Lead Borate Glasses and Glass-Ceramics Singly Doped with Dy3+ for White LEDs
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Glasses
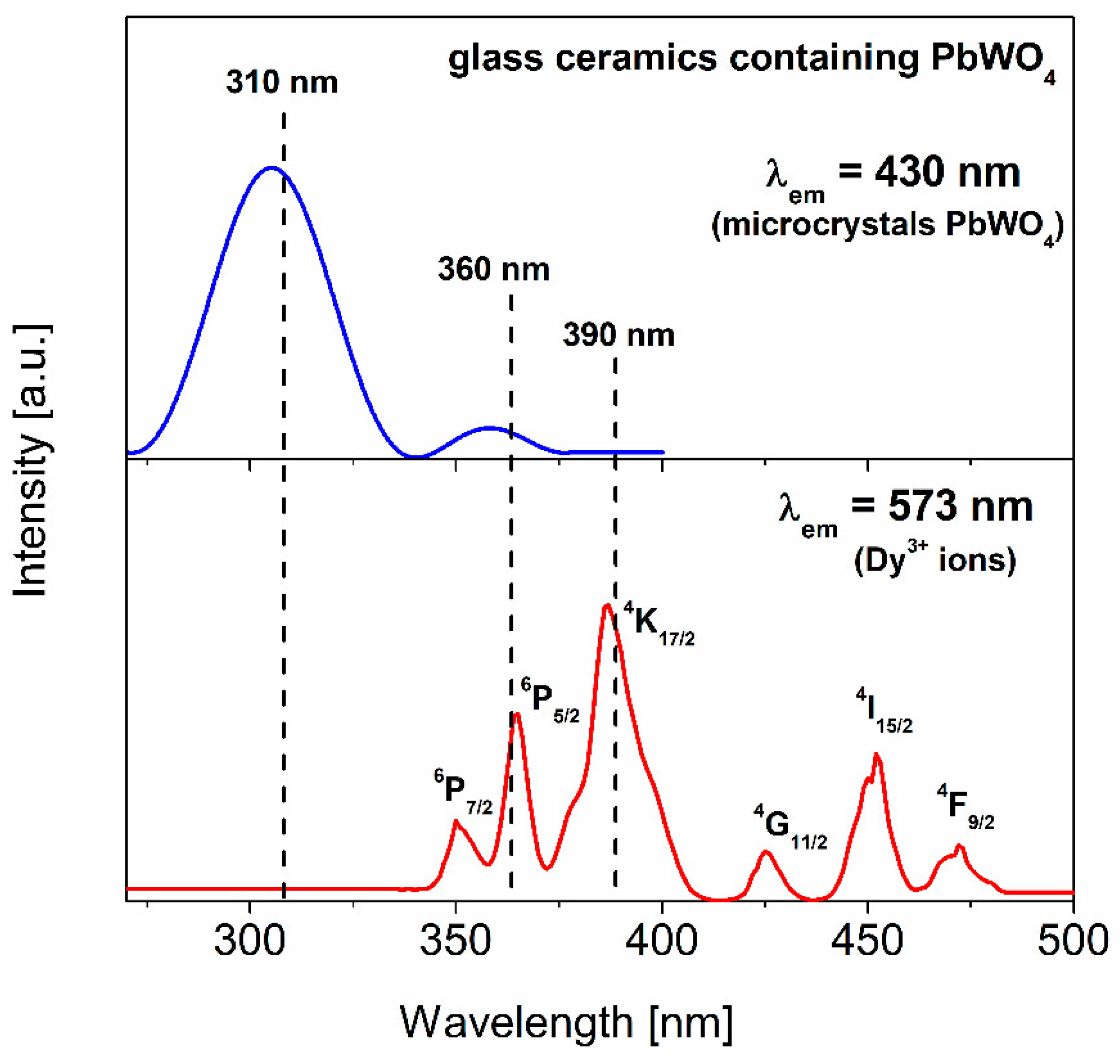
3.2. Glass-Ceramics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Linganna, K.; Sreedhar, V.B.; Jayasankar, C.K. Luminescence properties of Tb3+ ions in zinc fluorophosphate glasses for green laser applications. Mater. Res. Bull. 2015, 67, 196–200. [Google Scholar] [CrossRef]
- Lalla, E.A.; Rodríguez-Mendoza, U.R.; Lozano-Gorrín, A.D.; Sanz-Arranz, A.; Rull, F.; Lavín, V. Nd3+-doped TeO2–PbF2–AlF3 glasses for laser applications. Opt. Mater. 2016, 51, 35–41. [Google Scholar] [CrossRef]
- Madhu, A.; Eraiah, B.; Manasa, P.; Srinatha, N. Nd3+-doped lanthanum lead boro-tellurite glass for lasing andamplification applications. Opt. Mater. 2018, 75, 357–366. [Google Scholar] [CrossRef]
- Venkataiah, G.; Babu, P.; Martín, I.R.; Krishnaiah, K.V.; Suresh, K.; Lavín, V.; Jayasankar, C.K. Spectroscopic studies on Yb3+-doped tungsten-tellurite glasses for laser applications. J. Non Cryst. Solids 2018, 479, 9–15. [Google Scholar] [CrossRef]
- Deopa, N.; Rao, A.S.; Choudhary, A.; Saini, S.; Navhal, A.; Jayasimhadri, M.; Haranath, D.; Prakash, G.V. Photoluminescence investigations on Sm3+ ions doped borate glasses for tricolor w-LEDs and lasers. Mater. Res. Bull. 2018, 100, 206–212. [Google Scholar] [CrossRef]
- Caldiño, U.; Lira, A.; Meza-Rocha, A.N.; Pasquini, E.; Pelli, S.; Speghini, A.; Bettinelli, M.; Righini, G.C. White light generation in Dy3+-and Ce3+/Dy3+-doped zinc–sodium–aluminosilicate glasses. J. Lumin. 2015, 167, 327–332. [Google Scholar] [CrossRef]
- Meza-Rocha, A.N.; Lozada-Morales, R.; Speghini, A.; Bettinelli, M.; Caldiño, U. White light generation in Tb3+/Eu3+/Dy3+ triply-doped Zn(PO3)2 glass. Opt. Mater. 2016, 51, 128–132. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, P.; Singh, G.P.; Arora, D.; Kumar, S.; Singh, D.P. White light emission of Ce3+ sensitized Sm3+ doped lead alumino borate glasses. J. Lumin. 2016, 180, 190–197. [Google Scholar] [CrossRef]
- Segawa, H.; Hirosaki, N. Optical properties of zinc borate glasses dispersed with Eu-doped SiAlON for white LED applications. Ceram. Int. 2018, 44, 4783–4788. [Google Scholar] [CrossRef]
- Bengisu, M. Borate glasses for scientific and industrial applications: A review. J. Mater. Sci. 2016, 51, 2199–2242. [Google Scholar] [CrossRef]
