Rapid Thermal Annealing of Double Perovskite Thin Films Formed by Polymer Assisted Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Precursor Solutions Preparation
2.2. Thin Films Growth
2.3. Characterization of Structural and Physical Properties
3. Results and Discussion
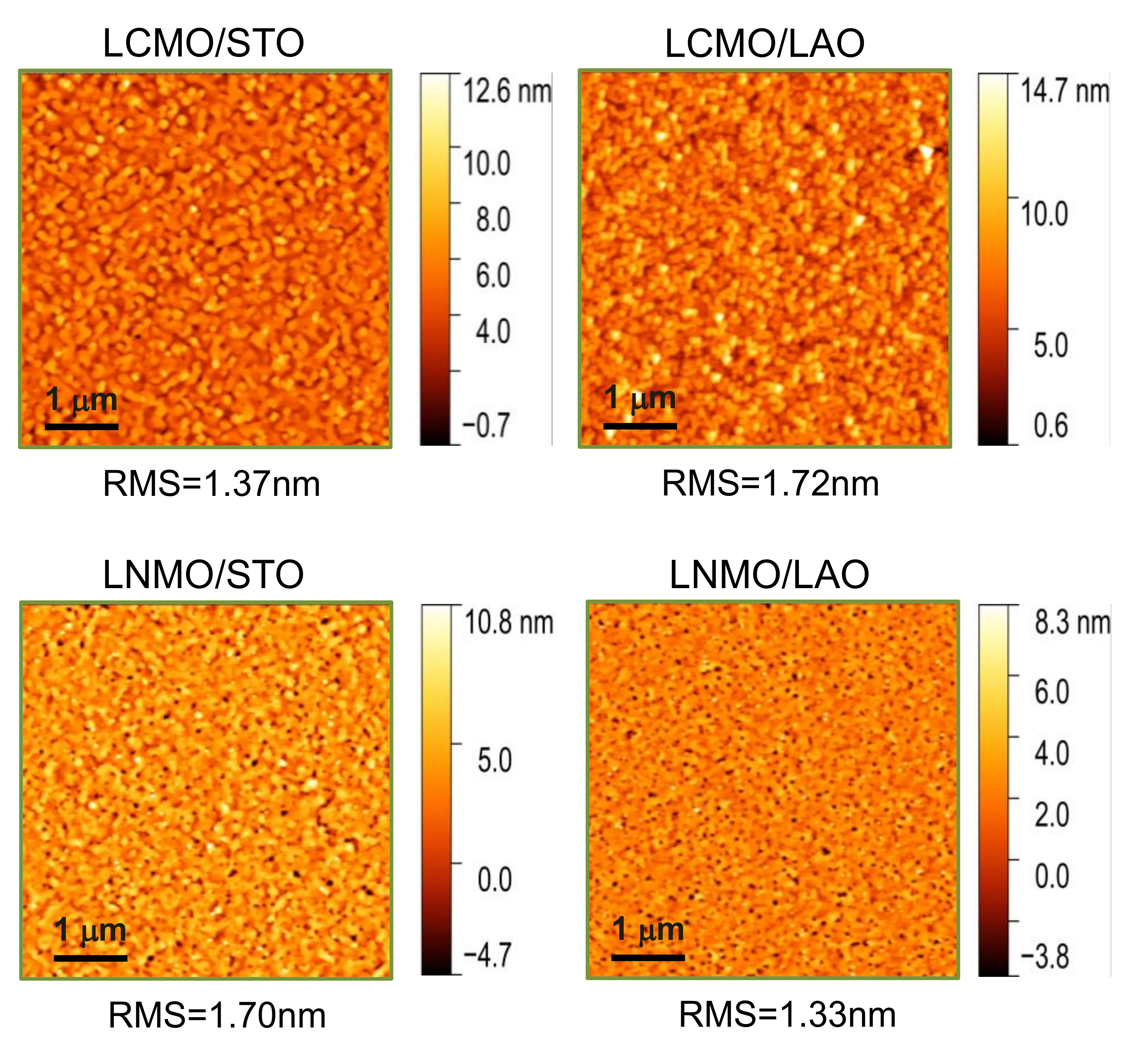
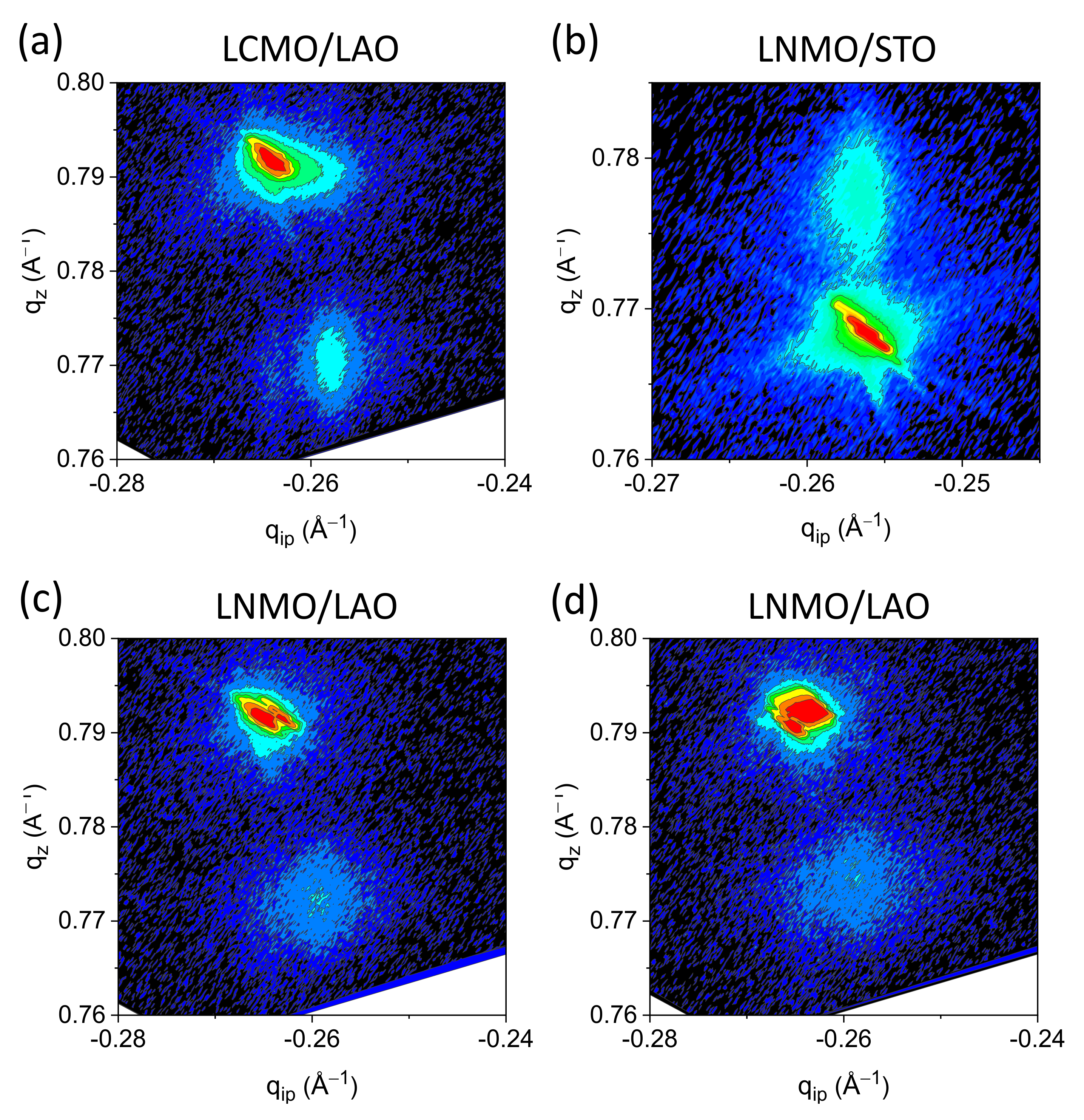
3.1. Structural Characterization
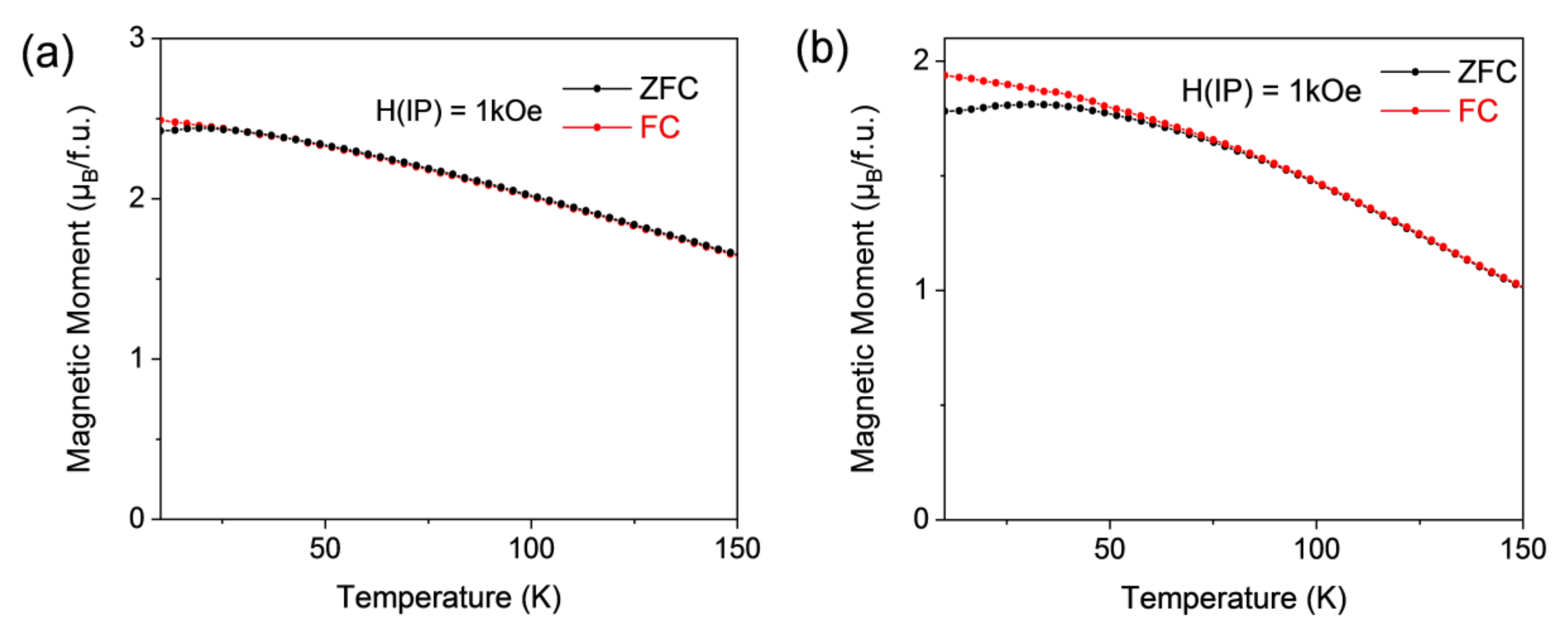
3.2. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cho, A.; Arthur, J. Molecular beam epitaxy. Prog. Solid State Chem. 1975, 10, 157–191. [Google Scholar] [CrossRef]
- Schlom, D.G. Perspective: Oxide molecular-beam epitaxy rocks! APL Mater. 2015, 3, 062403. [Google Scholar] [CrossRef] [Green Version]
- Safi, I. Recent aspects concerning DC reactive magnetron sputtering of thin films: A review. Surf. Coat. Technol. 2000, 127, 203–218. [Google Scholar] [CrossRef]
- Galceran, R.; Frontera, C.; Balcells, L.; Cisneros-Fernández, J.; López-Mir, L.; Roqueta, J.; Santiso, J.; Bagués, N.; Bozzo, B.; Pomar, A.; et al. Engineering the microstructure and magnetism of La2CoMnO6−δ thin films by tailoring oxygen stoichiometry. Appl. Phys. Lett. 2014, 105, 242401. [Google Scholar] [CrossRef] [Green Version]
- Blank, D.H.; Koster, G.; Rijnders, A.J.; Van Setten, E.; Slycke, P.; Rogalla, H. Epitaxial growth of oxides with pulsed laser interval deposition. J. Cryst. Growth 2000, 211, 98–105. [Google Scholar] [CrossRef]
