Synthesis of Porous Proton Ion Conducting Solid Polymer Blend Electrolytes Based on PVA: CS Polymers: Structural, Morphological and Electrochemical Properties
Abstract
1. Introduction
2. Experimental
2.1. Material and Preparation of Blended SPE Films
2.2. XRD and FESEM Study
2.3. Electrical Impedance Spectroscopy EIS
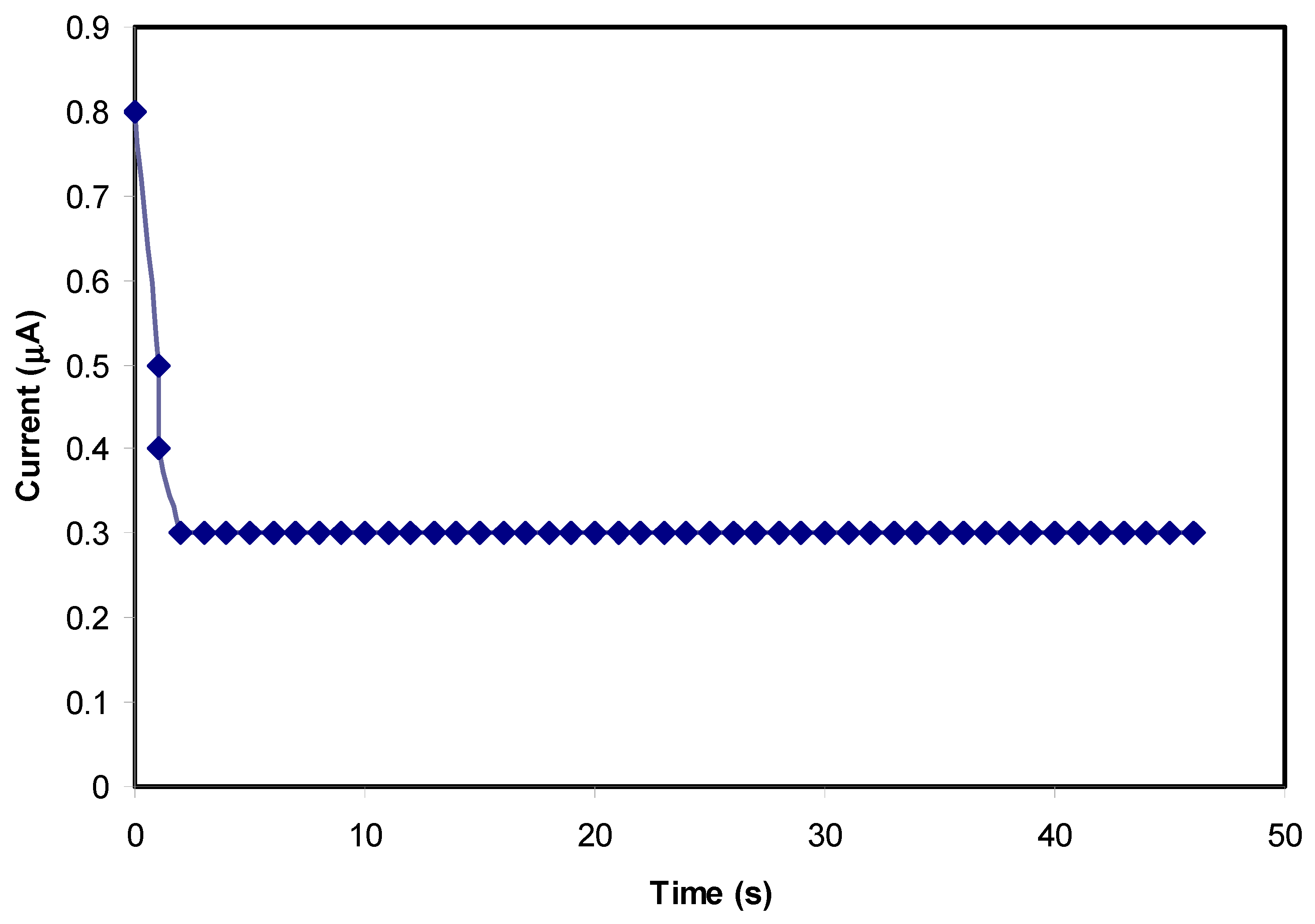
2.4. Transfer Number Measurement (TNM)
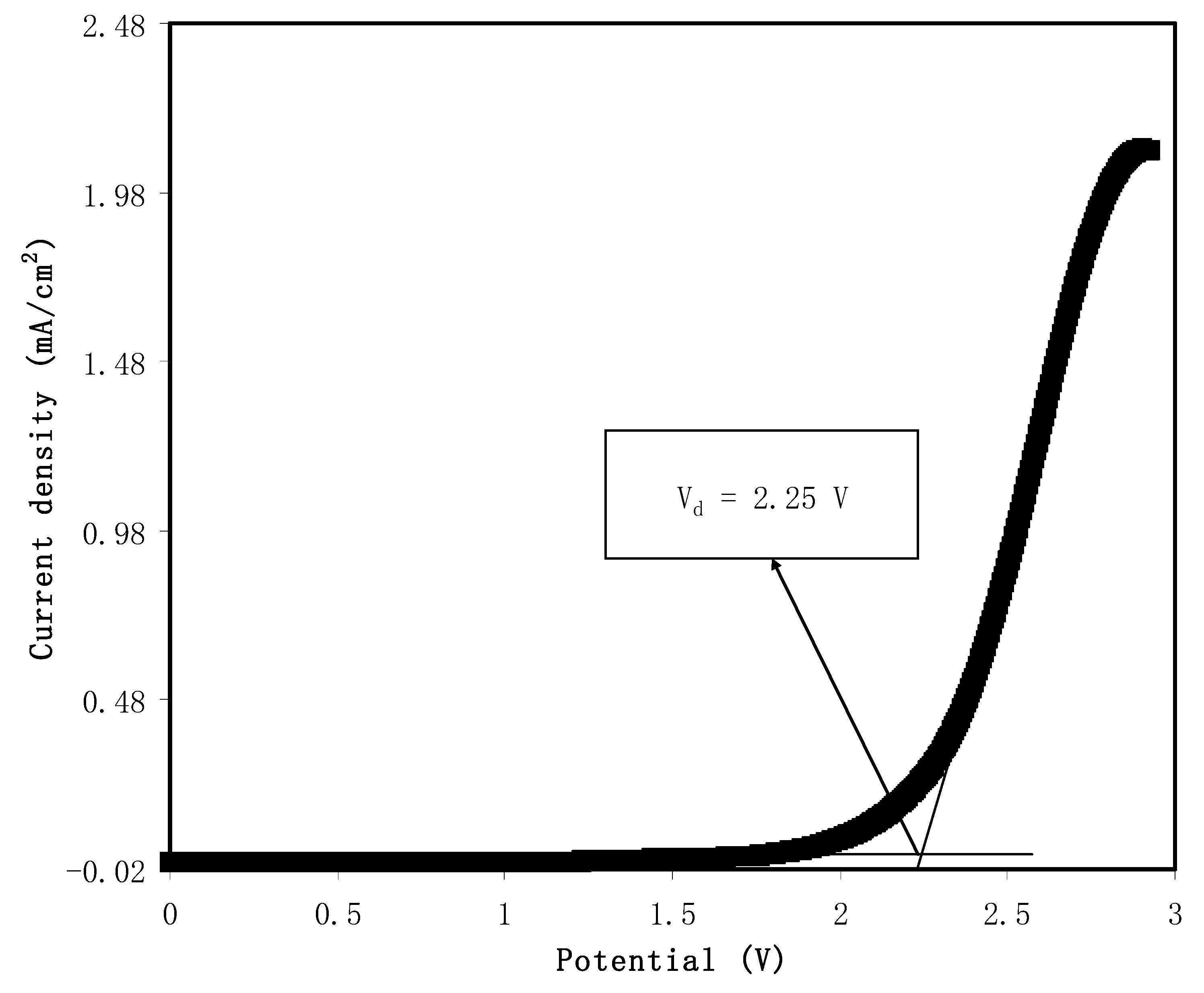
2.5. Linear Sweep Voltammetry LSV
3. Result and Discussions
3.1. XRD Analysis
3.2. Morphology Study
3.3. Impedance Analysis
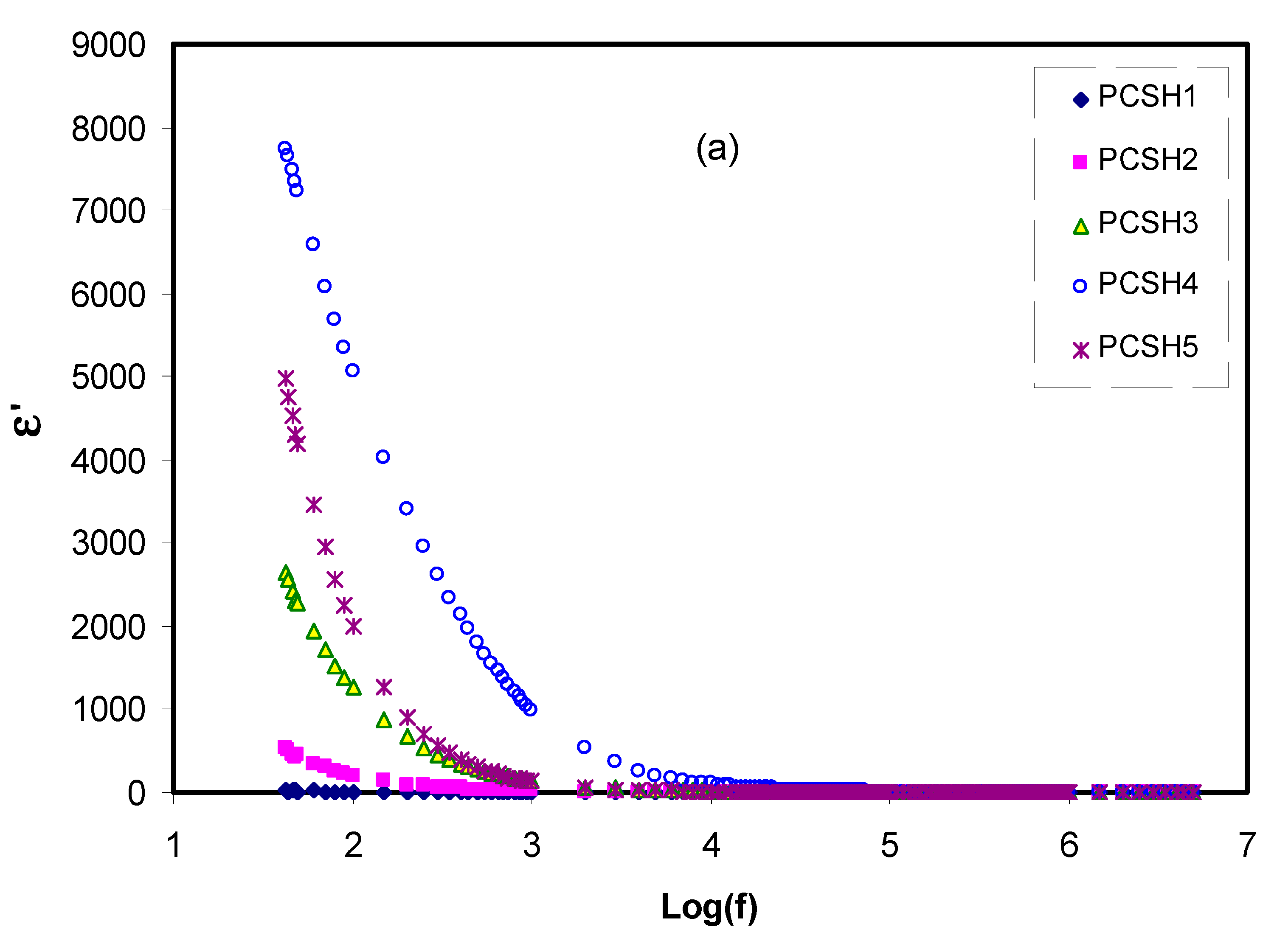
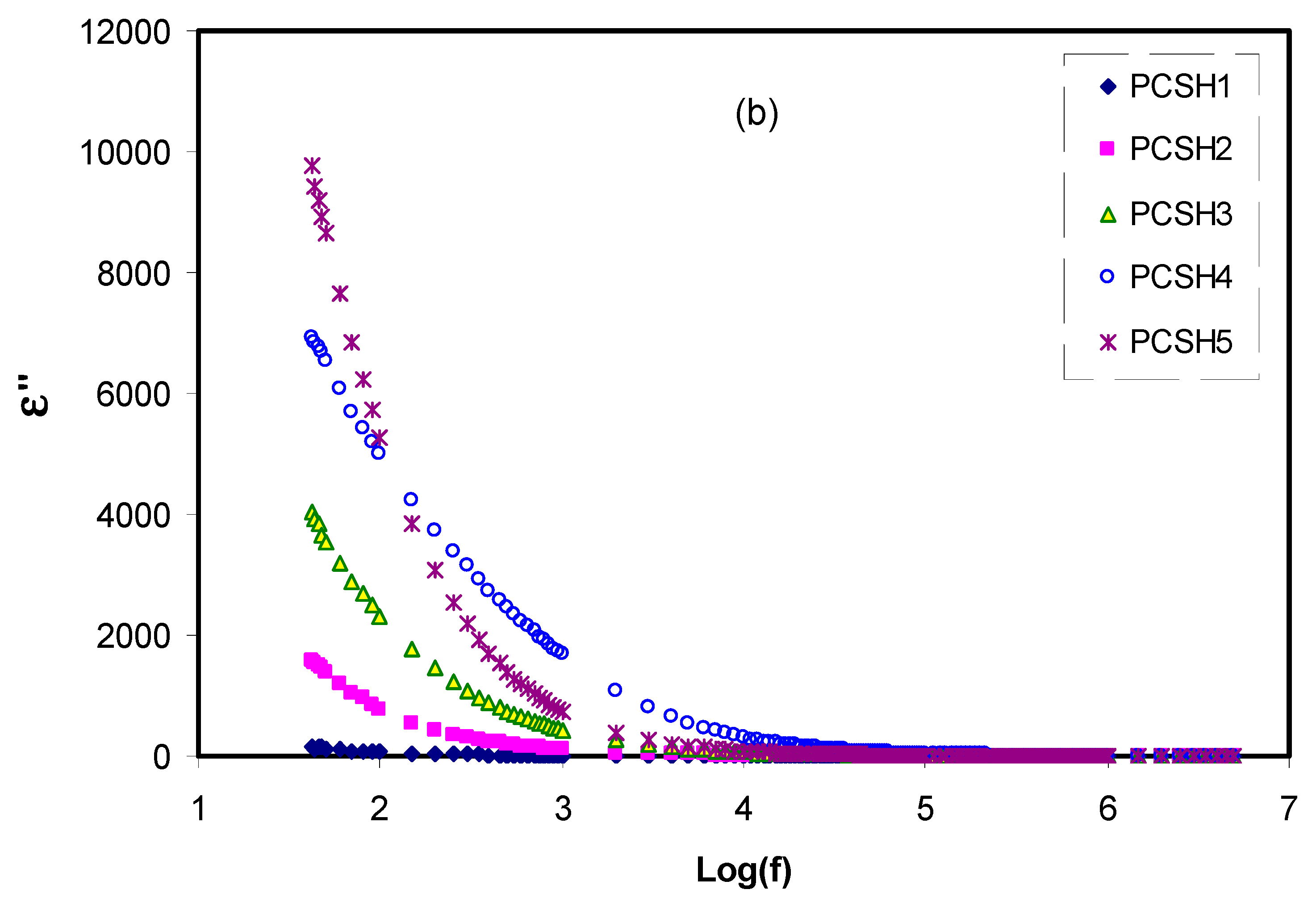
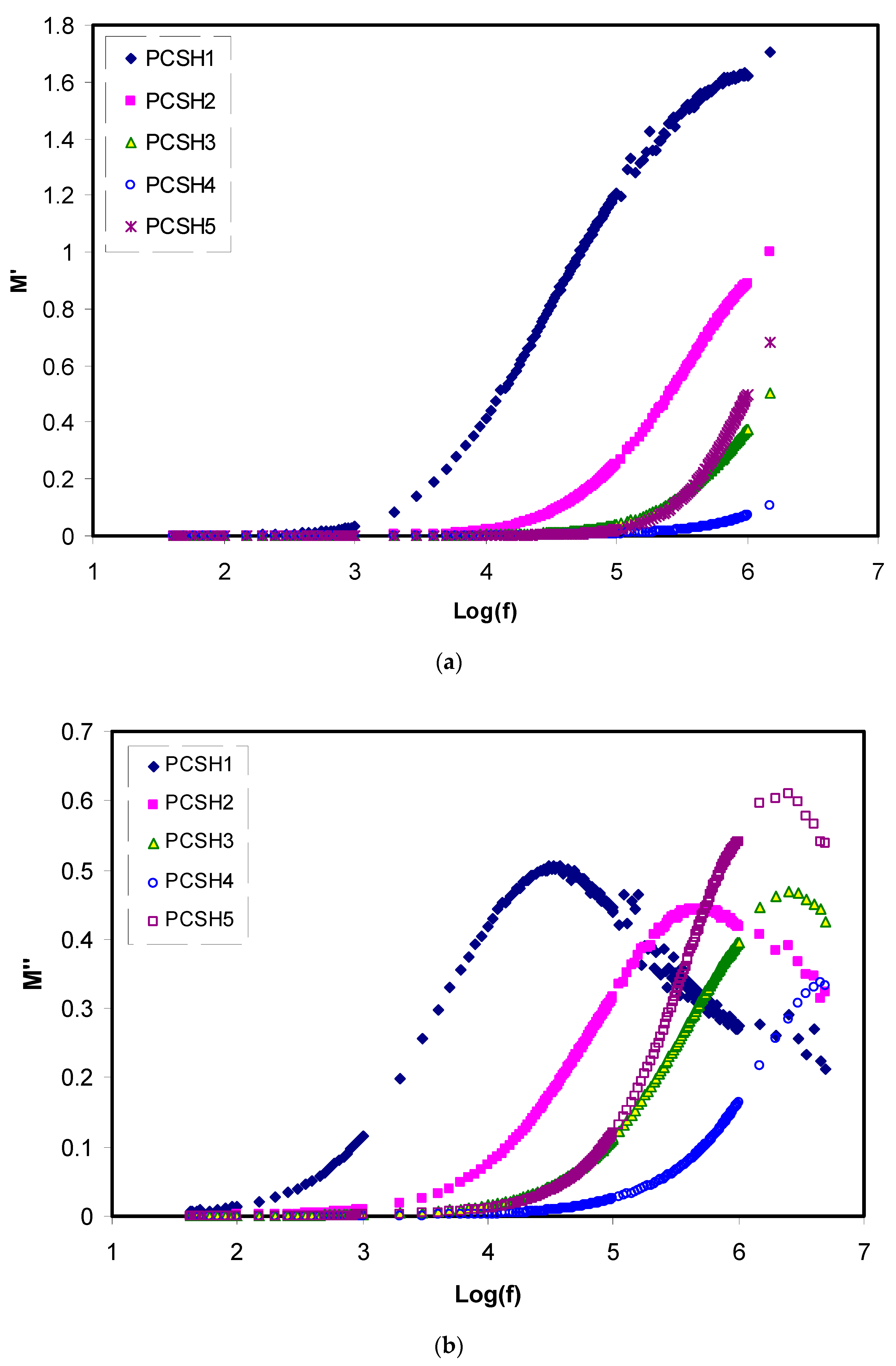
3.4. Dielectric and Electrical Modulus Study
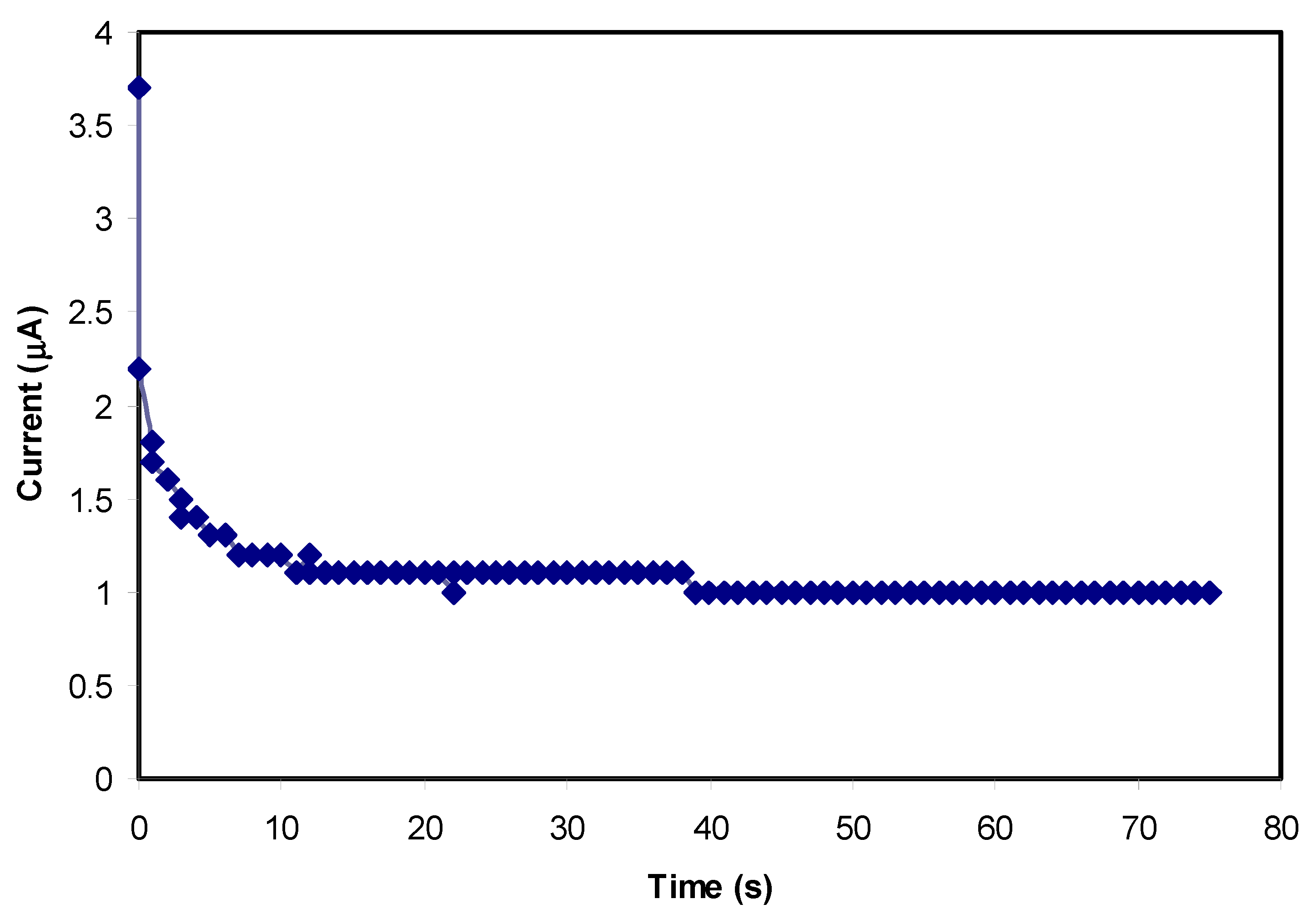
3.5. Transfer Number Measurement (TNM) Study
3.6. Linear Sweep Voltammetry (LSV) Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aziz, S.B.; Marif, R.B.; Brza, M.A.; Hamsan, M.H.; Kadir, M.F. Employing of Trukhan model to estimate ion transport parameters in PVA based solid polymer electrolyte. Polymers 2019, 11, 1694. [Google Scholar] [CrossRef] [PubMed]
- Hema, M.; Selvasekarapandian, S.; Arunkumar, D.; Sakunthala, A.; Nithya, H. FTIR, XRD and ac impedance spectroscopic study on PVA based polymer electrolyte doped with NH4X (X = Cl, Br, I). J. Non-Cryst. Solids 2009, 355, 84–90. [Google Scholar] [CrossRef]
- Yang, C.C.; Lin, S.J.; Wu, G.M. Study of ionic transport properties of alkaline poly(vinyl) alcohol-based polymer electrolytes. Mater. Chem. Phys. 2005, 92, 251–255. [Google Scholar] [CrossRef]
- Saroj, A.L.; Singh, R.K. Thermal, dielectric and conductivity studies on PVA/Ionic liquid [EMIM][EtSO4] based polymer electrolytes. J. Phys. Chem. Solids 2012, 73, 162–168. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, R.M.; Rasheed, M.A.; Ahmed, H.M. Role of ion dissociation on DC conductivity and silver nanoparticle formation in PVA:AgNt based polymer electrolytes: Deep insights to ion transport mechanism. Polymers 2017, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Polu, A.R.; Kumar, R. Preparation and characterization of pva based solid polymer electrolytes for electrochemical cell applications. Chin. J. Polym. Sci. 2013, 31, 641–648. [Google Scholar] [CrossRef]
- Farah, N.; Ng, H.; Numan, A.; Liew, C.-W.; Latip, N.; Ramesh, K.; Ramesh, S. Solid polymer electrolytes based on poly(vinyl alcohol) incorporated with sodium salt and ionic liquid for electrical double layer capacitor. Mater. Sci. Eng. B 2019, 251, 114468. [Google Scholar] [CrossRef]
- Aziz, S.B.; Kadir, M.F.Z.; Abidin, Z.H.Z. Structural, morphological and electrochemical impedance study of CS: LiTf based solid polymer electrolyte: Reformulated arrhenius equation for ion transport study. Int. J. Electrochem. Sci. 2016, 11, 9228–9244. [Google Scholar] [CrossRef]
- Wang, J.; Song, S.; Gao, S.; Muchakayala, R.; Liu, R.; Ma, Q. Mg-ion conducting gel polymer electrolyte membranes containing biodegradable chitosan: Preparation, structural, electrical and electrochemical properties. Polym. Test. 2017, 62, 278–286. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Hussein, S.A. Role of Silver Salts Lattice Energy on Conductivity Drops in Chitosan Based Solid Electrolyte: Structural, Morphological and Electrical Characteristics. J. Electron. Mater. 2018, 47, 3800–3808. [Google Scholar] [CrossRef]
