Effect of SiO2–Al2O3 Glass Composite Coating on the Oxidation Behavior of Ti60 Alloy
Abstract
:1. Introduction
2. Experimental Procedures
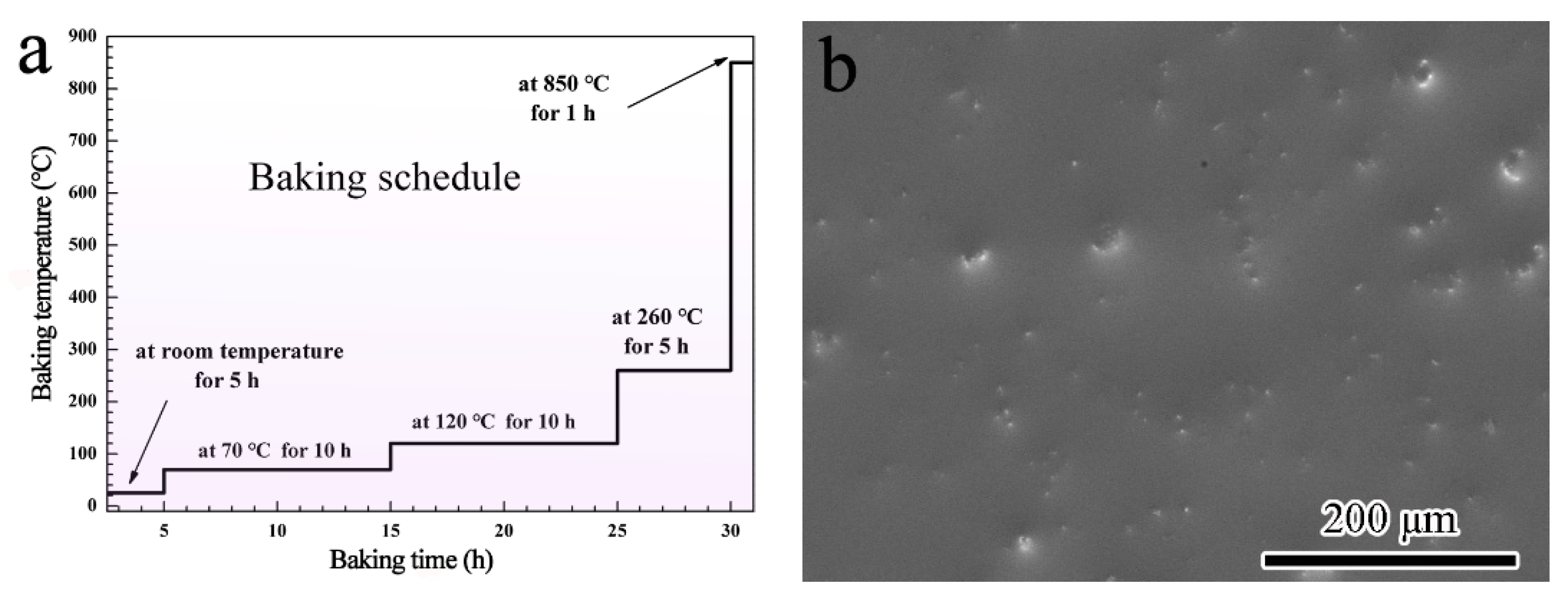
2.1. Coating Preparation
2.2. Oxidation Tests
2.3. Analytical Characterization
3. Results
4. Discussion
4.1. Effect of the Composite Coating on the Oxidation Resistance of Bare Ti60 Substrate
4.2. Interfacial Reaction between the Composite Coating and Ti60 Substrate
4.3. The Developing Trends of SiO2–Al2O3 Glass Composite Coating
- To optimize the composition and structure of the coating, e.g., by adjusting the ratio of SiO2 to K2O, optimizing the distribution, content and the size of the ceramic particles. These are ways to further lower the diffusion rate of oxygen in the coating and to achieve further improved oxidation resistance.