- Opel, M. Spintronic oxides grown by laser-MBE. J. Phys. D Appl. Phys. 2011, 45, 33001. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, R.W.; Schneller, T.; Waser, R. Chemical solution deposition of electronic oxide films. Comptes Rendus Chim. 2004, 7, 433–461. [Google Scholar] [CrossRef]
- Schneller, T.; Waser, R.; Kosec, M.; Payne, D. Chemical Solution Deposition of Functional Oxide Thin Films; Springer Science and Business Media LLC: Berlin, Germany, 2013; pp. 1–796. [Google Scholar]
- Calzada, M.L. Sol-Gel Electroceramic Thin Films. In The Sol-Gel Handbook; Levy, D., Zayat, M., Eds.; Wiley: Hoboken, NY, USA, 2015; pp. 841–882. [Google Scholar]
- Jia, Q.X.; McCleskey, T.M.; Burrell, A.K.; Lin, Y.; Collis, G.E.; Wang, H.; Li, A.D.Q.; Foltyn, S.R. Polymer-assisted deposition of metal-oxide films. Nat. Mater. 2004, 3, 529–532. [Google Scholar] [CrossRef]
- Bassiri-Gharb, N.; Bastani, Y.; Bernal, A. Chemical solution growth of ferroelectric oxide thin films and nanostructures. Chem. Soc. Rev. 2014, 43, 2125–2140. [Google Scholar] [CrossRef]
- Han, H.; Chen, Y.; Wang, Z. Effect of heating rates on the crystallization process of (111)-oriented lead zirconate titanate thin films prepared by the sol–gel method. Ceram. Int. 2015, 41, 15208–15216. [Google Scholar] [CrossRef]
