Mater. 2014, 54, 550–557. [Google Scholar] [CrossRef]
- Wiliński, D.; Łukowski, P.; Rokicki, G. Polymeric superplasticizers based on polycarboxylates for ready-mixed concrete: Current state of the art. Polimery 2016, 61, 474–481. [Google Scholar] [CrossRef][Green Version]
- Sobolev, K.; Flores, I.; Torres-Martinez, L.; Valdez, P.; Zarazua, E.; Cuellar, E. Engineering of SiO2 nanoparticles for optimal performance in nano cement-based materials. In Nanotechnology in Construction; Springer: Berlin/Heidelberg, Germany, 2009; pp. 139–148. [Google Scholar]
- Jo, B.-W.; Kim, C.-H.; Tae, G.; Park, J.-B. Characteristics of cement mortar with nano-SiO2 particles. Constr. Build. Mater. 2007, 21, 1351–1355. [Google Scholar] [CrossRef]
- Haruehansapong, S.; Pulngern, T.; Chucheepsakul, S. Effect of the particle size of nanosilica on the compressive strength and the optimum replacement content of cement mortar containing nano-SiO2. Constr. Build. Mater. 2014, 50, 471–477. [Google Scholar] [CrossRef]
- Li, H.; Xiao, H.; Yuan, J.; Ou, J. Microstructure of cement mortar with nanoparticles. Compos. Part B Eng. 2004, 35, 185–189. [Google Scholar] [CrossRef]
- Zhang, M.-H.; Islam, J.; Peethamparan, S. Use of nano-silica to increase early strength and reduce setting time of concretes with high volumes of slag. Cem. Concr. Comp. 2012, 34, 650–662. [Google Scholar] [CrossRef]