- Osman, Z.; Ibrahim, Z.A.; Arof, A.K. Conductivity enhancement due to ion dissociation in plasticized chitosan based polymer electrolytes. Carbohydr. Polym. 2001, 44, 167–173. [Google Scholar] [CrossRef]
- Aziz, N.A.; Majid, S.R.; Arof, A.K. Synthesis and characterizations of phthaloyl chitosan-based polymer electrolytes. J. Non-Cryst. Solids 2012, 358, 1581–1590. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.A.; Mishra, K.; Hamsan, M.H.; Karim, W.O.; Abdullah, R.M.; Abdulwahid, R.T. Fabrication of high performance energy storage EDLC device from proton conducting methylcellulose: Dextran polymer blend electrolytes. J. Mater. Res. Technol. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Samsudin, A.S.; Lai, H.M.; Isa, M.I.N. Biopolymer materials based carboxymethyl cellulose as a proton conducting biopolymer electrolyte for application in rechargeable proton battery. Electrochim. Acta 2014, 129, 1–13. [Google Scholar] [CrossRef]
- Salleh, N.S.; Aziz, S.B.; Aspanut, Z.; Kadir, M.F.Z. Electrical impedance and conduction mechanism analysis of biopolymer electrolytes based on methyl cellulose doped with ammonium iodide. Ionics 2016, 22, 2157–2167. [Google Scholar] [CrossRef]
- Kesharwani, P.; Sahu, D.K.; Mahipal, Y.K.; Agrawal, R.C. Conductivity enhancement in K+-ion conducting dry Solid Polymer Electrolyte (SPE): [PEO: KNO3]: A consequence of KI dispersal and nano-ionic effect. Mater. Chem. Phys. 2017, 193, 524–531. [Google Scholar] [CrossRef]
- Pratap, R.; Singh, B.; Chandra, S. Polymeric rechargeable solid-state proton battery. J. Power Sources 2016, 161, 702–706. [Google Scholar] [CrossRef]
- Bandara, T.M.W.J.; Karunathilaka, D.G.N.; Ratnasekera, J.L.; Ajith De Silva, L.; Herath, A.C.; Mellander, B.E. Electrical and complex dielectric behaviour of composite polymer electrolyte based on PEO, alumina and tetrapropylammonium iodide. Ionics 2017, 23, 1711–1719. [Google Scholar] [CrossRef]
- Santamaria, M.; Pecoraro, C.; Di Franco, F.; Di Quarto, F. Phosphomolybdic acid and mixed phosphotungstic/phosphomolybdic acid chitosan membranes as polymer electrolyte for H2/O2 fuel cells. Int. J. Hydrogen Energy 2017, 42, 6211–6219. [Google Scholar] [CrossRef]
- Gagliardi, G.G.; Ibrahim, A.; Borello, D.; El-Kharouf, A. Composite Polymers Development and Application for Polymer Electrolyte Membrane Technologies—A Review. Molecules 2020, 25, 1712. [Google Scholar] [CrossRef]
- Higashihara, T.; Matsumoto, K.; Ueda, M. Sulfonated aromatic hydrocarbon polymers as proton exchange membranes for fuel cells. Polymers 2009, 50, 5341–5357. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Jia, X.; Li, W.; Zhang, Q.; Fan, L.; Ding, H.; Yang, H.; Yu, X.; Li, X.; et al. Graphene Armored with a Crystal Carbon Shell for Ultrahigh-Performance Potassium Ion Batteries and Aluminum Batteries. ACS Nano 2019, 13, 10631–10642. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Ding, H.; Chen, S.; Yu, X.; Lu, B. Carbon Nanoscrolls for Aluminum Battery. ACS Nano 2018, 12, 8456–8466. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, S.; Wang, J.; Lu, B. Carbon foam with microporous structure for high performance symmetric potassium dual-ion capacitor. J. Energy Chem. 2020, 43, 129–138. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Luacd, B. Plum pudding model inspired KVPO4F@3DC as high-voltage and hyperstable cathode for potassium ion batteries. Sci. Bull. 2020, 65, 1242–1251. [Google Scholar] [CrossRef]