- Kirdsiri, K.; Ramakrishna, R.R.; Damdee, B.; Kim, H.J.; Kaewjaeng, S.; Kothan, S.; Kaewkhao, J. Investigations of optical and luminescence features of Sm3+ doped Li2O-MO-B2O3 (M = Mg/Ca/Sr/Ba) glasses mixed with different modifier oxides as an orange light emitting phosphor for WLED’s. J. Alloys Compd. 2018, 749, 197–204. [Google Scholar] [CrossRef]
- Martins, A.L.J.; Feitosa, C.A.C.; Santos, W.Q.; Jacinto, C.; Santos, C.C. Influence of BaX2 (X = Cl, F) and Er2O3 concentration on the physical and optical properties of barium borate glasses. Phys. B Condens. Matter 2019, 558, 146–153. [Google Scholar] [CrossRef]
- Swapna, K.; Shamshad, S.; Srinivasa Rao, A.; Jayasimhadri, M.; Sasikala, T.; Moorthy, L.R. Visible fluorescence characteristics of Dy3+ doped zinc alumino bismuth borate glasses for optoelectronic devices. Ceram. Int. 2013, 39, 8459–8465. [Google Scholar] [CrossRef]
- Li, B.; Li, D.; Pun, E.Y.B.; Lin, H. Dy3+ doped tellurium-borate glass phosphors for laser-driven white illumination. J. Lumin. 2019, 206, 70–78. [Google Scholar] [CrossRef]
- Jayasimhadri, M.; Jang, K.; Lee, H.S.; Chen, B.; Yi, S.S.; Jeong, J.H. White light generation from Dy3+-doped ZnO–B2O3–P2O5 glasses. J. Appl. Phys. 2009, 106, 013105. [Google Scholar] [CrossRef]
- Pawar, P.P.; Munishwar, S.R.; Gedam, R.S. Intense white light luminescent Dy3+ doped lithium borate glasses for W-LED: A correlation between physical, thermal, structural and optical properties. Solid State Sci. 2017, 64, 41–50. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Vijayakumar, R.; Marimuthu, K. Luminescence studies on Dy3+ doped calcium boro-tellurite glasses for white light applications. Phys. B Condens. Matter 2017, 521, 347–354. [Google Scholar] [CrossRef]
- Hegde, V.; Dwaraka Viswanath, C.S.; Mahato, K.K.; Kamath, S.D. Warm white light and colour tunable characteristics of Dy3+ co-doped with Eu3+ and Pr3+ zinc sodium bismuth borate glasses for solid state lighting applications. Mater. Chem. Phys. 2019, 234, 369–377. [Google Scholar] [CrossRef]
- Narwal, P.; Dahiya, M.S.; Yadav, A.; Hooda, A.; Agarwal, A.; Khasa, S. Improved white light emission in Dy3+ doped LiF–CaO–Bi2O3–B2O3 glasses. J. Non-Cryst. Solids 2018, 498, 470–479. [Google Scholar] [CrossRef]
- Babu, K.V.; Cole, S. Luminescence properties of Dy3+-doped alkali lead alumino borosilicate glasses. Ceram. Int. 2018, 44, 9080–9090. [Google Scholar] [CrossRef]