- Aly, M.; Hashmi, M.; Olabi, A.; Messeiry, M.; Abadir, M.; Hussain, A. Effect of colloidal nano-silica on the mechanical and physical behaviour of waste-glass cement mortar. Mater. Design 2012, 33, 127–135. [Google Scholar] [CrossRef]
- Qing, Y.; Zenan, Z.; Deyu, K.; Rongshen, C. Influence of nano-SiO2 addition on properties of hardened cement paste as compared with silica fume. Constr. Build. Mater. 2007, 21, 539–545. [Google Scholar] [CrossRef]
- Pan, Z.; He, L.; Qiu, L.; Korayem, A.; Li, G.; Zhu, J.; Collins, F.; Li, D.; Duan, W.; Wang, M. Mechanical properties and microstructure of a graphene oxide–cement composite. Cement Concrete Comp. 2015, 58, 140–147. [Google Scholar] [CrossRef]
- Horszczaruk, E.; Mijowska, E.; Kalenczuk, R.; Aleksandrzak, M.; Mijowska, S. Nanocomposite of cement/graphene oxide—Impact on hydration kinetics and Young’s modulus. Constr. Build. Mater. 2015, 78, 234–242. [Google Scholar] [CrossRef]
- Xia, Z.; Pezzini, S.; Treossi, E.; Giambastiani, G.; Corticelli, F.; Morandi, V.; Zanelli, A.; Bellani, V.; Palermo, V. The exfoliation of graphene in liquids by electrochemical, chemical, and sonication-assisted techniques: A nanoscale study. Adv. Funct. Mater. 2013, 23, 4684–4693. [Google Scholar] [CrossRef]