- Queraltó, A.; De La Mata, M.; Arbiol, J.; Obradors, X.; Puig, T. Disentangling Epitaxial Growth Mechanisms of Solution Derived Functional Oxide Thin Films. Adv. Mater. Interfaces 2016, 3, 1600392. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, R.W. Chemical Solution Deposition of Perovskite Thin Films. Chem. Mater. 1997, 9, 2325–2340. [Google Scholar] [CrossRef]
- Gunawan, R.; Jung, M.; Seebauer, E.; Braatz, R. Optimal control of rapid thermal annealing in a semiconductor process. J. Process. Control. 2004, 14, 423–430. [Google Scholar] [CrossRef]
- Vasala, S.; Karppinen, M. A2B′B″O6 perovskites: A review. Prog. Solid State Chem. 2015, 43, 1–36. [Google Scholar] [CrossRef]
- Anderson, M.; Greenwood, K.; Taylor, G.; Poeppelmeier, K. B-cation arrangements in double perovskites. Prog. Solid State Chem. 1993, 22, 197–233. [Google Scholar] [CrossRef]
- Wang, H.; Gazquez, J.; Frontera, C.; Chisholm, M.F.; Pomar, A.; Martinez, B.; Mestres, N. Spontaneous cationic ordering in chemical-solution-grown La2CoMnO6 double perovskite thin films. NPG Asia Mater. 2019, 11, 44. [Google Scholar] [CrossRef]
- Wang, H.; Frontera, C.; Herrero-Martín, J.; Pomar, A.; Roura-Grabulosa, P.; Martínez, B.; Mestres, N. Aqueous Chemical Solution Deposition of Functional Double Perovskite Epitaxial Thin Films. Chem. A Eur. J. 2020, 26, 9338–9347. [Google Scholar] [CrossRef]
- Kareev, M.; Prosandeev, S.; Liu, J.; Gan, C.; Kareev, A.; Freeland, J.W.; Xiao, M.; Chakhalian, J. Atomic control and characterization of surface defect states of TiO2 terminated SrTiO3 single crystals. Appl. Phys. Lett. 2008, 93, 061909. [Google Scholar] [CrossRef]