- Fan, L.; Ma, R.; Zhang, Q.; Jia, X.; Lu, B. Graphite Anode for a Potassium-Ion Battery with Unprecedented Performance. Angew. Chem. Int. Ed. 2019, 58, 10500–10505. [Google Scholar] [CrossRef]
- Buraidah, M.; Arof, A. Characterization of chitosan/PVA blended electrolyte doped with NH4I. J. Non-Cryst. Solids 2011, 357, 3261–3266. [Google Scholar] [CrossRef]
- Kadir, M.; Aspanut, Z.; Majid, S.; Arof, A.K. FTIR studies of plasticized poly(vinyl alcohol)–chitosan blend doped with NH4NO3 polymer electrolyte membrane. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 1068–1074. [Google Scholar] [CrossRef]
- Mobarak, N.; Ahmad, A.; Abdullah, M.; Ramli, N.; Rahman, M.Y.A. Conductivity enhancement via chemical modification of chitosan based green polymer electrolyte. Electrochim. Acta 2013, 92, 161–167. [Google Scholar] [CrossRef]
- Pandele, A.M.; Ionita, M.; Crica, L.; Dinescu, S.; Costache, M.; Iovu, H. Synthesis, characterization, and in vitro studies of graphene oxide/chitosan–polyvinyl alcohol films. Carbohydr. Polym. 2014, 102, 813–820. [Google Scholar] [CrossRef]
- Yilmaz, E. Chitosan: A Versatile Biomaterial. Adv. Exp. Med. Biol. 2004, 553, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Ramya, R.; Venkatesan, J.; Kim, S.-K.; Sudha, P.N. Biomedical Applications of Chitosan: An Overview. J. Biomater. Tissue Eng. 2012, 2, 100–111. [Google Scholar] [CrossRef]
- Rosli, N.A.H.; Shyuan, L.K.; Wong, W.Y.; Yunus, R.M.; Khoon, L.T.; Ahmad, A.; Chong, S.T. Review of Chitosan-Based Polymers as Proton Exchange Membranes and Roles of Chitosan-Supported Ionic Liquids. Int. J. Mol. Sci. 2020, 21, 632. [Google Scholar] [CrossRef]
- Ikram, S.; Ahmed, S.; Ali, S.W.; Agarwal, H. Chitosan-Based Polymer Electrolyte Membranes for Fuel Cell Applications. In Organic-Inorganic Composite Polymer Electrolyte Membranes; Springer: Berlin/Heidelberg, Germany, 2017; Volume 377, pp. 381–398. [Google Scholar]
- Ramirez-Salgado, J. Study of basic biopolymer as proton membrane for fuel cell systems. Electrochim. Acta 2007, 52, 3766–3778. [Google Scholar] [CrossRef]
- Kadir, M.F.Z.; Majid, S.R.; Arof, A.K. Plasticized chitosan-PVA blend polymer electrolyte based proton battery. Electrochim. Acta 2010, 55, 1475–1482. [Google Scholar] [CrossRef]
- Srivastava, N.; Chandra, S. Studies on a new proton conducting polymer system: Poly(ethylene succinate) + NH4ClO4. Eur. Polym. J. 2000, 36, 421–433. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdulwahid, R.T.; Hamsan, M.H.; Brza, M.A.; Abdullah, R.M.; Kadir, M.F.; Muzakir, S.K. Structural, Impedance, and EDLC Characteristics of Proton Conducting Chitosan-Based Polymer Blend Electrolytes with High Electrochemical Stability. Molecules 2019, 24, 3508. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.B.; Abdulwahid, R.T.; Hamsan, M.H.; Brza, M.A.; Abdullah, R.M.; Kadir, M.F.; Muzakir, S.K. Structural, Impedance and Electrochemical Characteristics of Electrical Double Layer Capacitor Devices Based on Chitosan. Polymers 2020, 12, 1411. [Google Scholar] [CrossRef]
- Amran, N.N.A.; Manan, N.S.A.; Kadir, M.F.Z. The effect of LiCF3SO3 on the complexation with potato starch-chitosan blend polymer electrolytes. Ionics 2016, 22, 1647–1658. [Google Scholar] [CrossRef]