- Mallur, S.B.; Czarnecki, T.; Adhikari, A.; Babu, P.K. Compositional dependence of optical band gap and refractive index in lead and bismuth borate glasses. Mater. Res. Bull. 2015, 68, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Mallur, S.B.; Ooi, H.G.; Ferrell, S.K.; Babu, P.K. Effect of metal and semiconducting nanoparticles on the optical properties of Dy3+ ions in lead borate glasses. Mater. Res. Bull. 2017, 92, 52–64. [Google Scholar] [CrossRef]
- Othman, H.A.; Elkholy, H.S.; Hager, I.Z. Structural and optical investigation of undoped and Sm3+ doped lead oxyfluoroborate glasses. Mater. Res. Bull. 2017, 89, 210–216. [Google Scholar] [CrossRef]
- Deopa, N.; Rao, A.S.; Mahamuda, S.; Gupta, M.; Jayasimhadri, M.; Haranath, D.; Prakash, G.V. Spectroscopic studies of Pr3+ doped lithium lead alumino borate glasses for visible reddish orange luminescent device applications. J. Alloys Compd. 2017, 708, 911–921. [Google Scholar] [CrossRef]
- Deopa, N.; Rao, A.S. Spectroscopic studies of single near ultraviolet pumped Tb3+ doped lithium lead alumino borate glasses for green lasers and tricolour w-LEDs. J. Lumin. 2018, 194, 56–63. [Google Scholar] [CrossRef]
- Zur, L.; Kos, A.; Górny, A.; Sołtys, M.; Pietrasik, E.; Pisarska, J.; Goryczka, T.; Pisarski, W.A. Influence of acceptor concentration on crystallization behavior and luminescence properties of lead borate glasses co-doped with Dy3+ and Tb3+ ions. J. Alloys Compd. 2018, 749, 561–566. [Google Scholar] [CrossRef]
- Pisarska, J.; Kos, A.; Sołtys, M.; Górny, A.; Pietrasik, E.; Pisarski, W.A. Spectroscopy and energy transfer in Tb3+/Sm3+ co-doped lead borate glasses. J. Lumin. 2018, 195, 87–95. [Google Scholar] [CrossRef]
- Rani, P.R.; Venkateswarlu, M.; Mahamuda, S.; Swapna, K.; Deopa, N.; Rao, A.S.; Prakash, G.V. Structural, absorption and photoluminescence studies of Sm3+ ions doped barium lead alumino fluoro borate glasses for optoelectronic device applications. Mater. Res. Bull. 2019, 110, 159–168. [Google Scholar] [CrossRef]
- Jamalaiah, B.C.; Moorthy, L.R.; Seo, H.J. Effect of lead oxide on optical properties of Dy3+ ions in PbO–H3BO3–TiO2–AlF3 glasses. J. Non Cryst. Solids 2012, 358, 204–209. [Google Scholar] [CrossRef]
- Jyothi, L.; Upender, G.; Kuladeep, R.; Rao, D.N. Structural, thermal, optical properties and simulation of white light of titanium-tungstate-tellurite glasses doped with dysprosium. Mater. Res. Bull. 2014, 50, 424–431. [Google Scholar] [CrossRef]