- Peyvandi, A.; Soroushian, P.; Balachandra, A.; Sobolev, K. Enhancement of the durability characteristics of concrete nanocomposite pipes with modified graphite nanoplatelets. Constr. Build. Mater. 2013, 47, 111–117. [Google Scholar] [CrossRef]
- Babak, F.; Abolfazl, H.; Alimorad, R.; Parviz, G. Preparation and mechanical properties of graphene oxide: Cement nanocomposites. Sci. World J. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Yan, S.; He, P.; Jia, D.; Yang, Z.; Duan, X.; Wang, S.; Zhou, Y. Effect of reduced graphene oxide content on the microstructure and mechanical properties of graphene–geopolymer nanocomposites. Ceram. Int. 2016, 42, 752–758. [Google Scholar] [CrossRef]
- Du, H.; Pang, S. Enhancement of barrier properties of cement mortar with graphene nanoplatelet. Cem. Concr. Res. 2015, 76, 10–19. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; White, J.; Xing, B. Graphene in the aquatic environment: Adsorption, dispersion, toxicity and transformation. Environ. Sci. Technol. 2014, 48, 9995–10009. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, X.; Ge, C.; Li, Q.; Guo, L.; Shu, X.; Liu, J. Investigation of the effectiveness of PC@GO on the reinforcement for cement composites. Constr. Build. Mater. 2016, 113, 470–478. [Google Scholar] [CrossRef]
- Yu, M.-F.; Lourie, O.; Dyer, M.; Moloni, K.; Kelly, T.; Ruoffet, R. Strength and breaking mechanism of multiwalled carbon nanotubes under tensile load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef]