- Stamenov, P.; Coey, J. Sample size, position, and structure effects on magnetization measurements using second-order gradiometer pickup coils. Rev. Sci. Instrum. 2006, 77, 15106. [Google Scholar] [CrossRef]
- Carcano, G.; Ceriani, M.; Soglio, F. Spin Coating with High Viscosity Photoresist on Square Substrates—Applications in the Thin Film Hybrid Microwave Integrated Circuit Field. Microelectron Int. 1993, 10, 12–20. [Google Scholar] [CrossRef]
- Kim, M.K.; Moon, J.Y.; Choi, H.Y.; Oh, S.H.; Lee, N.; Choi, Y.J. Investigation of the magnetic properties in double perovskite R2CoMnO6single crystals (R = rare earth: La to Lu). J. Phys. Condens Matter 2015, 27, 426002. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Burgess, J.; Street, S.; Gupta, A.; Calvarese, T.G.; Subramanian, M.A. Growth of epitaxial thin films of the ordered double perovskite La2NiMnO6 on different substrates. Appl. Phys. Lett. 2006, 89, 022509. [Google Scholar] [CrossRef]
- Goodenough, J.B. An interpretation of the magnetic properties of the perovskite-type mixed crystals La1−xSrxCoO3−λ. J. Phys. Chem. Solids 1958, 6, 287–297. [Google Scholar] [CrossRef]
- Kanamori, J. Superexchange interaction and symmetry properties of electron orbitals. J. Phys. Chem. Solids 1959, 10, 87–98. [Google Scholar] [CrossRef]