- Mishra, L.; Sharma, A.; Vishwakarma, A.K.; Jha, K.; Jayasimhadri, M.; Ratnam, B.V.; Jang, K.; Rao, A.S.; Sinh, R.K. White light emission and color tunability of dysprosium doped barium silicate glasses. J. Lumin. 2016, 169, 121–127. [Google Scholar] [CrossRef]
- Jha, K.; Jayasimhadri, M. Spectroscopic investigation on thermally stable Dy3+ doped zinc phosphate glasses for white light emitting diodes. J. Alloys Compd. 2016, 688, 833–840. [Google Scholar] [CrossRef]
- Lakshminarayana, G.; Baki, S.O.; Lira, A.; Kityk, I.V.; Caldiño, U.; Kaky, K.M.; Mahdi, M.A. Structural, thermal and optical investigations of Dy3+-doped B2O3–WO3–ZnO–Li2O–Na2O glasses for warm white light emitting applications. J. Lumin. 2017, 186, 283–300. [Google Scholar] [CrossRef]
- Khan, I.; Rooh, G.; Rajaramakrishna, R.; Srisittipokakun, N.; Wongdeeying, C.; Kiwsakunkran, N.; Wantana, N.; Kim, H.J.; Kaewkhao, J.; Tuscharoen, S.; et al. Photoluminescence and white light generation of Dy2O3 doped Li2O-BaO-Gd2O3- SiO2 for white light LED. J. Alloys Compd. 2019, 774, 244–254. [Google Scholar] [CrossRef]
- Kesavulu, C.R.; Jayasankar, C.K. White light emission in Dy3+-doped lead fluorophosphate glasses. Mater. Chem. Phys. 2011, 130, 1078–1085. [Google Scholar] [CrossRef]
- Linganna, K.; Rao, C.S.; Jayasankar, C.K. Optical properties and generation of white light in Dy3+-doped lead phosphate glasses. J. Quant. Spectrosc. Radiat. Transf. 2013, 118, 40–48. [Google Scholar] [CrossRef]
- Ahamed, S.Z.A.; Reddy, C.M.; Raju, B.D.P. Structural, thermal and optical investigations of Dy3+ ions doped lead containing lithium fluoroborate glasses for simulation of white light. Opt. Mater. 2013, 35, 1385–1394. [Google Scholar] [CrossRef]
- Damak, K.; El Sayed, Y.; Rüssel, C.; Maâlej, R. White light generation from Dy3+ doped tellurite glass. J. Quant. Spectrosc. Radiat. Transf. 2014, 134, 55–63. [Google Scholar] [CrossRef]
- Babu, M.R.; Rao, N.M.; Babu, A.M.; Jaidass, N.; Moorthy, C.K.; Moorthy, L.R. Effect of Dy3+ ions concentration on optical properties of lead borosilicate glasses for white light emission. Optik 2016, 127, 3121–3126. [Google Scholar] [CrossRef]
- Mohammed, A.B.; Lakshminarayana, G.; Baki, S.O.; Halimah, M.K.; Kityk, I.V.; Mahdi, M.A. Structural, thermal, optical and dielectric studies of Dy3+: B2O3-ZnO-PbO-Na2O-CaO glasses for white LEDs application. Opt. Mater. 2017, 73, 686–694. [Google Scholar] [CrossRef]
- Sun, X.; Wu, S.; Liu, X.; Gao, P.; Huang, S. Intensive white light emission from Dy3+-doped Li2B4O7 glasses. J. Non Cryst. Solids 2013, 368, 51–54. [Google Scholar] [CrossRef]