- Fischer, G.; Li, V. Effect of fiber reinforcement on the response of structural members. Eng. Fract. Mech. 2007, 74, 258–272. [Google Scholar] [CrossRef]
- Al-Rub, R.; Ashour, A.; Tyson, B. On the aspect ratio effect of multiwalled carbon nanotube reinforcements on the mechanical properties of cementitious nanocomposites. Constr. Build. Mater. 2012, 35, 647–655. [Google Scholar] [CrossRef]
- Siddique, R.; Mehta, A. Effect of carbon nanotubes on properties of cement mortars. Constr. Build. Mater. 2014, 50, 116–129. [Google Scholar] [CrossRef]
- Sikora, P.; Elrahman, M.; Chung, S.-Y.; Cendrowski, K.; Mijowska, E.; Stephan, D. Mechanical and microstructural properties of cement pastes containing carbon nanotubes and carbon nanotube-silica core-shell structures, exposed to elevated temperature. Cem. Concr. Comp. 2019, 95, 193–204. [Google Scholar] [CrossRef]
- Czarnecki, L.; Łukowski, P. An Usability Approach to Technical Evaluation of the Polymer Coatings for Concrete Substrate. In Proceedings of the 2nd International RILEM Symposium on Adhesion Between Polymers and Concrete, Dresden, Germany, 14–17 September 1999; Volume 9, pp. 173–180. [Google Scholar]
- Sobolkina, A.; Mechtcherine, V.; Khavrus, V.; Maier, D.; Mende, M.; Ritschel, M.; Leonhardt, A. Dispersion of carbon nanotubes and its influence on the mechanical properties of the cement matrix. Cem. Concr. Comp. 2012, 34, 1104–1113. [Google Scholar] [CrossRef]