- Anderson, P.W. Antiferromagnetism. Theory of Superexchange Interaction. Phys. Rev. 1950, 79, 350–356. [Google Scholar] [CrossRef]
- Balcells, L.; Navarro, J.; Bibes, M.; Roig, A.; Martınez, B.; Fontcuberta, J. Cationic ordering control of magnetization in Sr2FeMoO6 double perovskite. Appl. Phys. Lett. 2001, 78, 781–783. [Google Scholar] [CrossRef] [Green Version]
- Truong, K.D.; Singh, M.K.; Jandl, S.; Fournier, P. Influence of Ni/Mn cation order on the spin-phonon coupling in multifunctional La2NiMnO6 epitaxial films by polarized Raman spectroscopy. Phys. Rev. B 2009, 80, 134424. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.P.; Grygiel, C.; Sheets, W.C.; Boullay, P.; Hervieu, M.; Prellier, W.; Mercey, B.; Simon, C.; Raveau, B. Absence of long-range Ni/Mn ordering in ferromagnetic La2NiMnO6 thin films. Appl. Phys. Lett. 2007, 91, 012503. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Charpentier, S.; Truong, K.D.; Fournier, P. Evidence of bidomain structure in double-perovskite La2CoMnO6 thin films. Appl. Phys. Lett. 2007, 90, 211915. [Google Scholar] [CrossRef]
- Galceran, R.; López-Mir, L.; Bozzo, B.; Cisneros-Fernández, J.; Santiso, J.; Balcells, L.; Frontera, C.; Martínez, B. Strain-induced perpendicular magnetic anisotropy in La2CoMnO6−ɛ thin films and its dependence on film thickness. Phys. Rev. B 2016, 93, 144417. [Google Scholar] [CrossRef] [Green Version]