- Mahamuda, S.; Swapna, K.; Packiyaraj, P.; Rao, A.S.; Prakash, G.V. Lasing potentialities and white light generation capabilities of Dy3+ doped oxy-fluoroborate glasses. J. Lumin. 2014, 153, 382–392. [Google Scholar] [CrossRef]
- Annapoorani, K.; Karthikeyan, P.; Basavapoornima, C.; Marimuthu, K. Investigations on the optical properties of Dy3+ ions doped potassium aluminium telluroborate glasses for white light applications. J. Non Cryst. Solids 2017, 476, 128–136. [Google Scholar] [CrossRef]
- Shamshad, L.; Rooh, G.; Kirdsiri, K.; Srisittipokakun, N.; Damdee, B.; Kim, H.J.; Kaewkhao, J. Photoluminescence and white light generation behavior of lithium gadolinium silicoborate glasses. J. Alloys Compd. 2017, 695, 2347–2355. [Google Scholar] [CrossRef]
- Kesavulu, C.R.; Kim, H.J.; Lee, S.W.; Kaewkhao, J.; Chanthima, N.; Tariwong, Y. Physical, vibrational, optical and luminescence investigations of Dy3+-doped yttrium calcium silicoborate glasses for cool white LED applications. J. Alloys Compd. 2017, 726, 1062–1071. [Google Scholar] [CrossRef]
- Kaur, S.; Vishwakarma, A.K.; Deopa, N.; Prasad, A.; Jayasimhadri, M.; Rao, A.S. Spectroscopic studies of Dy3+ doped borate glasses for cool white light generation. Mater. Res. Bull. 2018, 104, 77–82. [Google Scholar] [CrossRef]
- Zaman, F.; Rooh, G.; Srisittipokakun, N.; Ahmad, T.; Khan, I.; Shoaib, M.; Rajagukguk, J.; Kaewkhao, J. Comparative investigations of gadolinium based borate glasses doped with Dy3+ for white light generations. Solid State Sci. 2019, 89, 50–56. [Google Scholar] [CrossRef]
- Pisarska, J.; Pisarski, W.A. Lanthanide—Doped borate glasses for optical applications: A review. Chapter 4. In Handbook on Borates: Chemistry, Production and Applications; Chung, M.P., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2010; pp. 107–158. [Google Scholar]
- Pisarski, W.A.; Dominiak-Dzik, G.; Ryba-Romanowski, W.; Pisarska, J. Role of PbO substitution by PbF2 on structural behavior and luminescence of rare earth doped lead borate glass. J. Alloys Compd. 2008, 451, 220–222. [Google Scholar] [CrossRef]
- Pisarska, J. Optical properties of lead borate glasses containing Dy3+ ions. J. Phys. Condens. Matter 2009, 21, 285101. [Google Scholar] [CrossRef]
- Pisarska, J.; Czopek, I.; Lisiecki, R.; Ryba-Romanowski, W.; Goryczka, T.; Pisarski, W.A. PbWO4 formation during controlled crystallization of lead borate glasses. Ceram. Int. 2013, 39, 9151–9156. [Google Scholar] [CrossRef]