- Yazdanbakhsh, A.; Grasley, Z.; Tyson, B.; Al-Rub, R. Carbon nano filaments in cementitious materials: Some issues on dispersion and interfacial bond. Proc. Am. Concr. Inst. 2009, 267, 21–34. [Google Scholar]
- Yamada, K.; Takahashi, T.; Hanehara, S.; Matsuhisa, M. Effects of the chemical structure on the properties of polycarboxylate-type superplasticizer. Cem. Concr. Res. 2000, 30, 197–207. [Google Scholar] [CrossRef]
- European Committee for Standardization. BS-EN 197-1:2011. Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements; European Committee for Standardization: Brussels, Belgium, 2012. [Google Scholar]
- European Committee for Standardization. BS-EN 196-1:2016. Methods of Testing Cement. Determination of Strength; European Committee for Standardization: Brussels, Belgium, 2016. [Google Scholar]
- European Committee for Standardization. BS-EN 1008:2002. Mixing Water for Concrete. Specification for Sampling, Testing and Assessing the Suitability of Water, Including Water Recovered from Processes in the Concrete Industry, as Mixing Water for Concrete; European Committee for Standardization: Brussels, Belgium, 2002. [Google Scholar]
- Senff, L.; Hotza, D.; Repette, W.; Ferreira, V.; Labrincha, J. Mortars with nano-SiO2 and micro-SiO2 investigated by experimental design. Constr. Build. Mater. 2010, 24, 1432–1437. [Google Scholar] [CrossRef]
- Korayem, A.; Tourani, N.; Zakertabrizi, M.; Sabziparvar, A.; Duan, W. A review of dispersion of nanoparticles in cementitious matrices: Nanoparticle geometry perspective. Constr. Build. Mater. 2017, 153, 346–357. [Google Scholar] [CrossRef]
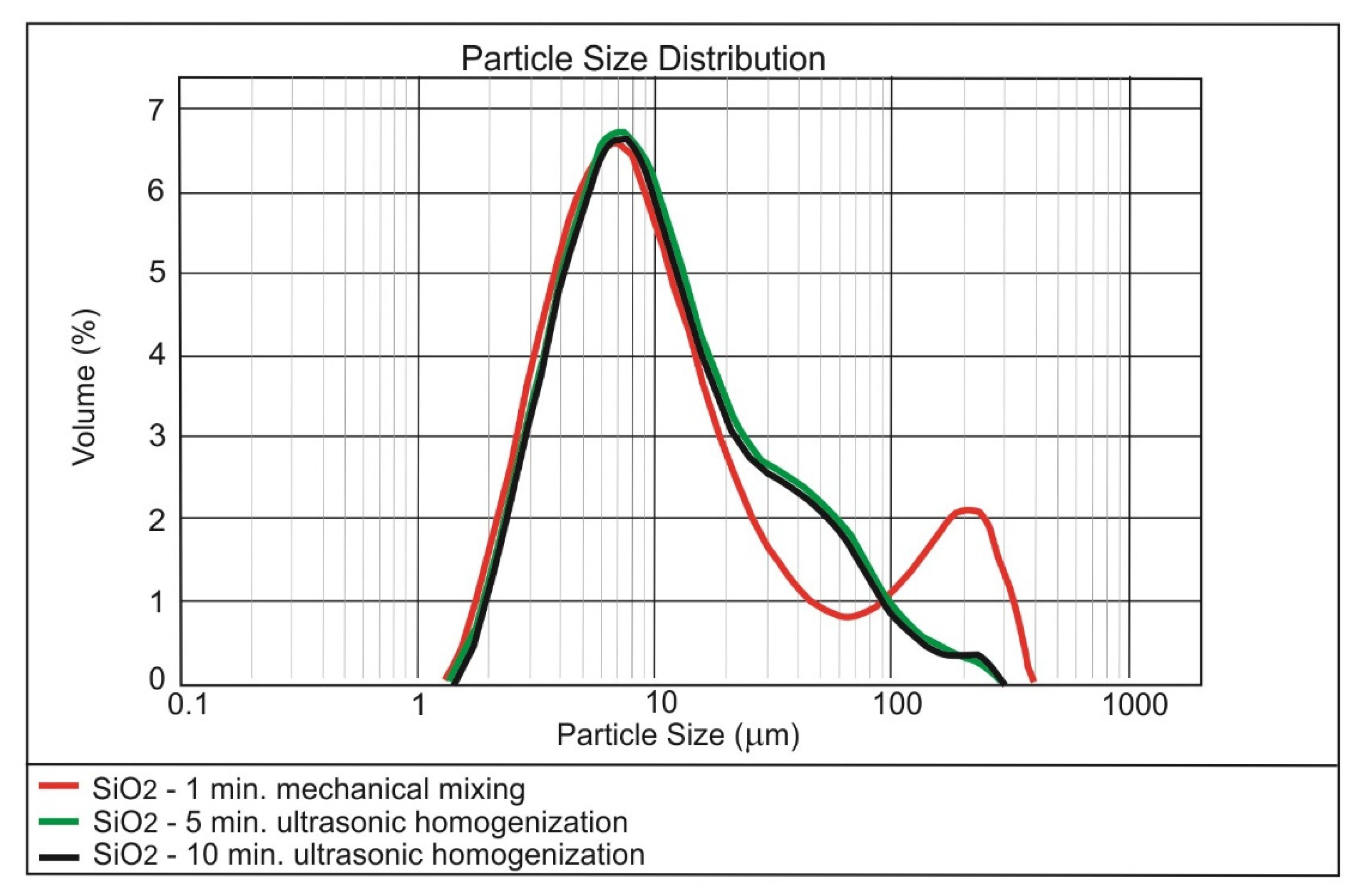

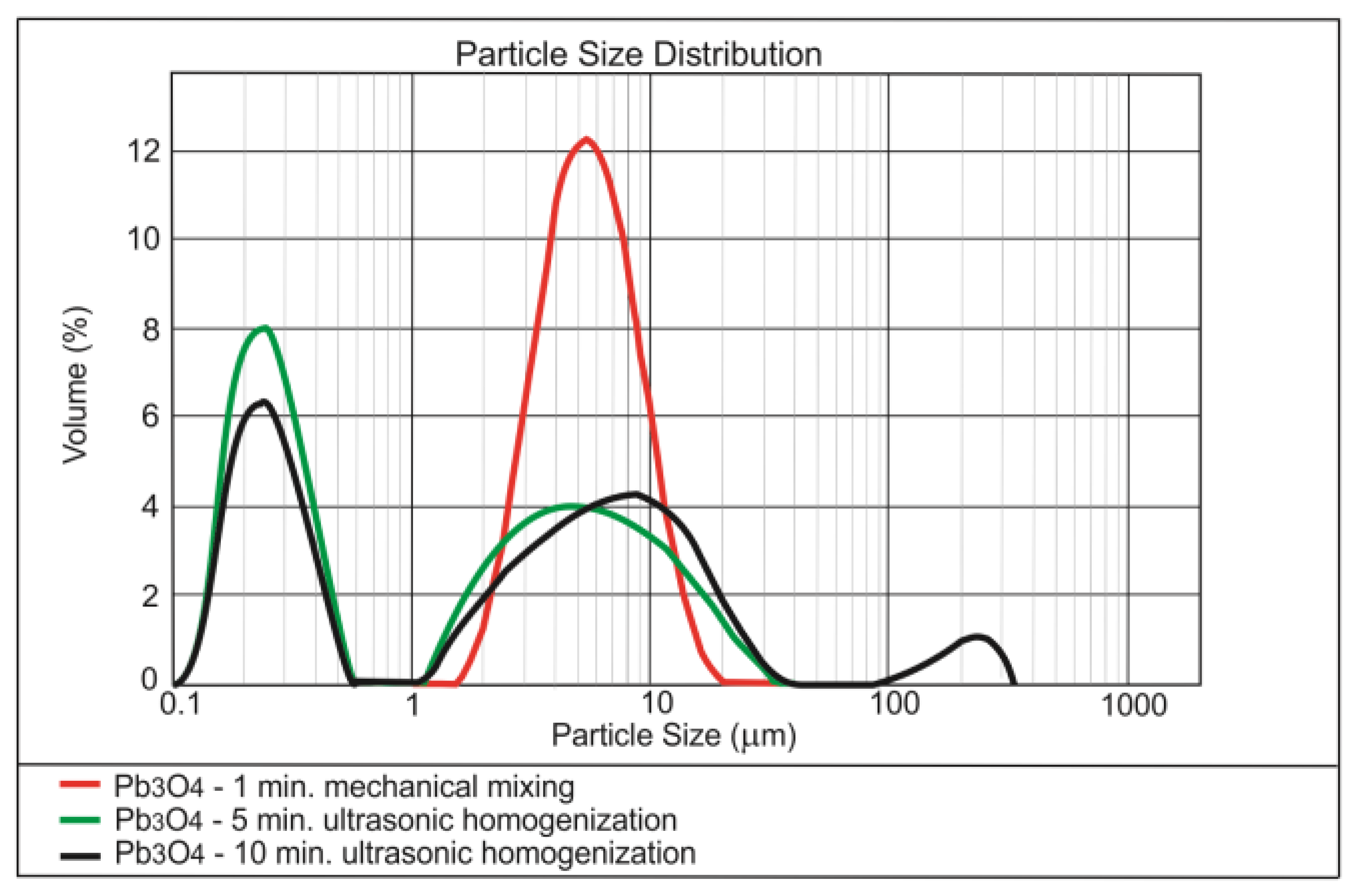
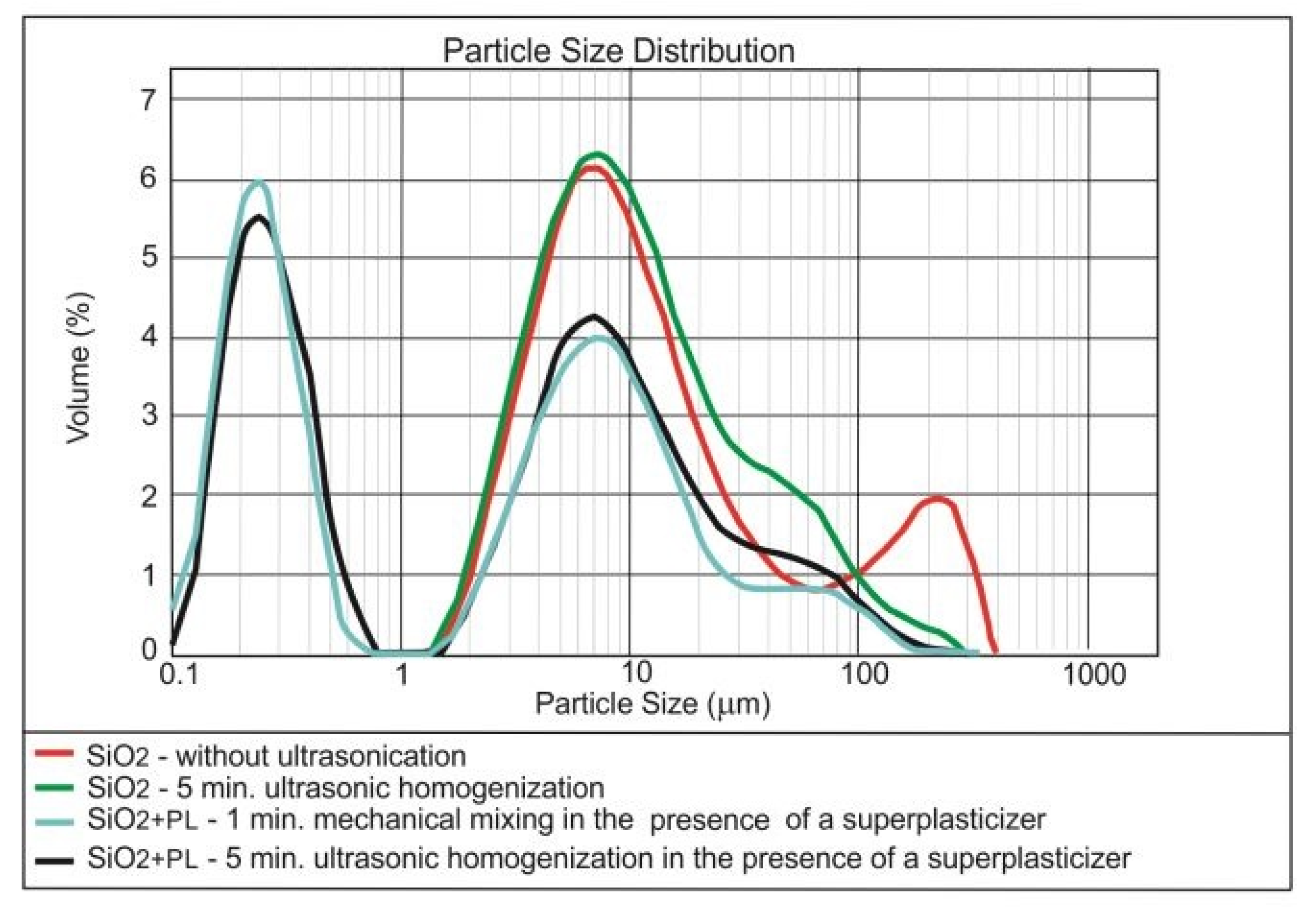

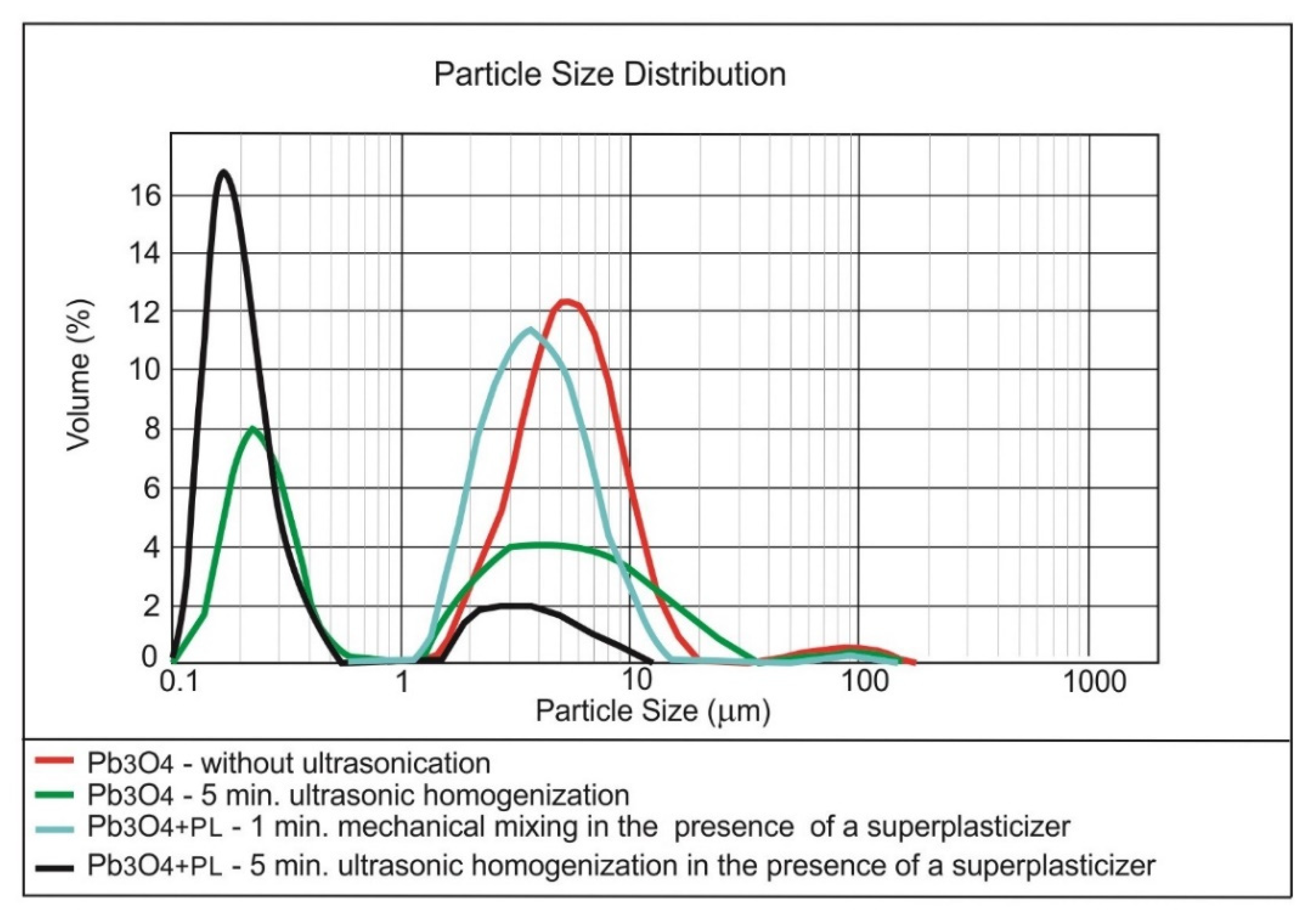
| Method | Form of Nanoparticles | Mechanism | Advantages | Disadvantages |
|---|---|---|---|---|
| Grinding | Powder | Grinding as fine as possible | The method is useful for the large volumes of nanoparticles | The method is slow and low-efficient |
| Magnetic and mechanical stirring | In liquid media | The stirrer rotating at the high speed, sufficient to create a vortex | The method is relatively inexpensive | The method is low-effective, re-agglomeration usually occurs when stopping to stir |
| High-speed homogenisation | In liquid media | High rate of rotation draws the material to the head, where it is mixed intensively The centrifugal force directs the material to the edges of the head, where it is mechanically cut inside the gap between the rotor and the stator | The method is useful for the large volumes of the liquids | The method is rarely used, mainly for the graphene oxide (GO) and other 2D nanomaterials |