- López-Mir, L.; Galceran, R.; Herrero-Martin, J.; Bozzo, B.; Cisneros-Fernández, J.; Miner, E.V.P.; Pomar, A.; Balcells, L.; Martínez, B.; Frontera, C. Magnetic anisotropy and valence states in La2Co1−xMn1+xO6 (x ≈ 0.23) thin films studied by x-ray absorption spectroscopy techniques. Phys. Rev. B 2017, 95, 224434. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, D.; Mandal, P.; Mathieu, R.; Hazarika, A.; Rajan, S.; Sundaresan, A.; Waghmare, U.V.; Knut, R.; Karis, O.; Nordblad, P.; et al. Near-Room-Temperature Colossal Magnetodielectricity and Multiglass Properties in Partially Disordered La2NiMnO6. Phys. Rev. Lett. 2012, 108, 127201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, S.; Govinda, S.; Goyal, M.; Mukherjee, S.; Pal, B.; Saha, R.; Sundaresan, A.; Jana, S.; Karis, O.; Freeland, J.W.; et al. Effect of anti-site disorder on magnetism in La2NiMnO6. Phys. Rev. B 2018, 97, 165137. [Google Scholar] [CrossRef] [Green Version]
- Nasir, M.; Kumar, S.; Patra, N.; Bhattacharya, D.; Jha, S.N.; Basaula, D.R.; Bhatt, S.; Khan, M.; Liu, S.-W.; Biring, S.; et al. Role of Antisite Disorder, Rare-Earth Size, and Superexchange Angle on Band Gap, Curie Temperature, and Magnetization of R2NiMnO6 Double Perovskites. ACS Appl. Electron. Mater. 2019, 1, 141–153. [Google Scholar] [CrossRef]
- Dass, R.I.; Goodenough, J.B. Multiple magnetic phases of La2CoMnO6−δ (0 < ~δ < ~0.05). Phys. Rev. B 2003, 67, 014401. [Google Scholar] [CrossRef]