- Pisarska, J. Luminescence behavior of Dy3+ ions in lead borate glasses. Opt. Mater. 2009, 31, 1784–1786. [Google Scholar] [CrossRef]
- Narwal, P.; Dahiya, M.S.; Yadav, A.; Hooda, A.; Agarwal, A.; Khasa, S. Dy3+ doped LiCl–CaO–Bi2O3–B2O3 glasses for WLED applications. Ceram. Int. 2017, 43, 11132–11141. [Google Scholar] [CrossRef]
- Gökçe, M.; Koçyiğit, D. Spectroscopic investigations of Dy3+ doped borogermanate glasses for laser and wLED applications. Opt. Mater. 2019, 89, 568–575. [Google Scholar] [CrossRef]
- Hegde, V.; Chauhan, N.; Viswanath, C.S.D.; Kumar, V.; Mahato, K.K.; Kamath, S.D. Photoemission and thermoluminescence characteristics of Dy3+-doped zinc sodium bismuth borate glasses. Solid State Sci. 2019, 89, 130–138. [Google Scholar] [CrossRef]
- Pisarska, J.; Zur, L.; Pisarski, W.A. Optical spectroscopy of Dy3+ ions in heavy metal lead-based glasses and glass-ceramics. J. Mol. Struct. 2011, 993, 160–166. [Google Scholar] [CrossRef]
- Jayasankar, C.K.; Venkatramu, V.; Babu, S.S.; Babu, P. Luminescence properties of Dy3+ ions in a variety of borate and fluoroborate glasses containing lithium, zinc and lead. J. Alloys Compd. 2004, 374, 22–26. [Google Scholar] [CrossRef]
- Tobinaga, K.; Inoue, S.; Matsumura, Y. Synthesis of broad yellow phosphors by co-doping and realization of high quality of white light. Chem. Phys. Lett. 2019, 717, 11–15. [Google Scholar] [CrossRef]
- Deopa, N.; Rao, A.S. Photoluminescence and energy transfer studies of Dy3+ ions doped lithium lead alumino borate glasses for w-LED and laser applications. J. Lumin. 2017, 192, 832–841. [Google Scholar] [CrossRef]
- Fortes, L.M.; Santos, L.F.; Goncalves, M.C.; Almeida, R.M. Preparation and characterization of Er3+ doped TeO2-based oxyhalide glasses. J. Non Cryst. Solids 2003, 324, 150–158. [Google Scholar] [CrossRef]
- Bueno, L.A.; Messaddeq, Y.; Filho, F.A.; Ribeiro, S.J.L. Study of fluorine losses in oxyfluoride glasses. J. Non Cryst. Solids 2005, 351, 3804–3808. [Google Scholar] [CrossRef]
- Lin, L.; Ren, G.; Chen, M.; Liu, Y.; Yang, Q.B. Study of fluorine losses and spectroscopic properties of Er3+ doped oxyfluoride silicate glasses and glass ceramics. Opt. Mater. 2009, 31, 1439–1442. [Google Scholar] [CrossRef]
- Souza Filho, A.G.; Mendes Filho, J.; Melo, F.E.A.; Custodio, M.C.C.; Lebullenger, R.; Hernandes, A.C. Optical properties of Sm3+ doped lead fluoroborate glasses. J. Phys. Chem. Solids 2000, 61, 1535–1542. [Google Scholar] [CrossRef]