| High-pressure homogenisation | In liquid media | Increase of the velocity of liquids streams under pressure inside the micro-channels causes shear and cavitation | The method is highly effective | The method usually causes the rise of temperature and is expensive |
| Ultrasonication | In liquid media | Use of the ultrasonic waves energy and cavitation in the water | The method is relatively inexpensive | The method can change the nanoparticles structure due to the rise of temperature. Efficiency of the process is unstable |
| Cement Type | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | Na2O | K2O | SO3 | Loss on Ignition LOI | Specific Density [g/cm3] | Surface Area [cm2/g] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CEM I 42.5 R | 63.29 | 19.52 | 4.88 | 2.91 | 1.31 | 0.10 | 0.90 | 2.79 | 3.01 | 3.05 | 3956 |
| Mix Designation | Sand [g] | Cement [g] | Water [g] | Water/Cement | SiO2 [g] | SiO2/Cement | Superplasticiser/Cement |
|---|---|---|---|---|---|---|---|
| R | 1546 | 518 | 257 | 0.5 | 0.00 | 0.0% | 0.0% |
| Method A | 1546 | 518 | 257 | 0.5 | 15.6 | 3.0% | 0.0% |
| Method B | 1546 | 518 | 257 | 0.5 | 15.6 | 3.0% | 0.0% |
| Method C | 1546 | 518 | 257 | 0.5 | 15.6 | 3.0% | 1.5% |