- Shukur, M.F.; Kadir, M.F.Z. Hydrogen ion conducting starch-chitosan blend based electrolyte for application in electrochemical devices. Electrochim. Acta 2015, 158, 152–165. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hassan, A.Q.; Mohammed, S.J.; Karim, W.O.; Kadir, M.F.Z.; Tajuddin, H.A.; Chan, N.N.M.Y. Structural and optical characteristics of pva:C-dot composites: Tuning the absorption of ultra violet (uv) region. Nanomaterials 2019, 9, 216. [Google Scholar] [CrossRef]
- Mazuki, N.F.; Majeed, A.A.; Nagao, Y.; Samsudin, A.S. Studies on ionics conduction properties of modification CMC-PVA based polymer blend electrolytes via impedance approach. Polym. Test. 2020, 81, 106234. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abidin, Z.H.Z. Electrical and morphological analysis of chitosan:AgTf solid electrolyte. Mater. Chem. Phys. 2014, 144, 280–286. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Karim, W.O.; Kadir, M.F.Z.; Brza, M.A.; Abdullah, O.G. High proton conducting polymer blend electrolytes based on chitosan: Dextran with constant specific capacitance and energy density. Biomolecules 2019, 9, 267. [Google Scholar] [CrossRef]
- Liu, J.; Khanam, Z.; Muchakayala, R.; Song, S. Fabrication and characterization of Zn-ion-conducting solid polymer electrolyte films based on PVdF-HFP/Zn(Tf)2 complex system. J. Mater. Sci. Mater. Electron. 2020, 31, 6160–6173. [Google Scholar] [CrossRef]
- Machappa, T.; Prasad, M.A. AC conductivity and dielectric behavior of polyaniline/sodium metavenadate (PANI/NaVO3) composites. Phys. B Condens. Matter 2009, 404, 4168–4172. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Kadir, M.F.Z.; Karim, W.O.; Abdullah, R.M. Development of polymer blend electrolyte membranes based on chitosan: Dextran with high ion transport properties for EDLC application. Int. J. Mol. Sci. 2019, 20, 3369. [Google Scholar] [CrossRef]
- Siddaiah, T.; Ojha, P.; Gopal, N.O.; Ramu, C.; Nagabhushana, H. Thermal, structural, optical and electrical properties of PVA/MAA:EA polymer blend filled with different concentrations of Lithium Perchlorate. J. Sci. Adv. Mater. Devices 2018, 3, 456–463. [Google Scholar] [CrossRef]
- Kumar, D.A.; Selvasekarapandian, S.; Baskaran, R.; Savitha, T.; Nithya, H. Thermal, vibrational and Ac impedance studies on proton conducting polymer electrolytes based on poly(vinyl acetate). J. Non-Cryst. Solids 2012, 358, 531–536. [Google Scholar] [CrossRef]
- Perumal, P.; Christopher Selvin, P.; Selvasekarapandian, S.; Sivaraj, P. Structural and electrical properties of bio-polymer pectin with LiClO4 solid electrolytes for lithium ion polymer batteries. Mater. Today Proc. 2019, 8, 196–202. [Google Scholar] [CrossRef]
- Aziz, S.B.; Karim, W.O.; Ghareeb, H.O. The deficiency of chitosan: AgNO3 polymer electrolyte incorporated with titanium dioxide filler for device fabrication and membrane separation technology. J. Mater. Res. Technol. 2020, 9, 4692–4705. [Google Scholar] [CrossRef]
- Premalatha, M.; Vijaya, N.; Selvasekarapandian, S.; Selvalakshmi, S. Characterization of blend polymer PVA-PVP complexed with ammonium thiocyanate. Ionics 2016, 22, 1299–1310. [Google Scholar] [CrossRef]