- To investigate the corrosion behavior and mechanisms of the composite coating under molten salts as well as environments with high Cl− content.
- To adequately understand the reactions of each components at high temperature and seek more economical components, such as kaolin, which makes the composite coating oxidation-resistant and low-cost.
5. Conclusions
- The composite coating exhibits a dense structure after baking. It is adherent to the Ti60 substrate after oxidation due to their similar CTE and occurring of interfacial reaction at the coating/substrate interface.
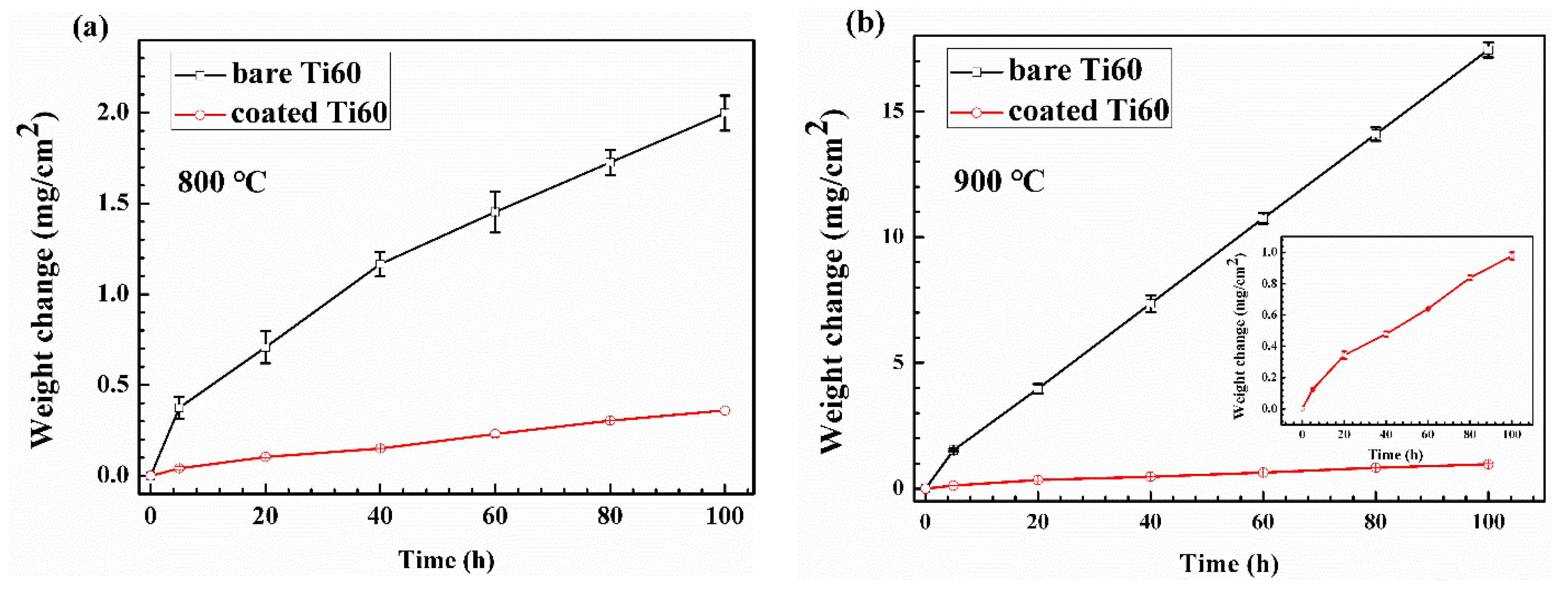
- A much better oxidation resistance is achieved on Ti60 alloy with composite coating. The mass gain of the coated alloy is about a half and to a fifth of the bare alloy after oxidation for 100 h at 800 °C and 900 °C, respectively.