- Guo, H.Z.; Gupta, A.; Zhang, J.; Varela, M.; Pennycook, S.J. Effect of oxygen concentration on the magnetic properties of La2CoMnO6 thin films. Appl. Phys. Lett. 2007, 91, 202509. [Google Scholar] [CrossRef]
- Shi, J.; Wang, C.; Xu, Z.; Shen, Q.; Zhang, L. Enhanced electrical and magnetic properties of post-annealed plasma-activated-sintered La2CoMnO6 ceramics. Ceram. Int. 2019, 45, 20855–20859. [Google Scholar] [CrossRef]
- Spurgeon, S.R.; Du, Y.; Droubay, T.; Devaraj, A.; Sang, X.; Longo, P.; Yan, P.; Kotula, P.G.; Shutthanandan, V.; Bowden, M.E.; et al. Competing Pathways for Nucleation of the Double Perovskite Structure in the Epitaxial Synthesis of La2MnNiO6. Chem. Mater. 2016, 28, 3814–3822. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Nasir, M.; Khan, M.; Kumar, S.; Bhatt, S.; Patra, N.; Bhattacharya, D.; Jha, S.N.; Biring, S.; Sen, S. The effect of high temperature annealing on the antisite defects in ferromagnetic La2NiMnO6 double perovskite. J. Magn. Magn. Mater. 2019, 483, 114–123. [Google Scholar] [CrossRef]
- Wu, S.-Q.; Cheng, S.; Lu, L.; Liu, M.; Jin, X.-W.; Cheng, S.; Mi, S. B-site ordering and strain-induced phase transition in double-perovskite La2NiMnO6 films. Sci. Rep. 2018, 8, 2516. [Google Scholar] [CrossRef] [Green Version]
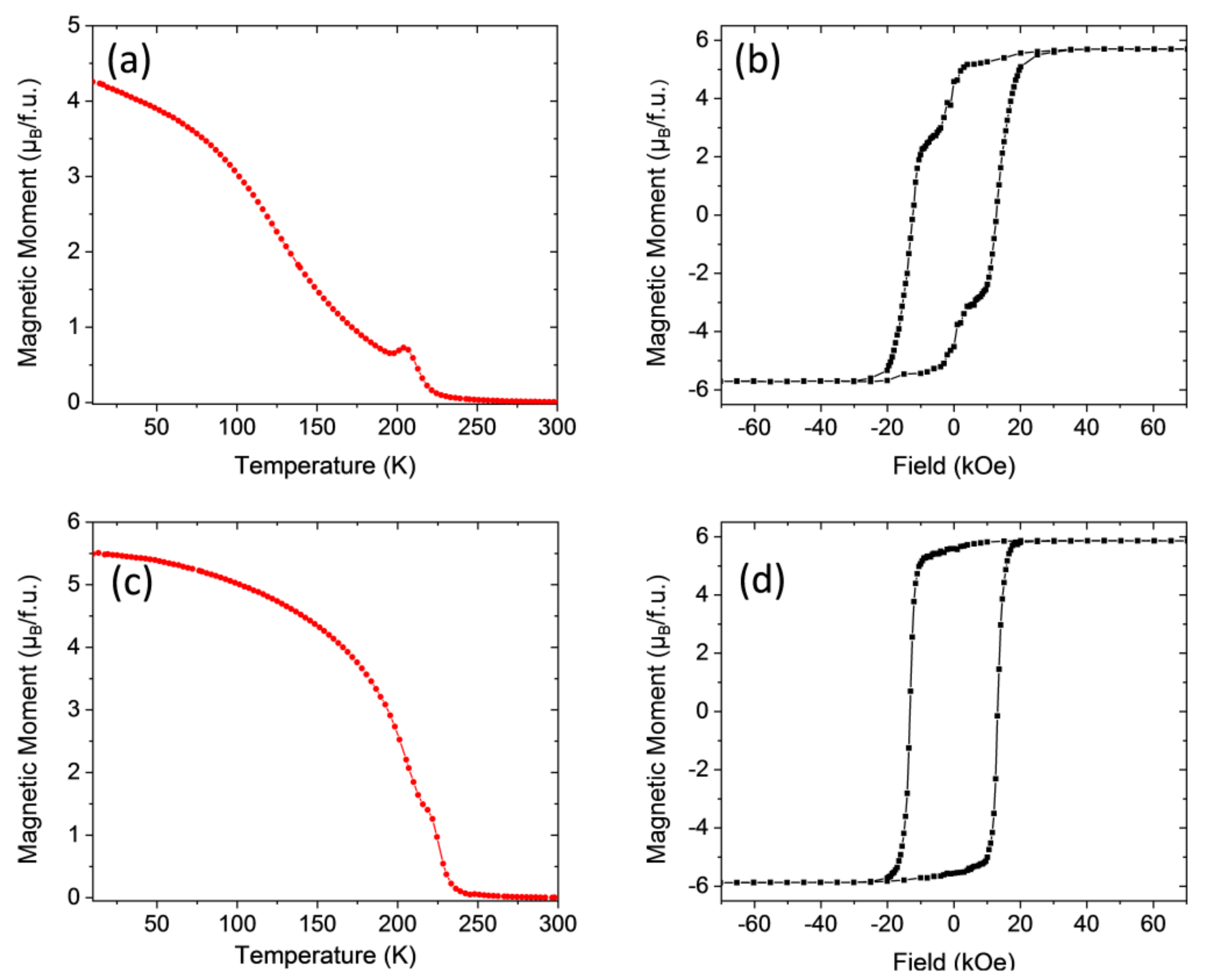
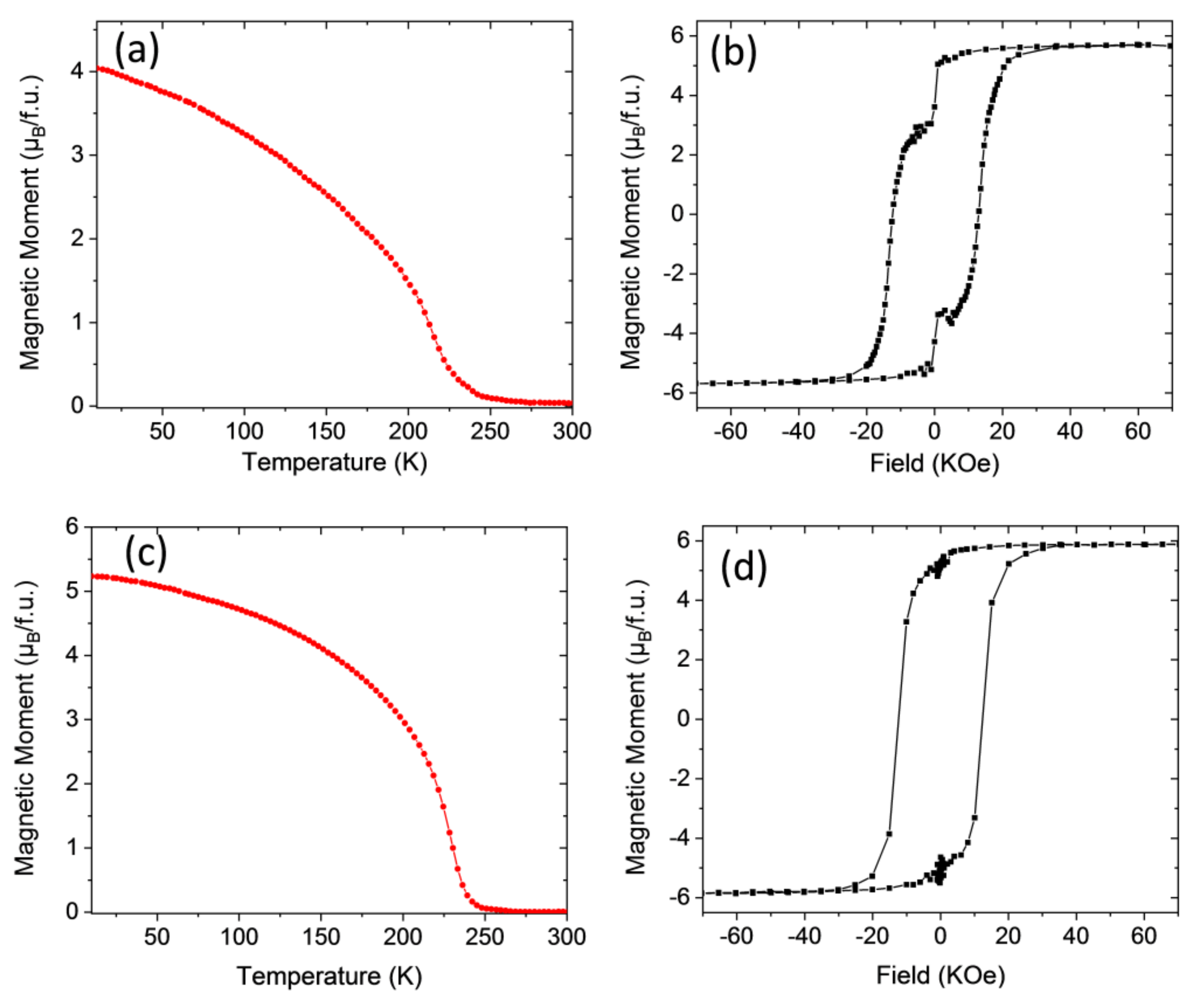
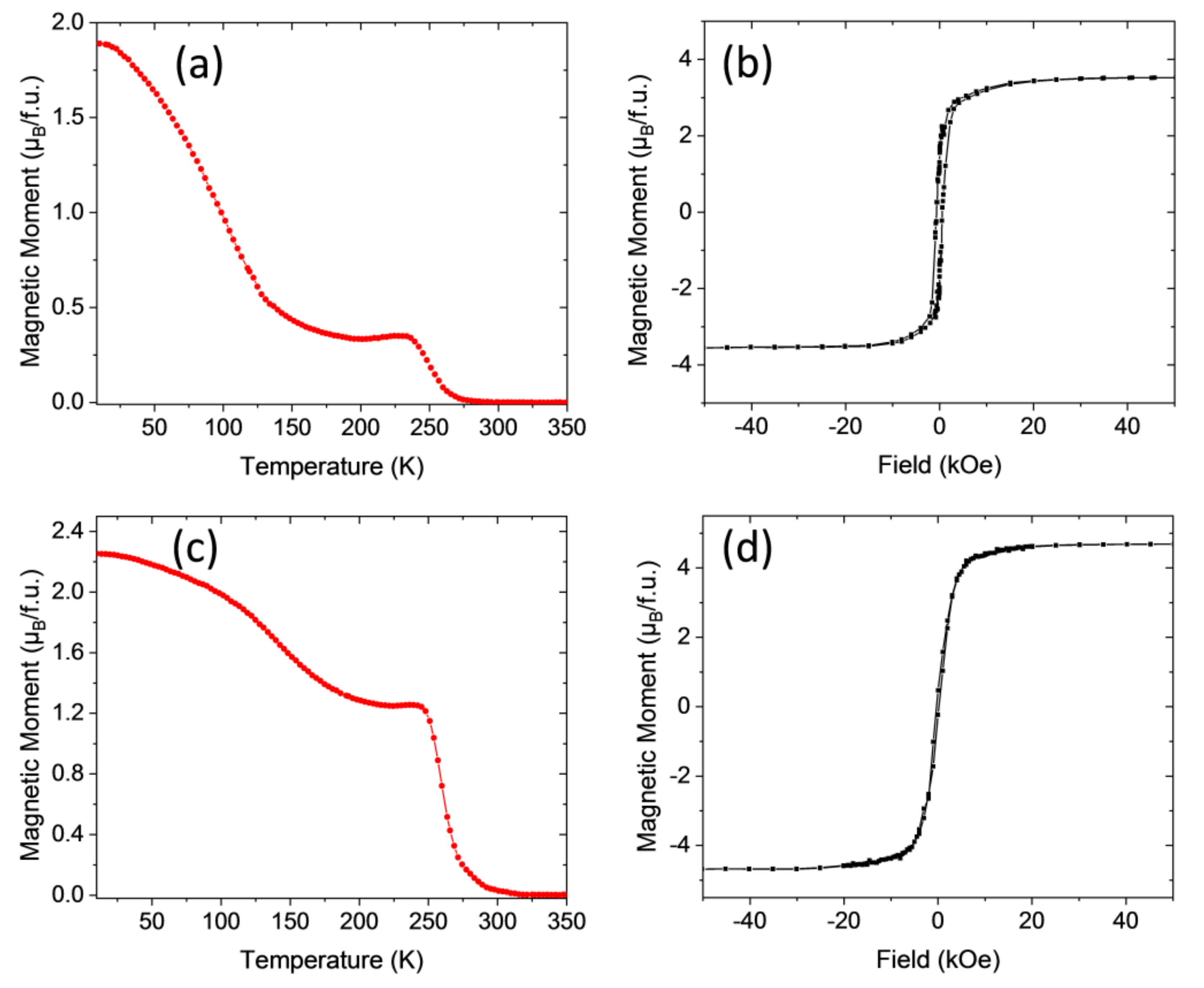