| Sample Designation | R | Method A | Method B | Method C |
|---|---|---|---|---|
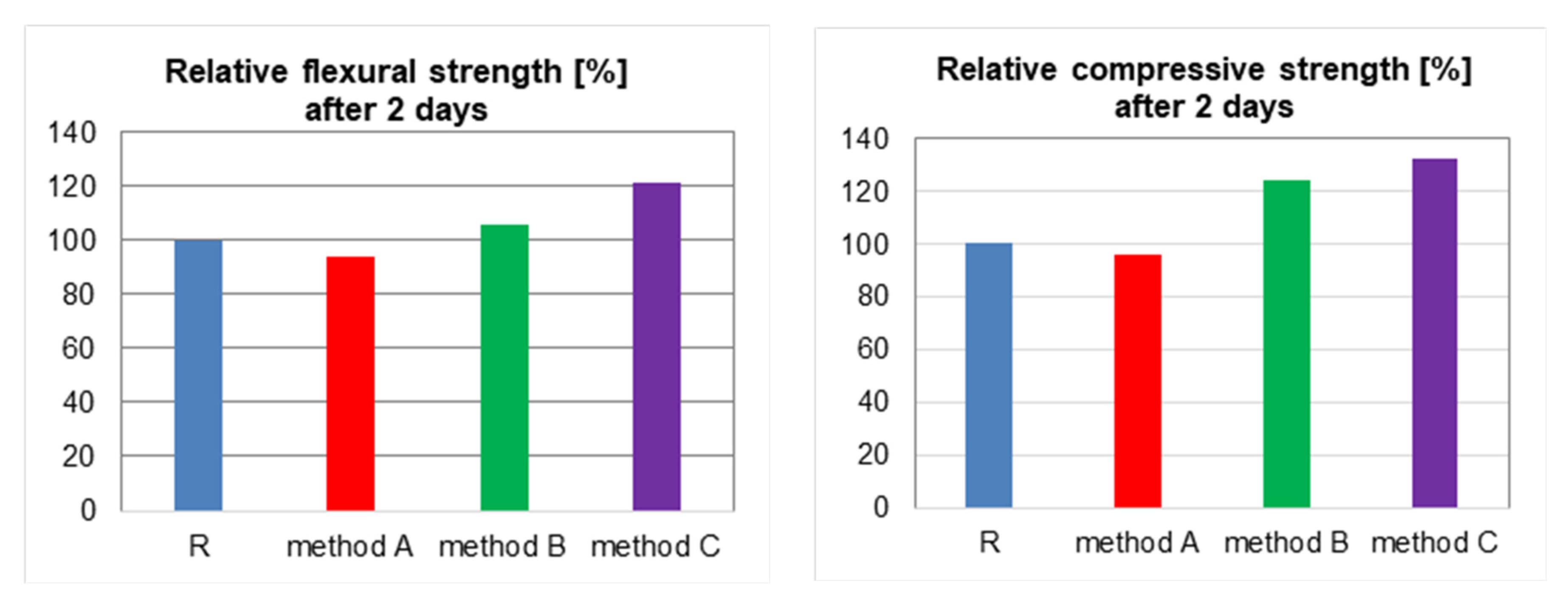
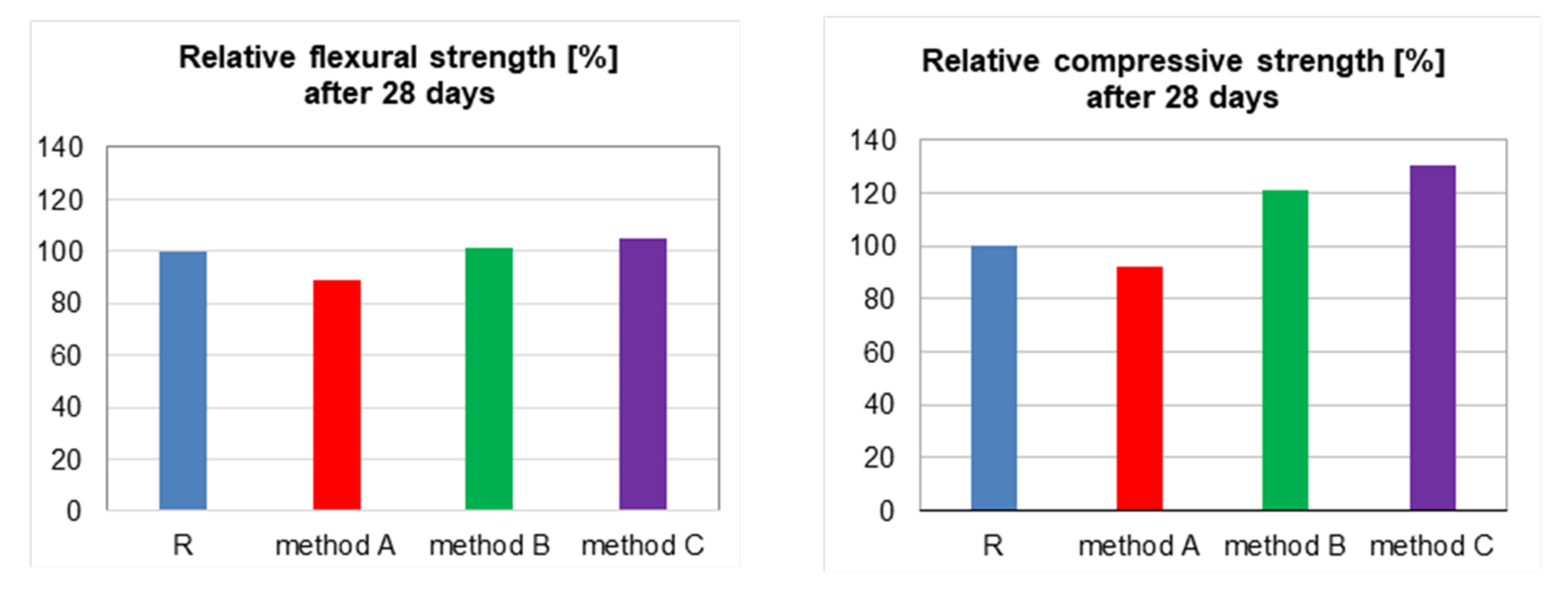
| Flexural strength after 2 days, MPa | 5.70 | 5.36 | 6.04 | 6.89 |
| Flexural strength after 28 days, MPa | 7.62 | 6.78 | 7.70 | 8.01 |
| Compressive strength after 2 days, MPa | 31.53 | 30.27 | 39.09 | 41.62 |
| Compressive strength after 28 days, MPa | 52.18 | 48.01 | 63.14 | 67.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horszczaruk, E.; Łukowski, P.; Seul, C. Influence of Dispersing Method on the Quality of Nano-Admixtures Homogenization in Cement Matrix. Materials 2020, 13, 4865. https://doi.org/10.3390/ma13214865
Horszczaruk E, Łukowski P, Seul C. Influence of Dispersing Method on the Quality of Nano-Admixtures Homogenization in Cement Matrix. Materials. 2020; 13(21):4865. https://doi.org/10.3390/ma13214865
Chicago/Turabian StyleHorszczaruk, Elżbieta, Paweł Łukowski, and Cyprian Seul. 2020. "Influence of Dispersing Method on the Quality of Nano-Admixtures Homogenization in Cement Matrix" Materials 13, no. 21: 4865. https://doi.org/10.3390/ma13214865
APA StyleHorszczaruk, E., Łukowski, P., & Seul, C. (2020). Influence of Dispersing Method on the Quality of Nano-Admixtures Homogenization in Cement Matrix. Materials, 13(21), 4865. https://doi.org/10.3390/ma13214865