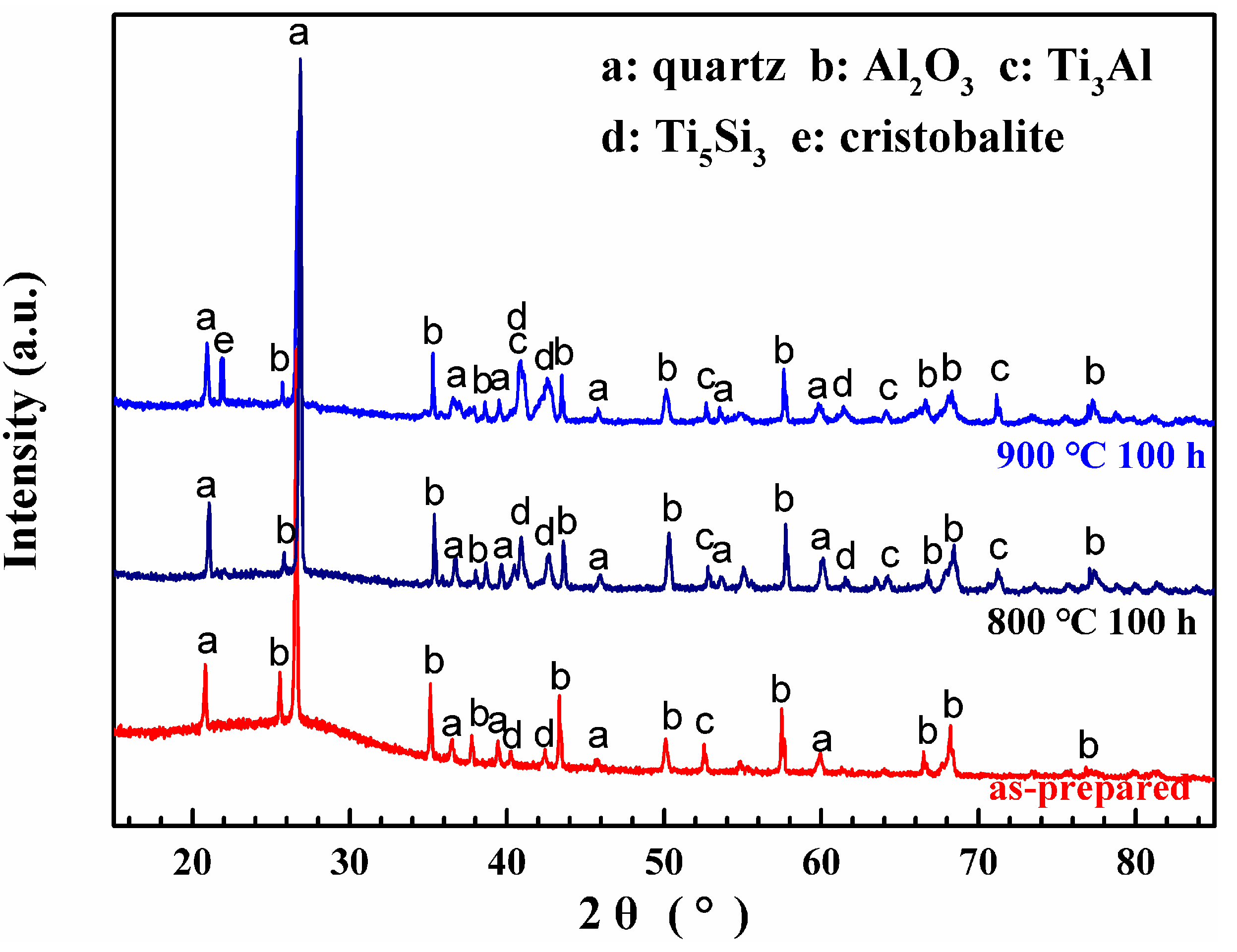
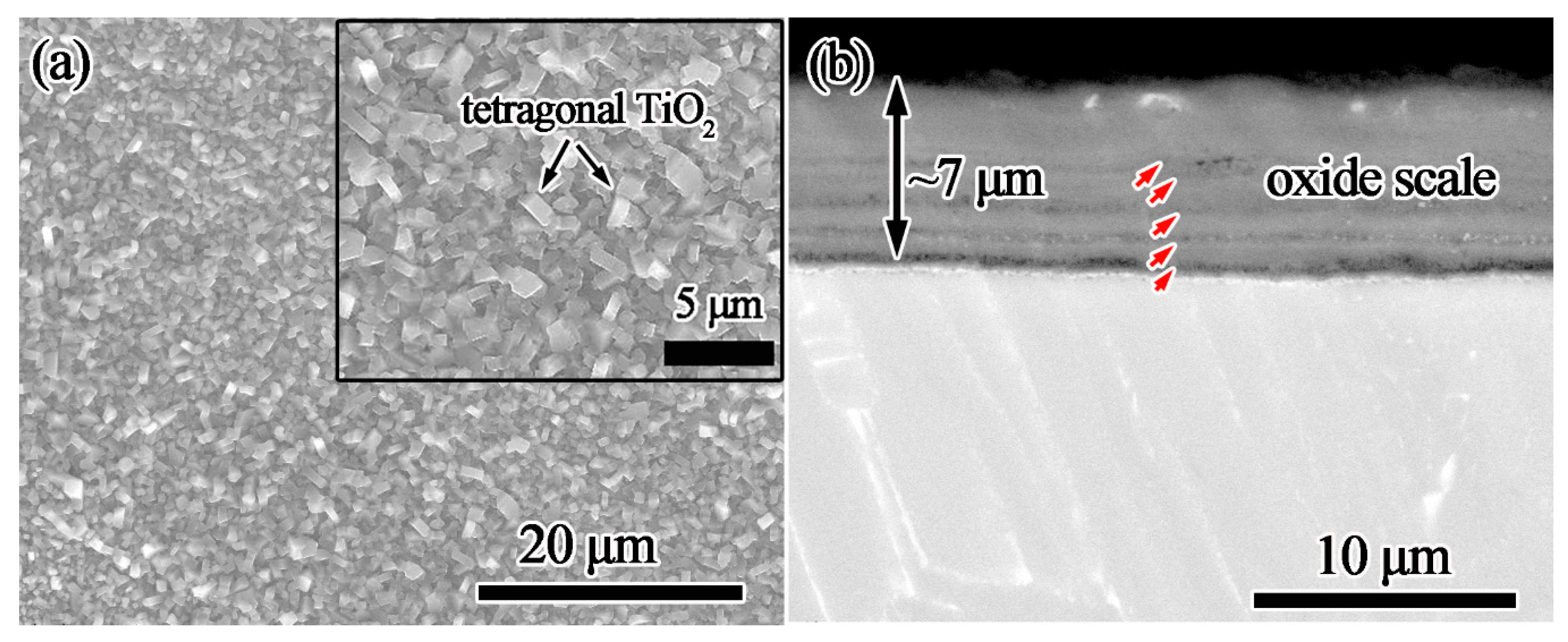
- Coating/alloy interfacial reactions occur during oxidation tests and a multilayered structure forms. This complex structure includes composite-coating/Al2O3-layer/Ti5Si3-layer/Ti3Al-layer/substrate.