| Sample | Annealing Conditions Ramp, Temperature, Dwell Time, Oxygen | Curie Temperature, Tc (K) | Saturation Magnetization, Ms (μB/f.u.) |
|---|---|---|---|
| LCMO/STO–1 | RTA 20 °C/s, 900 °C, 20 min, static oxygen | 230 | 5.7 |
| LCMO/STO–1R | CTA 5 °C/min, 700 °C, 240 min, 0.5 L/min O2 | 240 | 5.9 |
| LCMO/LAO–1 | RTA 20 °C/s, 900 °C, 10 min, static oxygen | 225 | 5.7 |
| LCMO/LAO–1R | CTA 5 °C/min, 700 °C, 240 min, 0.5 L/min O2 | 240 | 5.8 |
| LNMO/STO−1 | RTA 20 °C/s, 900 °C, 20 min, static oxygen | 255 | 3.7 |
| LNMO/STO−1R | CTA 5 °C/min, 800 °C, 180 min, 0.5 L/min O2 | 270 | 4.3 |
| LNMO/LAO−1 | RTA 20 °C/s, 900 °C, 10 min, static oxygen | 265 | 3.5 |
| LNMO/LAO−1R | CTA 5 °C/min, 800 °C, 180 min, 0.5 L/min O2 | 275 | 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Frontera, C.; Martínez, B.; Mestres, N. Rapid Thermal Annealing of Double Perovskite Thin Films Formed by Polymer Assisted Deposition. Materials 2020, 13, 4966. https://doi.org/10.3390/ma13214966
Wang H, Frontera C, Martínez B, Mestres N. Rapid Thermal Annealing of Double Perovskite Thin Films Formed by Polymer Assisted Deposition. Materials. 2020; 13(21):4966. https://doi.org/10.3390/ma13214966
Chicago/Turabian StyleWang, Hailin, Carlos Frontera, Benjamín Martínez, and Narcís Mestres. 2020. "Rapid Thermal Annealing of Double Perovskite Thin Films Formed by Polymer Assisted Deposition" Materials 13, no. 21: 4966. https://doi.org/10.3390/ma13214966