- Hager, I.Z. Optical properties of lithium barium haloborate glasses. J. Phys. Chem. Solids 2009, 70, 210–217. [Google Scholar] [CrossRef]
- Pisarska, J.; Żur, L.; Pisarski, W.A. Visible luminescence of dysprosium ions in oxyhalide lead borate glasses. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 705–707. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Pisarska, J.; Lisiecki, R.; Grobelny, Ł.; Dominiak-Dzik, G.; Ryba-Romanowski, W. Erbium-doped oxide and oxyhalide lead borate glasses for near-infrared broadband optical amplifiers. Phys. Lett. 2009, 472, 217–219. [Google Scholar] [CrossRef]
- Babu, P.; Jang, K.H.; Kim, E.S.; Shi, L.; Seo, H.J.; Rivera-López, F.; Rodríguez-Mendoza, U.R.; Lavín, V.; Vijaya, R.; Jayasankar, C.K.; et al. Spectral investigations on Dy3+-doped transparent oxyfluoride glasses and nanocrystalline glass ceramics. J. Appl. Phys. 2009, 105, 013516. [Google Scholar] [CrossRef]
- Babu, P.; Jang, K.H.; Rao, C.S.; Shi, L.; Jayasankar, C.K.; Lavín, V.; Seo, H.J. White light generation in Dy3+-doped oxyfluoride glass and transparent glass-ceramics containing CaF2 nanocrystals. Opt. Express 2011, 19, 1836–1841. [Google Scholar] [CrossRef]
- Ramachari, D.; Moorthy, L.R.; Jayasankar, C.K. Energy transfer and photoluminescence properties of Dy3+/Tb3+ co-doped oxyfluorosilicate glass–ceramics for solid-state white lighting. Ceram. Int. 2014, 40, 11115–11121. [Google Scholar] [CrossRef]
- Kaur, S.; Pandey, O.P.; Jayasankar, C.K.; Chopra, N. Spectroscopic, thermal and structural investigations of Dy3+ activated zinc borotellurite glasses and nano-glass-ceramics for white light generation. J. Non Cryst. Solids 2019, 521, 119472. [Google Scholar] [CrossRef]
- Ryu, J.H.; Yoon, J.W.; Shim, K.B. Blue luminescence of nanocrystalline PbWO4 phosphor synthesized via a citrate complex route assisted by microwave irradiation. Solid State Commun. 2005, 133, 657–661. [Google Scholar] [CrossRef]
- Huang, Y.; Seo, H.J.; Feng, Q.; Yuan, S. Effects of trivalent rare-earth ions on spectral properties of PbWO4 crystals. Mater. Sci. Eng. B 2005, 121, 103–107. [Google Scholar] [CrossRef]
- Pisarska, J.; Lisiecki, R.; Ryba-Romanowski, W.; Goryczka, T.; Pisarski, W.A. Unusual luminescence behavior of Dy3+-doped lead borate glass after heat treatment. Chem. Phys. Lett. 2010, 489, 198–201. [Google Scholar] [CrossRef]
- Shi, C.; Wei, Y.; Jang, X.; Zhou, D.; Guo, C.; Liao, J.; Tang, H. Spectral properties and thermoluminescence of PbWO4 crystals annealed in different atmospheres. Chem. Phys. Lett. 2000, 328, 1–4. [Google Scholar] [CrossRef]