Author Contributions
Funding
Conflicts of Interest
References
- Peters, M.; Kumpfert, J.; Ward, C.; Leyens, C. Titanium Alloys for Aerospace Applications. Adv. Eng. Mater. 2003, 5, 419–427. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, J.; Chen, C.; Weng, F. High temperature oxidation behavior and research status of modifications on improving high temperature oxidation resistance of titanium alloys and titanium aluminides: A review. J. Alloys Compd. 2016, 685, 784–798. [Google Scholar] [CrossRef]
- Gorynin, I. Titanium alloys for marine application. Mater. Sci. Eng. A 1999, 263, 112–116. [Google Scholar] [CrossRef]
- Veiga, C.; Davim, J.P.; Loureiro, A.J.R. Properties and applications of titanium alloys: A brief review. Rev. Adv. Mater. Sci. 2012, 32, 133–148. [Google Scholar]
- Boyer, R. An overview on the use of titanium in the aerospace industry. Mater. Sci. Eng. A 1996, 213, 103–114. [Google Scholar] [CrossRef]
- Fanning, J.C. Military Applications for β Titanium Alloys. J. Mater. Eng. Perform. 2005, 14, 686–690. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, F.; Wang, A.; Yu, H.; Chen, C. Microstructure and properties of Ti-Al coating and Ti-Al-Si system coatings on Ti-6Al-4V fabricated by laser surface alloying. Surf. Coat. Technol. 2017, 309, 805–813. [Google Scholar] [CrossRef]
- Liu, L.; Li, Z.; Hua, Y.; Hu, Z.; Lu, W.; Qiao, J. Reactive synthesis of oxidation-resistant coatings on pure Ti substrates via an Al/porous TiO2/Ti precursory stack. Surf. Coat. Technol. 2016, 289, 219–225. [Google Scholar] [CrossRef]
- Dai, J.; Li, S.; Zhang, H.; Yu, H.; Chen, C.; Li, Y. Microstructure and high-temperature oxidation resistance of Ti-Al-Nb coatings on a Ti-6Al-4V alloy fabricated by laser surface alloying. Surf. Coat. Technol. 2018, 344, 479–488. [Google Scholar] [CrossRef]
- Du, H.; Datta, P.; Lewis, D.; Burnell-Gray, J. Air oxidation behaviour of Ti-6Al-4V alloy between 650 and 850 °C. Corros. Sci. 1994, 36, 631–642. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, B.; Chen, M.; Feng, M.; Wang, J.; Zhu, S.; Wang, F. Self-healing metal-enamel composite coating and its protection for TiAl alloy against oxidation under thermal shock in NaCl solution. Corros. Sci. 2020, 167, 108526. [Google Scholar] [CrossRef]
- Feng, M.; Chen, M.; Yu, Z.; Lv, Z.; Zhu, S.; Wang, F. Comparative study of thermal shock behavior of the arc ion plating NiCrAlY and the enamel based composite coatings. Acta Metall. Sin. 2017, 53, 1636–1644. [Google Scholar]
- Zhou, L.; Huang, J.; Cao, L.; Hao, W.; Wu, W. A novel design of oxidation protective β-Y2Si2O7 nanowire toughened Y2SiO5/Y2O3-Al2O3-SiO2 glass ceramic coating for SiC coated carbon/carbon composites. Corros. Sci. 2018, 135, 233–242. [Google Scholar] [CrossRef]
- Das, S.; Datta, S.; Basu, D.; Das, G. Glass–ceramics as oxidation resistant bond coat in thermal barrier coating system. Ceram. Int. 2009, 35, 1403–1406. [Google Scholar] [CrossRef]
- Ghosh, S. Thermal behavior of glass–ceramic bond coat in a TBC system. Vacuum 2014, 101, 367–370. [Google Scholar] [CrossRef]