- Aziz, S.B.; Karim, O.W.; Qadir, K.W.; Qayyum, Z. Proton ion conducting solid polymer electrolytes based on chitosan incorporated with various amounts of barium titanate (BaTiO3). Int. J. Electrochem. Sci. 2018, 13, 6112–6125. [Google Scholar] [CrossRef]
- Shukur, M.F.; Hamsan, M.H.; Kadir, M.F.Z. Investigation of plasticized ionic conductor based on chitosan and ammonium bromide for EDLC application. Mater. Today Proc. 2019, 17, 490–498. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Kadir, M.F.Z.; Woo, H.J. Design of Polymer Blends Based on Chitosan:POZ with Improved Dielectric Constant for Application in Polymer Electrolytes and Flexible Electronics. Adv. Polym. Technol. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Ramesh, S.; Ng, H.M.; Shanti, R.; Ramesh, K. Studies on the Influence of Titania Content on the Properties of Poly(vinyl chloride)—Poly (acrylonitrile)-Based Polymer Electrolytes. Polym. Technol. Eng. 2013, 52, 1474–1481. [Google Scholar] [CrossRef]
- Salman, Y.A. Conductivity and Electrical Properties of Chitosan—Methylcellulose Blend Biopolymer Electrolyte Incorporated with Lithium Tetrafluoroborate. Int. J. Electrochem. Sci. 2018, 13, 3185–3199. [Google Scholar] [CrossRef]
- Pawlicka, A.; Tavares, F.C.; Dörr, D.S.; Cholant, C.M.; Ely, F.; Santos, M.J.L.; Avellaneda, C.O. Dielectric behavior and FTIR studies of xanthan gum-based solid polymer electrolytes. Electrochim. Acta 2019, 305, 232–239. [Google Scholar] [CrossRef]
- Aziz, S.B. Study of electrical percolation phenomenon from the dielectric and electric modulus analysis. Bull. Mater. Sci. 2015, 38, 1597–1602. [Google Scholar] [CrossRef]
- Gozzo, C.; Terezo, A.; Thaines, E.; Sales, A.; Freitas, R.G.; Sombra, A.S.B.; Costa, M. Effects of MgO on dielectric relaxation and phase transition of the ceramic matrix BaBi4Ti4O15. J. Sci. Adv. Mater. Devices 2019, 4, 170–179. [Google Scholar] [CrossRef]
- Aziz, S.B. Occurrence of electrical percolation threshold and observation of phase transition in chitosan(1−x):AgI x (0.05 ≤ x ≤ 0.2)-based ion-conducting solid polymer composites. Appl. Phys. A 2016, 122. [Google Scholar] [CrossRef]
- Noor, N.A.M.; Isa, M.I.N. Ionic Conductivity and Dielectric Properties of CMC Doped NH4SCN Solid Biopolymer Electrolytes. Adv. Mater. Res. 2015, 1107, 230–235. [Google Scholar] [CrossRef]
- Hadi, J.M.; Aziz, S.B.; Mustafa, M.S.; Brza, M.A. Electrochemical Impedance study of Proton Conducting Polymer Electrolytes based on PVC Doped with Thiocyanate and Plasticized with Glycerol. Int. J. Electrochem. Sci. 2020, 15, 4671–4683. [Google Scholar] [CrossRef]
- Monisha, S.; Mathavan, T.; Selvasekarapandian, S.; Benial, A.M.F.; Aristatil, G.; Mani, N.; Premalatha, M.; Selvasekarapandian, S. Investigation of bio polymer electrolyte based on cellulose acetate-ammonium nitrate for potential use in electrochemical devices. Carbohydr. Polym. 2017, 157, 38–47. [Google Scholar] [CrossRef]
- Noor, N.A.M.; Isa, M.I.N. Investigation on transport and thermal studies of solid polymer electrolyte based on carboxymethyl cellulose doped ammonium thiocyanate for potential application in electrochemical devices. Int. J. Hydrogen Energy 2019, 44, 8298–8306. [Google Scholar] [CrossRef]
- Ramlli, M.A.; Isa, M.I.N. Structural and ionic transport properties of protonic conducting solid biopolymer electrolytes based on carboxymethyl cellulose doped with ammonium fluoride. J. Phys. Chem. B 2016, 120, 11567–11573. [Google Scholar] [CrossRef]