- Yanes, A.C.; Del-Castillo, J. Enhanced emission via energy transfer in RE co-doped SiO2-KYF4 nano-glass-ceramics for white LEDs. J. Alloys Compd. 2016, 658, 170–176. [Google Scholar] [CrossRef]
- Kemere, M.; Rogulis, U.; Sperga, J. Luminescence and energy transfer in Dy3+/Eu3+ co-doped aluminosilicate oxyfluoride glasses and glass-ceramics. J. Alloys Compd. 2018, 735, 1253–1261. [Google Scholar] [CrossRef]
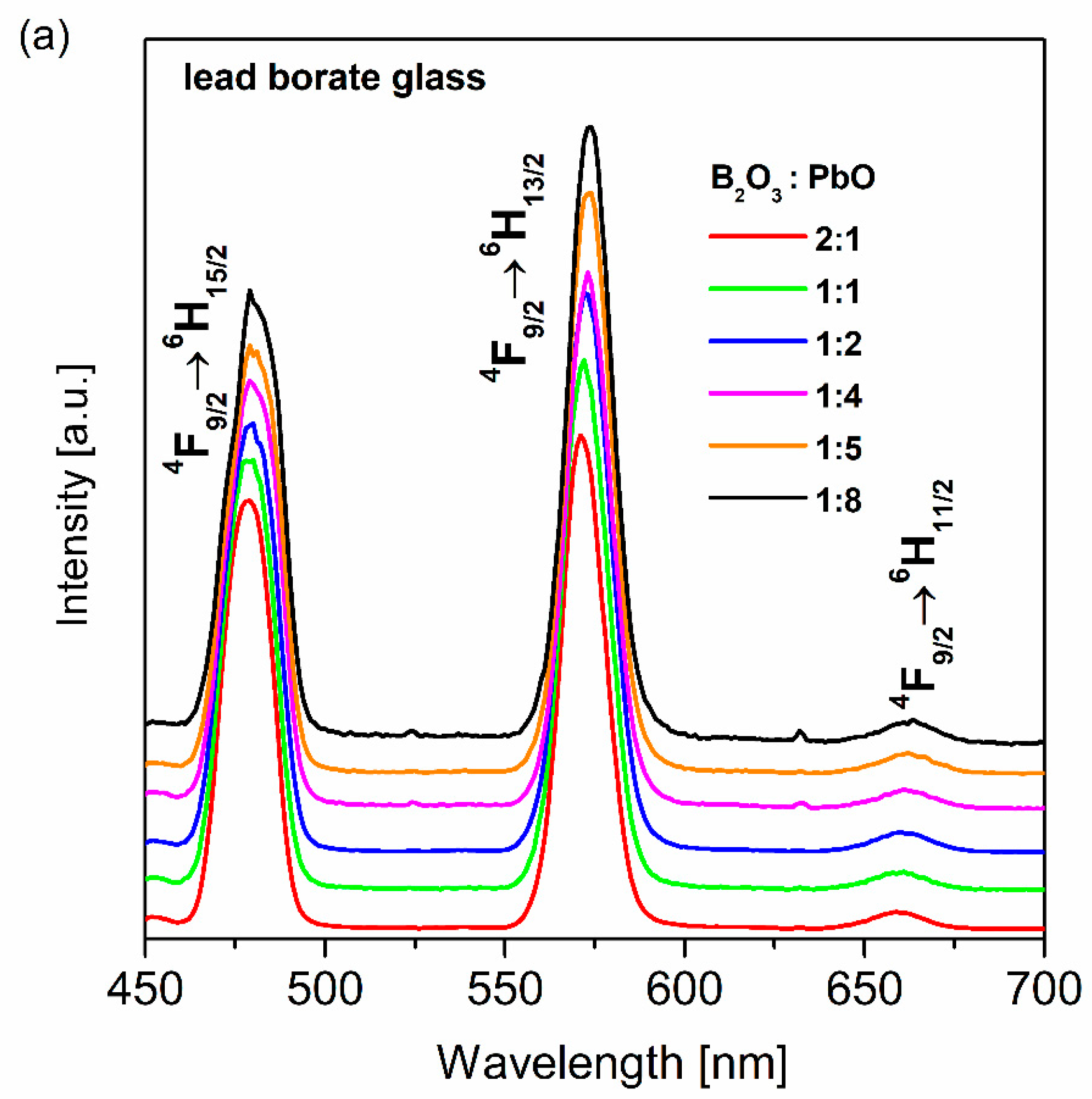
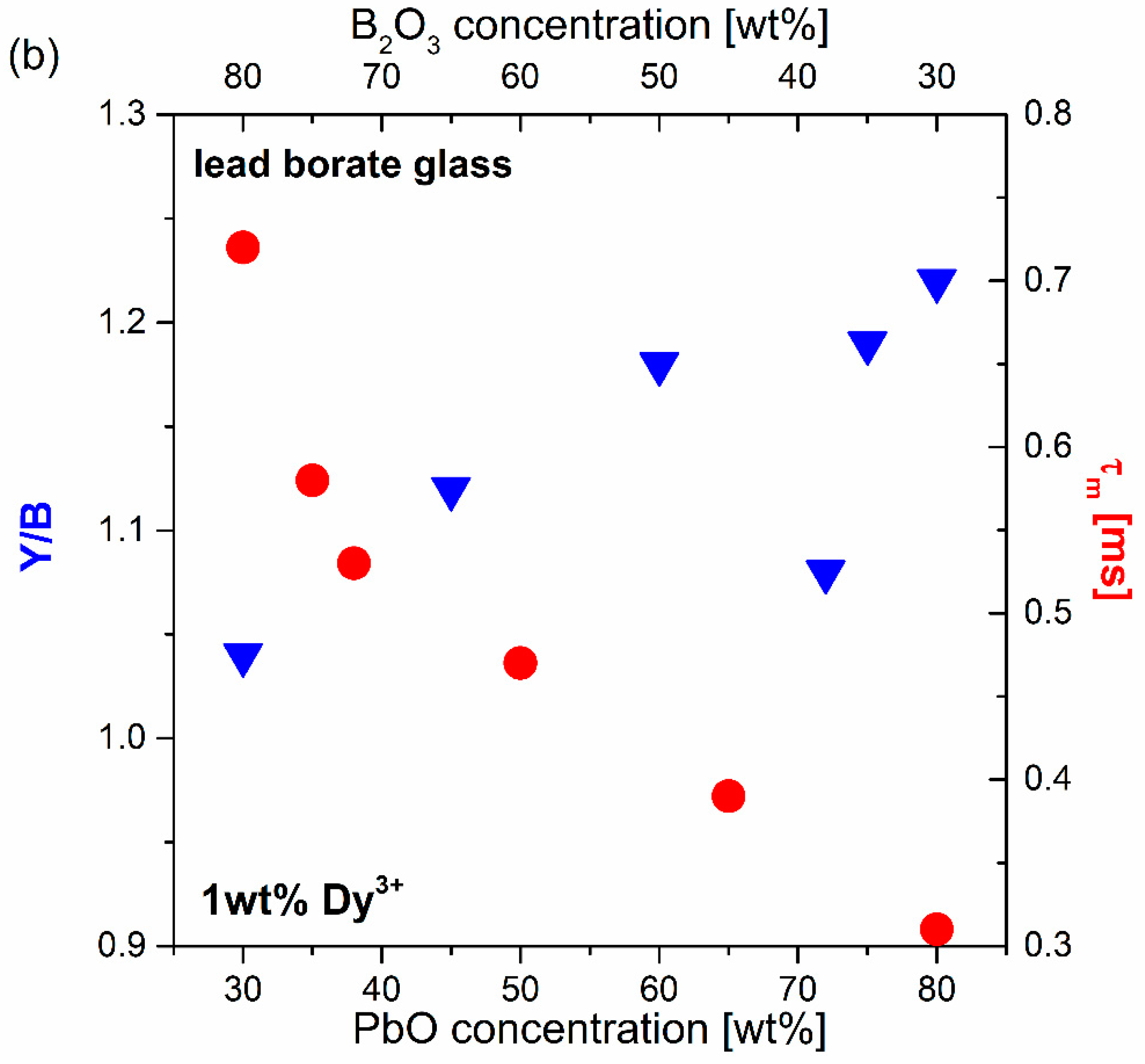

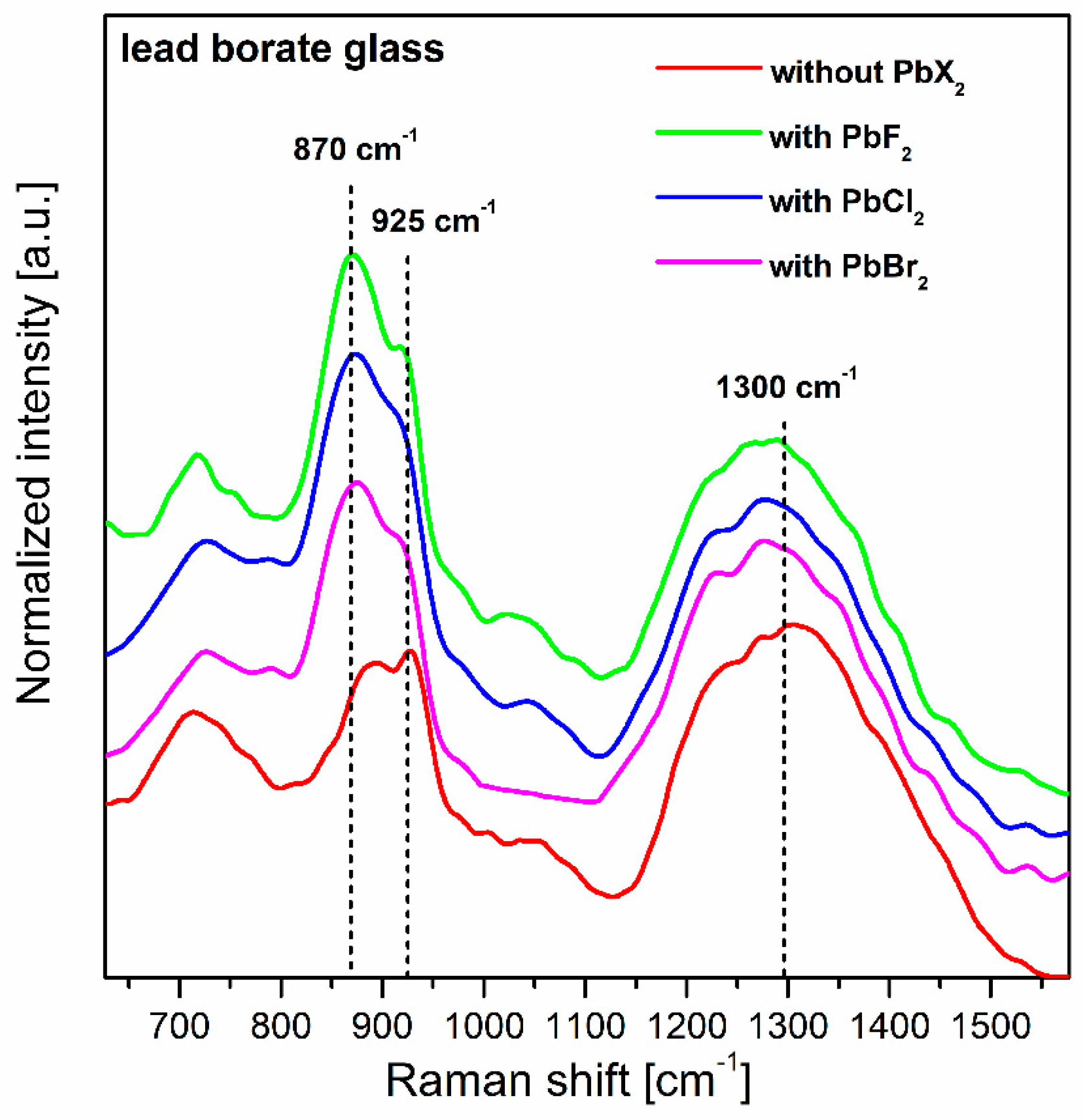
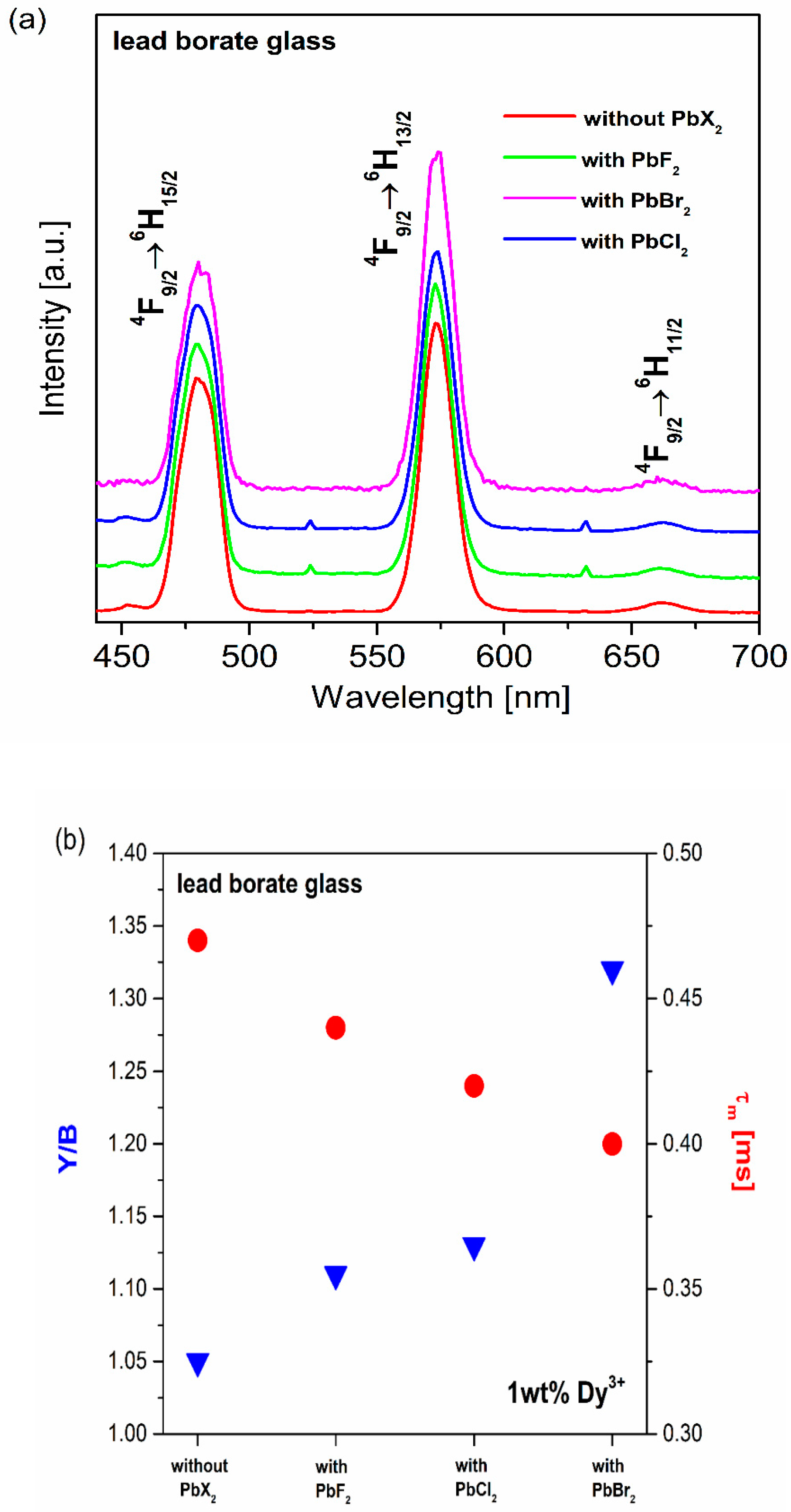

| Glass No. | Ratio | Chemical Composition (wt.%) | ||||
|---|---|---|---|---|---|---|
| B2O3:PbO | B2O3 | PbO | Al2O3 | WO3 | Dy2O3 | |
| (a) | 2:1 | 60 | 30 | 6 | 3 | 1 |
| (b) | 1:1 | 45 | 45 | 6 | 3 | 1 |
| (c) | 1:2 | 30 | 60 | 6 | 3 | 1 |
| (d) | 1:4 | 18 | 72 | 6 | 3 | 1 |
| (e) | 1:5 | 15 | 75 | 6 | 3 | 1 |
| (f) | 1:8 | 10 | 80 | 6 | 3 | 1 |
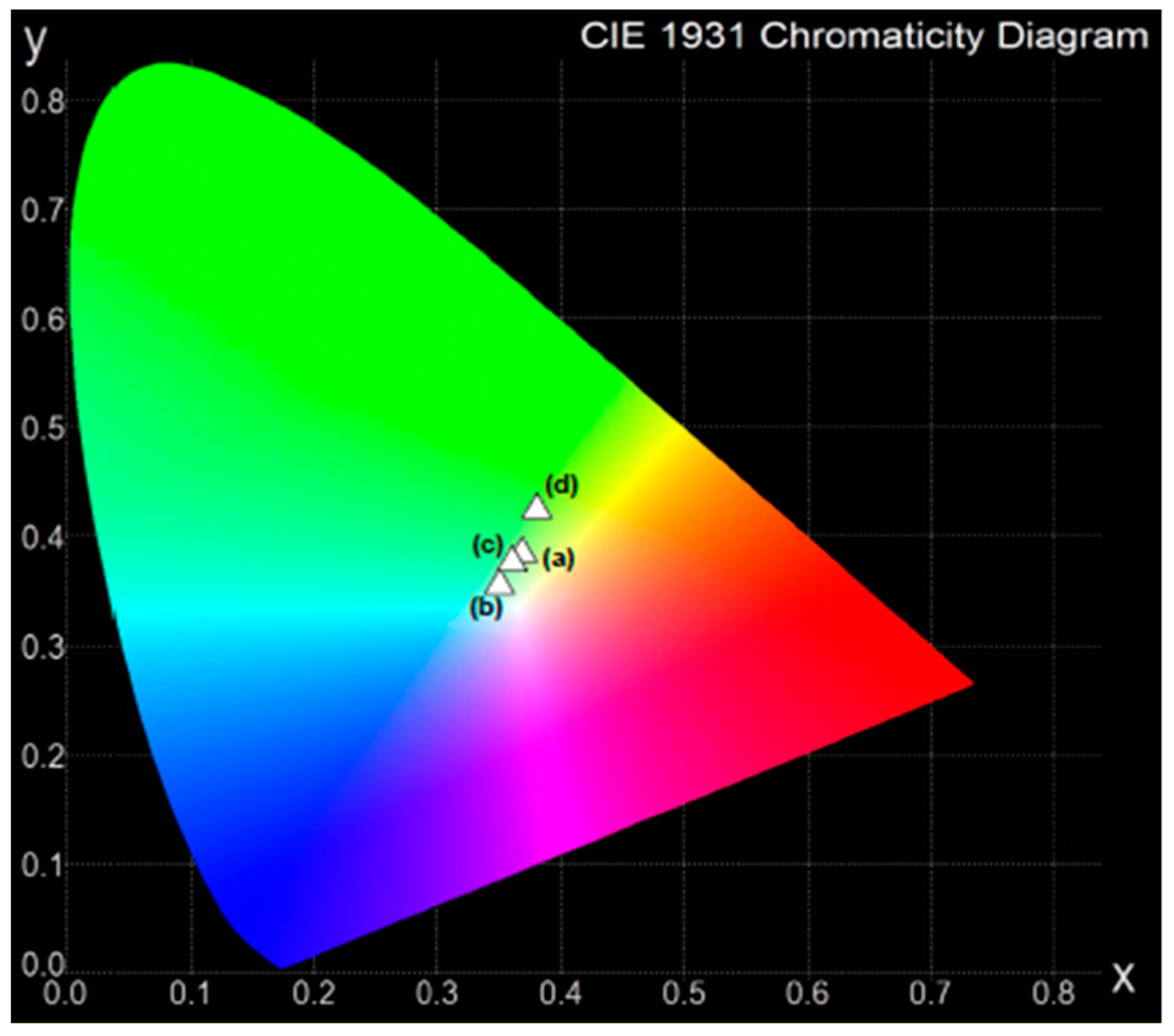
| Glass No. | Ratio | Y/B | CIE (x, y) |
|---|---|---|---|
| B2O3:PbO | |||
| (a) | 2:1 | 1.04 | (0.321, 0.352) |
| (b) | 1:1 | 1.12 | (0.319, 0.379) |
| (c) | 1:2 | 1.18 | (0.338, 0.386) |
| (d) | 1:4 | 1.08 | (0.331, 0.378) |
| (e) | 1:5 | 1.19 | (0.353, 0.391) |
| (f) | 1:8 | 1.22 | (0.375, 0.395) |
| Glass No. | PbX2 | Y/B | CIE (x, y) |
|---|---|---|---|
| (a) | without | 1.04 | (0.336, 0.381) |
| (b) | PbF2 | 1.11 | (0.318, 0.352) |
| (c) | PbCl2 | 1.13 | (0.328, 0.373) |
| (d) | PbBr2 | 1.32 | (0.348, 0.421) |
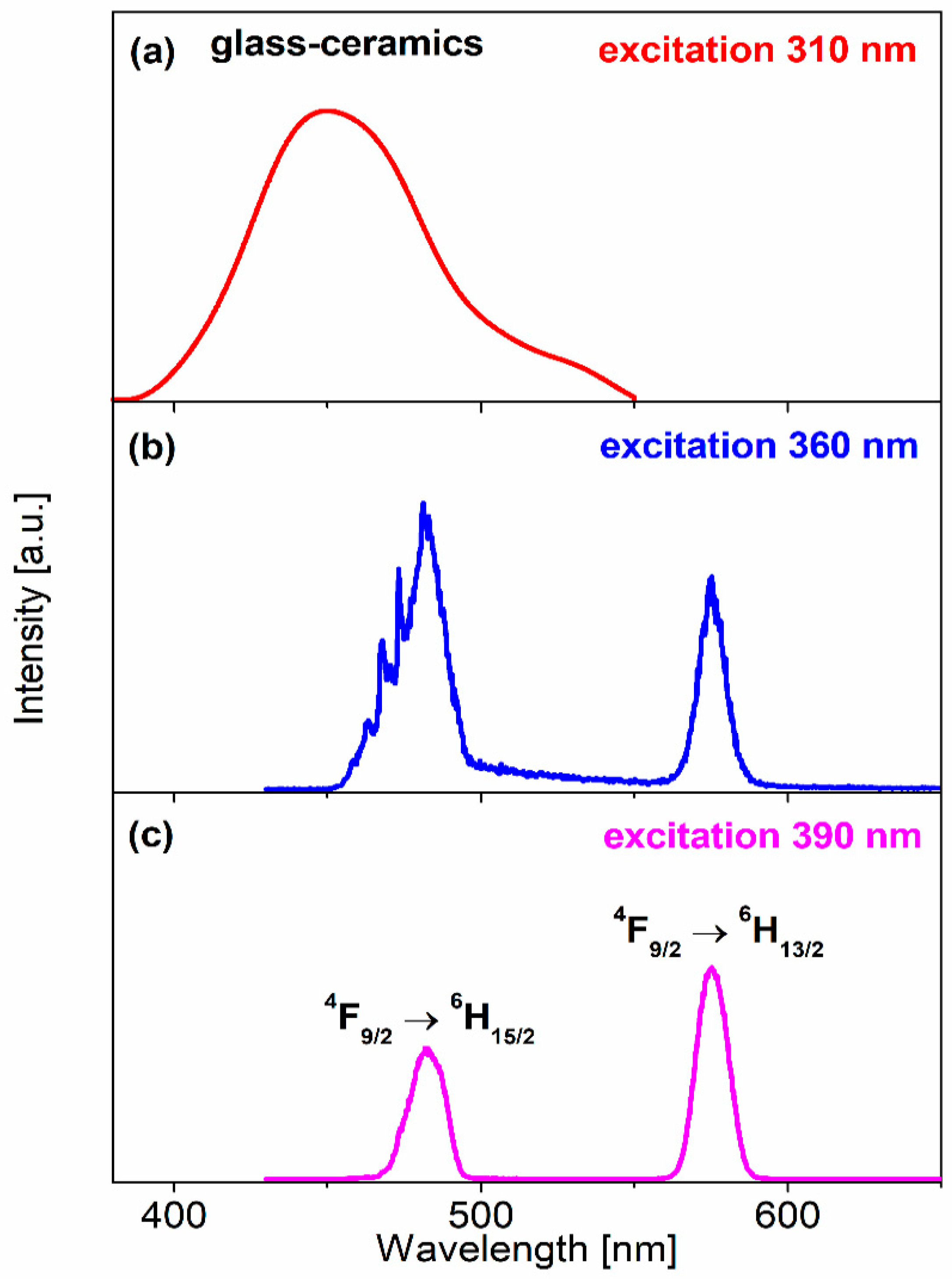
| Glass-Ceramics No. | λexc (nm) | CIE (x, y) |
|---|---|---|
| (a) | 310 | (0.142, 0.081) |
| (b) | 360 | (0.305, 0.317) |
| (c) | 390 | (0.370, 0.416) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górny, A.; Kuwik, M.; Pisarski, W.A.; Pisarska, J. Lead Borate Glasses and Glass-Ceramics Singly Doped with Dy3+ for White LEDs. Materials 2020, 13, 5022. https://doi.org/10.3390/ma13215022
Górny A, Kuwik M, Pisarski WA, Pisarska J. Lead Borate Glasses and Glass-Ceramics Singly Doped with Dy3+ for White LEDs. Materials. 2020; 13(21):5022. https://doi.org/10.3390/ma13215022
Chicago/Turabian StyleGórny, Agata, Marta Kuwik, Wojciech A. Pisarski, and Joanna Pisarska. 2020. "Lead Borate Glasses and Glass-Ceramics Singly Doped with Dy3+ for White LEDs" Materials 13, no. 21: 5022. https://doi.org/10.3390/ma13215022