- Manjuladevi, R.; Thamilselvan, M.; Selvasekarapandian, S.; Mangalam, R.; Premalatha, M.; Monisha, S. Mg-ion conducting blend polymer electrolyte based on poly(vinyl alcohol)-poly (acrylonitrile) with magnesium perchlorate. Solid State Ion. 2017, 308, 90–100. [Google Scholar] [CrossRef]
- Ahmad, N.H.B.; Isa, M.I.N.B.M. Proton conducting solid polymer electrolytes based carboxymethyl cellulose doped ammonium chloride: Ionic conductivity and transport studies. Int. J. Plast. Technol. 2015, 19, 47–55. [Google Scholar] [CrossRef]
- Sohaimy, M.I.H.; Isa, M.I.N. Ionic conductivity and conduction mechanism studies on cellulose based solid polymer electrolytes doped with ammonium carbonate. Polym. Bull. 2017, 74, 1371–1386. [Google Scholar] [CrossRef]
- Manjuladevi, R.; Thamilselvan, M.; Selvasekarapandian, S.; Christopher Selvin, P.; Mangalam, R.; Monisha, S. Preparation and characterization of blend polymer electrolyte film based on poly(vinyl alcohol)-poly(acrylonitrile)/MgCl2 for energy storage devices. Ionics 2018, 24, 1083–1095. [Google Scholar] [CrossRef]
- Kubota, S.; Ozaki, S.; Onishi, J.; Kano, K.; Shirai, O. Selectivity on Ion Transport across Bilayer Lipid Membranes in the Presence of Gramicidin A. Anal. Sci. 2009, 25, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Premalatha, M.; Mathavan, T.; Selvasekarapandian, S.; Selvalakshmi, S.; Monisha, S. Incorporation of NH4Br in Tamarind Seed Polysaccharide biopolymer and its potential use in electrochemical energy storage devices. Org. Electron. 2017, 50, 418–425. [Google Scholar] [CrossRef]
- Wang, G.X.; Yang, L.; Wang, J.Z.; Liu, H.K.; Dou, S.X. Enhancement of ionic conductivity of PEO based polymer electrolyte by the addition of nanosize ceramic powders. J. Nanosci. Nanotechnol. 2005, 5, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.S.; Teoh, K.H.; Liew, C.W.; Ramesh, S. Electric double layer capacitor based on activated carbon electrode and biodegradable composite polymer electrolyte. Ionics 2014, 20, 251–258. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Brza, M.A.; Kadir, M.F.Z.; Muzakir, S.K.; Abdulwahid, R.T. Effect of glycerol on EDLC characteristics of chitosan: Methylcellulose polymer blend electrolytes. J. Mater. Res. Technol. 2020, 9, 8355–8366. [Google Scholar] [CrossRef]
- Dhatarwal, P.; Choudhary, S.; Sengwa, R.J. Electrochemical performance of Li+-ion conducting solid polymer electrolytes based on PEO–PMMA blend matrix incorporated with various inorganic nanoparticles for the lithium ion batteries. Compos. Commun. 2018, 10, 11–17. [Google Scholar] [CrossRef]
- Saikia, D.; Wu, H.Y.; Pan, Y.C.; Lin, C.P.; Huang, K.P.; Chen, K.N.; Kao, H.M. Highly conductive and electrochemically stable plasticized blend polymer electrolytes based on PVdF-HFP and triblock copolymer PPG-PEG-PPG diamine for Li-ion batteries. J. Power Sources 2011, 196, 2826–2834. [Google Scholar] [CrossRef]
- Hamsan, M.; Aziz, S.B.; Kadir, M.; Brza, M.; Karim, W.O. The study of EDLC device fabricated from plasticized magnesium ion conducting chitosan-based polymer electrolyte. Polym. Test. 2020, 90, 106714. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.H.; Nofal, M.M.M.; San, S.; Abdulwahid, R.T.; Saeed, S.R.R.; Brza, M.A.; Kadir, M.F.Z.; Mohammed, S.J.; Al-Zangana, S. From Cellulose, Shrimp and Crab Shells to Energy Storage EDLC Cells: The Study of Structural and Electrochemical Properties of Proton Conducting Chitosan-Based Biopolymer Blend Electrolytes. Polymers 2020, 12, 1526. [Google Scholar] [CrossRef]
- Azli, A.A.; Manan, N.S.A.; Aziz, S.B.; Kadir, M.F.Z. Structural, impedance and electrochemical double-layer capacitor characteristics of improved number density of charge carrier electrolytes employing potato starch blend polymers. Ionics 2020, 26, 5773–5804. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.; Hamsan, M.; Kadir, M.; Muzakir, S.; Abdulwahid, R.T. Effect of ohmic-drop on electrochemical performance of EDLC fabricated from PVA: Dextran: NH4I based polymer blend electrolytes. J. Mater. Res. Technol. 2020, 9, 1–12. [Google Scholar] [CrossRef]
- Hadi, J.M.; Aziz, S.B.; Mustafa, M.S.; Hamsan, M.H.; Abdulwahid, R.T.; Kadir, M.F.; Ghareeb, H.O. Role of nano-capacitor on dielectric constant enhancement in PEO:NH4SCN:xCeO2 polymer nano-composites: Electrical and electrochemical properties. J. Mater. Res. Technol. 2020, 9, 9283–9294. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Illias, H.A.; Kadir, M.F.Z. Incorporation of NH4Br in PVA-chitosan blend-based polymer electrolyte and its effect on the conductivity and other electrical properties. Ionics 2014, 20, 1235–1245. [Google Scholar] [CrossRef]
- Kadir, M.F.Z.; Arof, A.K. Application of PVA–chitosan blend polymer electrolyte membrane in electrical double layer capacitor. Mater. Res. Innov. 2011, 15, s217–s220. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Karim, W.O.; Marif, A.S.; Abdulwahid, R.T.; Kadir, M.F.Z.; Brza, M.A. Study of impedance and solid-state double-layer capacitor behavior of proton (H+)-conducting polymer blend electrolyte-based CS:PS polymers. Ionics 2020, 26, 4635–4649. [Google Scholar] [CrossRef]
- Hadi, J.M.; Aziz, S.B.; Nofal, M.M.; Hussein, S.A.; Hamsan, M.H.; Brza, M.A.; Abdulwahid, R.T.; Kadir, M.F.Z.; Woo, H.J. Electrical, Dielectric Property and Electrochemical Performances of Plasticized Silver Ion-Conducting Chitosan-Based Polymer Nanocomposites. Membranes 2020, 10, 151. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Aziz, S.B.; Shukur, M.F.; Kadir, M.F.Z. Protonic cell performance employing electrolytes based on plasticized methylcellulose-potato starch-NH4NO3. Ionics 2019, 25, 559–572. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Shukur, M.F.; Hamsan, M.H.; Jumbri, K.; Kadir, M.F.Z. Plasticized solid polymer electrolyte based on natural polymer blend incorporated with lithium perchlorate for electrical double-layer capacitor fabrication. Ionics 2019, 25, 5473–5484. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Abdullah, R.M.; Abdulwahid, R.T.; Brza, M.A.; Marif, A.S.; Kadir, M.F.Z. Protonic EDLC cell based on chitosan (CS): Methylcellulose (MC) solid polymer blend electrolytes. Ionics 2020, 26, 1829–1840. [Google Scholar] [CrossRef]
- Hemalatha, R.; Alagar, M.; Selvasekarapandian, S.; Sundaresan, B.; Moniha, V. Studies of proton conducting polymer electrolyte based on PVA, amino acid proline and NH4SCN. J. Sci. Adv. Mater. Devices 2019, 4, 101–110. [Google Scholar] [CrossRef]
- Hemalatha, R.; Alagar, M.; Selvasekarapandian, S.; Sundaresan, B.; Moniha, V.; Boopathi, G.; Selvin, P.C. Preparation and characterization of proton-conducting polymer electrolyte based on PVA, amino acid proline, and NH4Cl and its applications to electrochemical devices. Ionics 2018, 25, 141–154. [Google Scholar] [CrossRef]
- Shuhaimi, N.E.A.; Teo, L.P.; Woo, H.J.; Majid, S.R.; Arof, A. Electrical double-layer capacitors with plasticized polymer electrolyte based on methyl cellulose. Polym. Bull. 2012, 69, 807–826. [Google Scholar] [CrossRef]
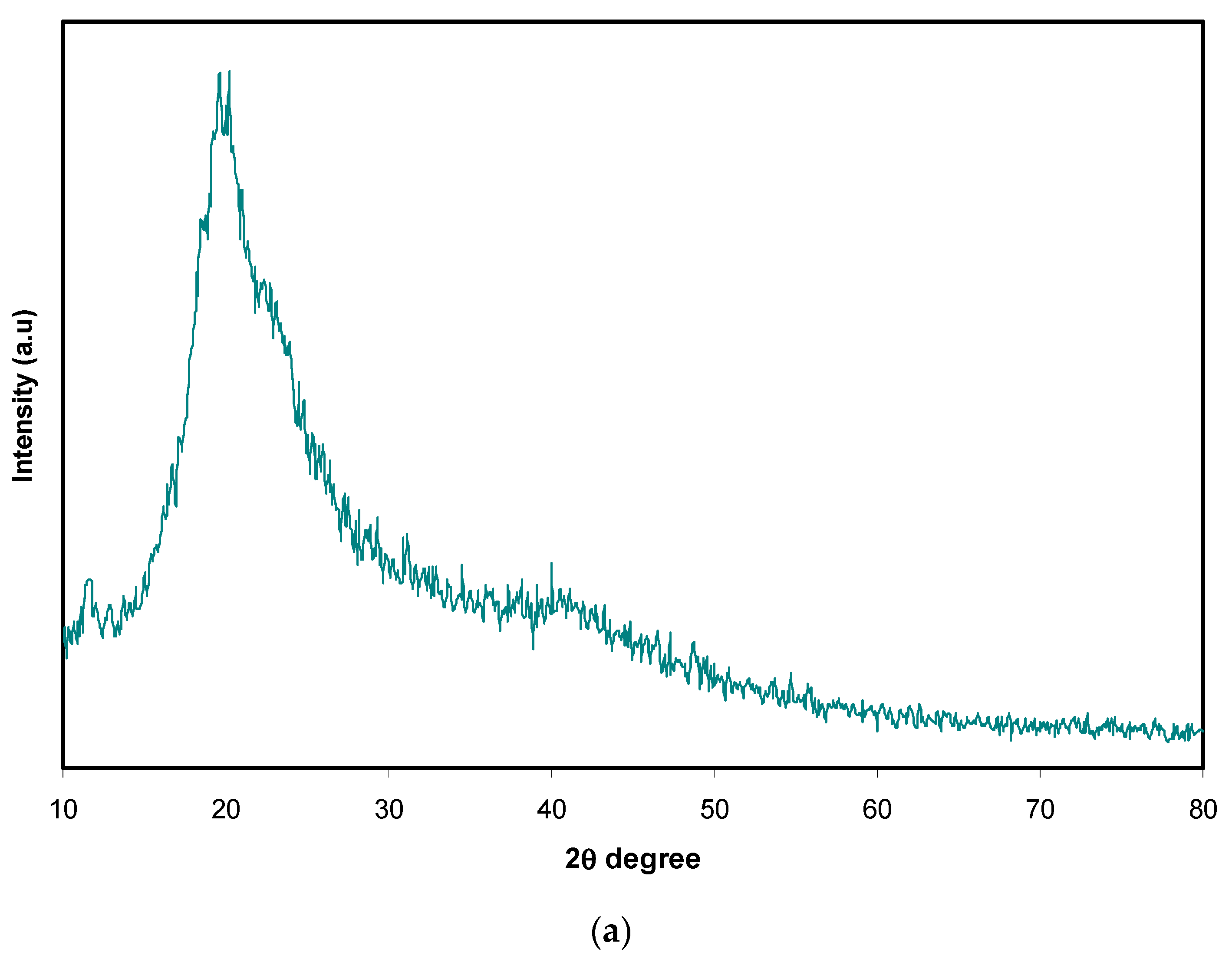
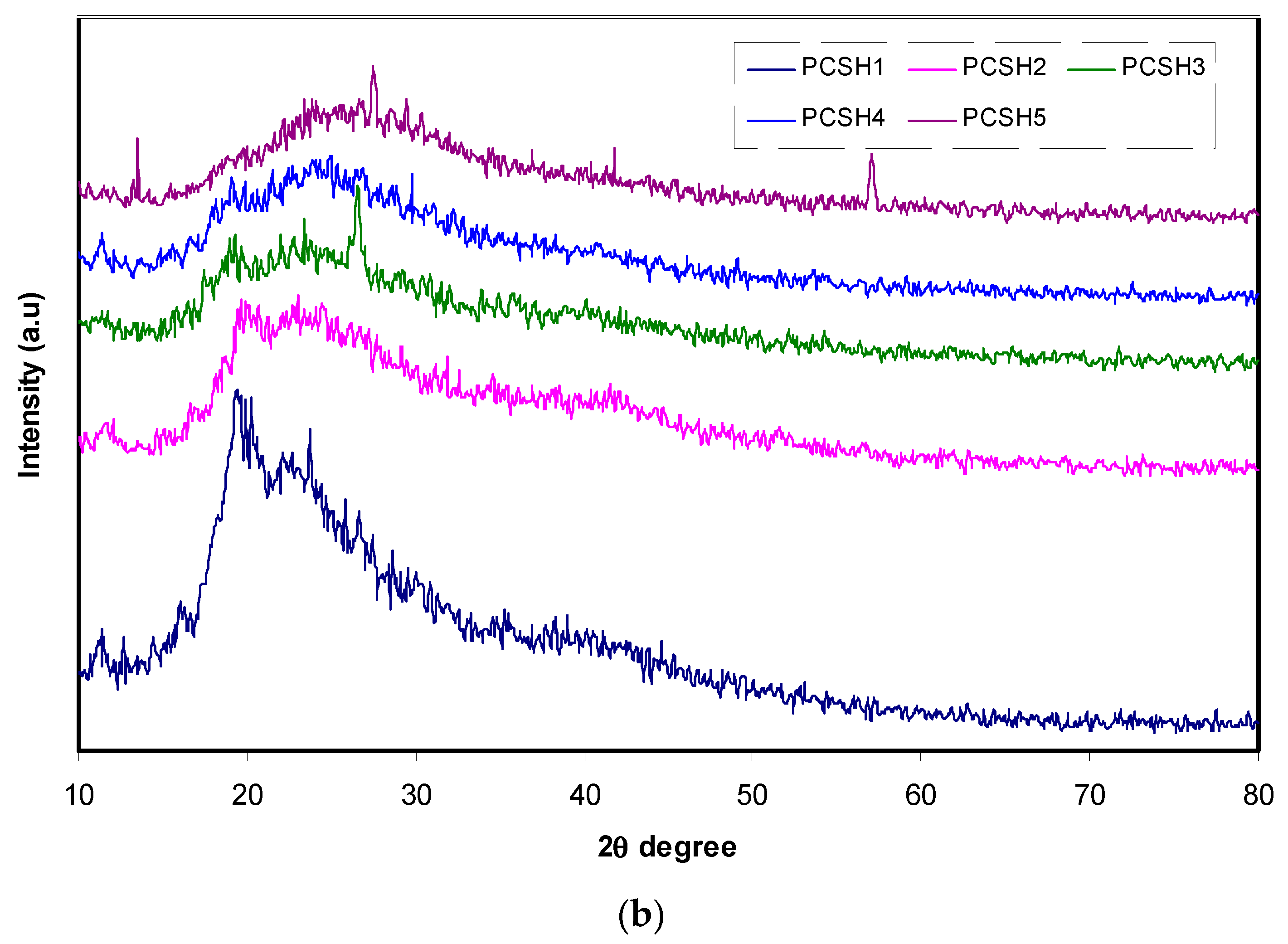
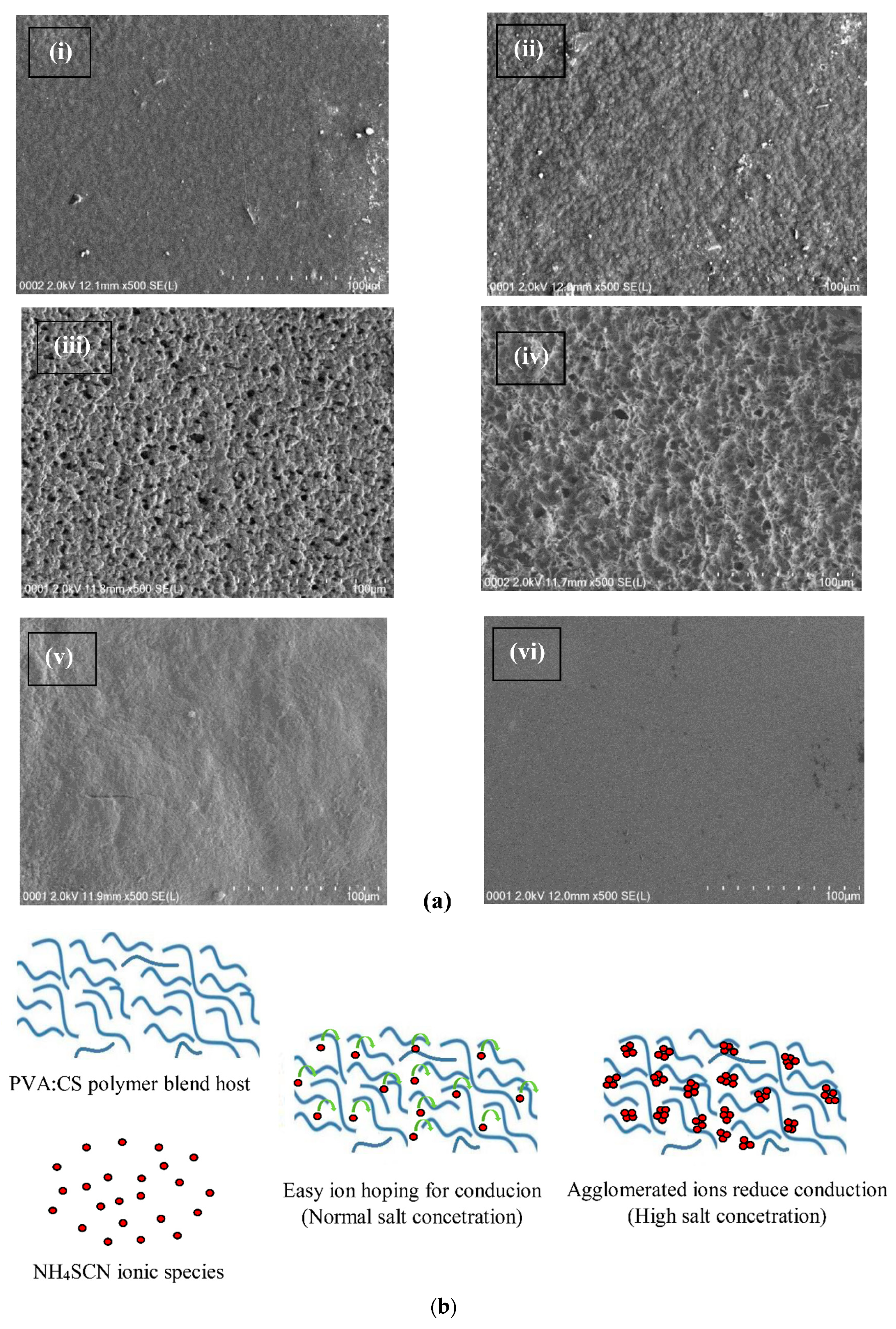
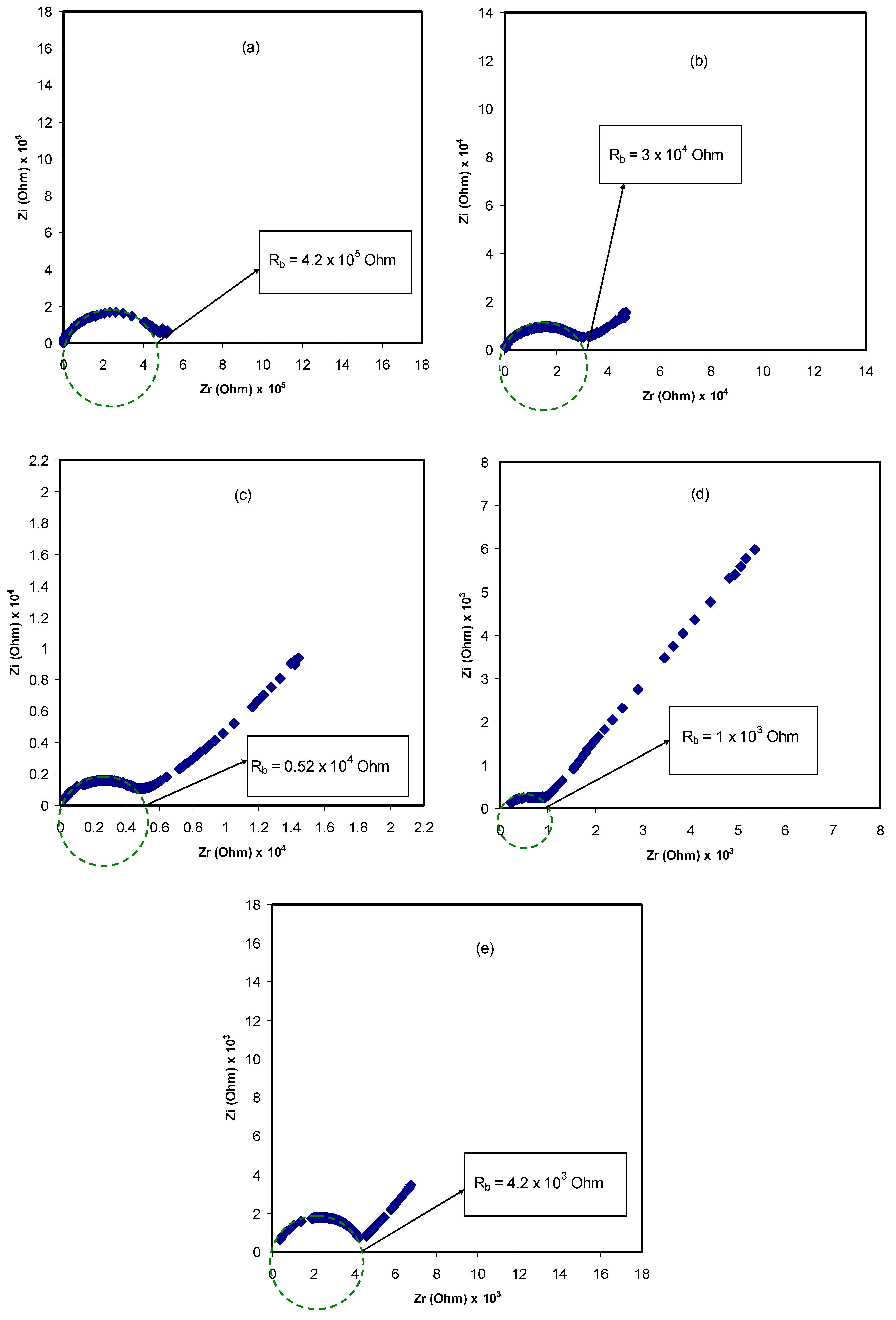
| Sample Designation | (PVA) (g) | (CS) (g) | NH4SCN wt% |
|---|---|---|---|
| PCSH1 | 0.5 | 0.5 | 10 |
| PCSH2 | 0.5 | 0.5 | 20 |
| PCSH3 | 0.5 | 0.5 | 30 |
| PCSH4 | 0.5 | 0.5 | 40 |
| PCSH5 | 0.5 | 0.5 | 50 |
| Sample Code | DC Conductivity (S/cm) |
|---|---|
| PCSH1 | (3.43 × 10−8) ± 0.32 |
| PCSH2 | (4.84 × 10−7) ± 0.54 |
| PCSH3 | (2.76 × 10−6) ± 0.29 |
| PCSH4 | (1.36 × 10−5) ± 0.19 |
| PCSH5 | (3.49 × 10−6) ± 0.39 |
| SPEs | Scan Rate mV s−1 | Electrode Material | Decomposition Voltage (V) | References |
|---|---|---|---|---|
| PVA/dextran/NH4I | 50 | Stainless steel | 1.3 | [82] |
| PEO/NH4SCN/CeO2 | 5 | Stainless steel | 1.4 | [83] |
| PVA/CS/NH4Br | 1 | Stainless steel | 1.57 | [84] |
| PVA/CS/NH4NO3/EC | 10 | Stainless steel | 1.7 | [85] |
| CS/PS/NH4F | 100 | Stainless steel | 1.78 | [86] |
| CS/AgNO3/Al2O3/glycerol | 10 | Stainless steel | 1.8 | [87] |
| MC/PS/NH4NO3/glycerol | 1 | Stainless steel | 1.88 | [88] |
| MC/PS/LiClO4 | 5 | Stainless steel | 2 | [89] |
| CS/MC/NH4I | 10 | Stainless steel | 2.1 | [90] |
| PVA/proline/NH4SCN | 1 | Stainless steel | 3.61 | [91] |
| PVA/proline/NH4Cl | 1 | Stainless steel | 3.10 | [92] |
| MC/NH4NO3/PEG | 1 | Stainless steel | 2.4 | [93] |
| PVA/CS/NH4SCN | 10 | Stainless steel | 2.25 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nofal, M.M.; Aziz, S.B.; Hadi, J.M.; Abdulwahid, R.T.; Dannoun, E.M.A.; Marif, A.S.; Al-Zangana, S.; Zafar, Q.; Brza, M.A.; Kadir, M.F.Z. Synthesis of Porous Proton Ion Conducting Solid Polymer Blend Electrolytes Based on PVA: CS Polymers: Structural, Morphological and Electrochemical Properties. Materials 2020, 13, 4890. https://doi.org/10.3390/ma13214890
Nofal MM, Aziz SB, Hadi JM, Abdulwahid RT, Dannoun EMA, Marif AS, Al-Zangana S, Zafar Q, Brza MA, Kadir MFZ. Synthesis of Porous Proton Ion Conducting Solid Polymer Blend Electrolytes Based on PVA: CS Polymers: Structural, Morphological and Electrochemical Properties. Materials. 2020; 13(21):4890. https://doi.org/10.3390/ma13214890
Chicago/Turabian StyleNofal, Muaffaq M., Shujahadeen B. Aziz, Jihad M. Hadi, Rebar T. Abdulwahid, Elham M. A. Dannoun, Ayub Shahab Marif, Shakhawan Al-Zangana, Qayyum Zafar, M. A. Brza, and M. F. Z. Kadir. 2020. "Synthesis of Porous Proton Ion Conducting Solid Polymer Blend Electrolytes Based on PVA: CS Polymers: Structural, Morphological and Electrochemical Properties" Materials 13, no. 21: 4890. https://doi.org/10.3390/ma13214890
APA StyleNofal, M. M., Aziz, S. B., Hadi, J. M., Abdulwahid, R. T., Dannoun, E. M. A., Marif, A. S., Al-Zangana, S., Zafar, Q., Brza, M. A., & Kadir, M. F. Z. (2020). Synthesis of Porous Proton Ion Conducting Solid Polymer Blend Electrolytes Based on PVA: CS Polymers: Structural, Morphological and Electrochemical Properties. Materials, 13(21), 4890. https://doi.org/10.3390/ma13214890