- Zheng, D.; Zhu, S.; Wang, F. Oxidation and hot corrosion behavior of a novel enamel-Al2O3 composite coating on K38G superalloy. Surf. Coat. Technol. 2006, 200, 5931–5936. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, F.; Li, Y.; Gu, Y.; Zhang, F.; Xiong, X. Oxidation mechanism of SiC–Zirconia–Glass ceramic coated carbon/carbon composites at 1123–1273 K. Mater. Res. Bull. 2017, 91, 189–196. [Google Scholar] [CrossRef]
- Li, H.-C.; Wang, D.; Meng, X.; Chen, C.-Z. Influence of heat treatments upon the mechanical properties and in vitro bioactivity of ZrO2-toughened MgO-CaO-SiO2-P2O5-CaF2 glass–ceramics. Biointerphases 2014, 9, 031014. [Google Scholar] [CrossRef]
- Banijamali, S.; Yekta, B.E.; Rezaie, H.R.; Marghussian, V. Crystallization and sintering characteristics of CaO–Al2O3–SiO2 glasses in the presence of TiO2, CaF2 and ZrO2. Themochim. Acta 2009, 488, 60–65. [Google Scholar] [CrossRef]
- Fu, K.; Wang, S.; Wang, G.; Wang, Y. The effects of calcium oxide on fluorapatite crystal morphology and mechanical property of functional glass-ceramics. Ceram. Int. 2018, 44, 20531–20538. [Google Scholar] [CrossRef]
- Wang, G.; Fu, K.; Wang, S.; Yang, B. Optimization of mechanical and tribological properties of a dental SiO2–Al2O3–K2O–CaO–P2O5 glass-ceramic. J. Mech. Behav. Biomed. Mater. 2020, 102, 103523. [Google Scholar] [CrossRef] [PubMed]
- Shirke, G.D.; Umarji, G.G.; Tarale, A.R.; Mathe, V.L.; Mulik, U.P.; Rane, S.B. Effect of firing temperature on microstructure and dielectric properties of chromium oxide based glass composite thick films on stainless steel substrate. J. Mater. Sci. Mater. Electron. 2018, 29, 9871–9878. [Google Scholar] [CrossRef]
- Li, W.-B.; Liu, L.-L.; Yang, Y.F.; Zhu, S.; Wang, F.-H. Hot Corrosion Behavior of SiO2–Al2O3–Glass Composite Coating on Ti–47Al–2Cr–2Nb Alloy: Diffusion Barrier for S and Cl. Acta Met. Sin. 2018, 32, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Chen, M.; Wang, C.; Zhu, S.; Wang, F. Preparation and oxidation behavior of SiO2–Al2O3–glass composite coating on Ti–47Al–2Cr–2Nb alloy. Surf. Coat. Technol. 2013, 218, 30–38. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F. Introduction to the High-Temperature Oxidation of Metals; Cambridge University Press (CUP): Cambridge, UK, 2006; pp. 82–98. [Google Scholar]
- Wang, F. The effect of nanocrystallization on the selective oxidation and adhesion of Al2O3 scales. Oxid. Met. 1997, 48, 215–224. [Google Scholar] [CrossRef]
- Han, R.; Tariq, N.U.H.; Li, J.; Kong, L.; Liu, J.; Shan, X.; Cui, X.; Xiong, T. A novel phosphate-ceramic coating for high temperature oxidation resistance of Ti65 alloys. Ceram. Int. 2019, 45, 23895–23901. [Google Scholar] [CrossRef]
- Holmquist, S.B. Conversion of Quartz to Tridymite. J. Am. Ceram. Soc. 1961, 44, 82–86. [Google Scholar] [CrossRef]
- Okamoto, H. O-Ti (Oxygen-Titanium). J. Phase Equilibria Diffus. 2011, 32, 473–474. [Google Scholar] [CrossRef]
- Lamkin, M.; Riley, F.; Fordham, R. Oxygen mobility in silicon dioxide and silicate glasses: A review. J. Eur. Ceram. Soc. 1992, 10, 347–367. [Google Scholar] [CrossRef]
- Dunn, T. Oxygen diffusion in three silicate melts along the join diopside-anorthite. Geochim. Cosmochim. Acta 1982, 46, 2293–2299. [Google Scholar] [CrossRef]
- Mrowec, S. On the mechanism of high temperature oxidation of metals and alloys. Corros. Sci. 1967, 7, 563–578. [Google Scholar] [CrossRef]
- Huang, B.; Li, C.; Shi, L.; Qiu, G.; Zuo, T. China materials engineering canon. Nonferrous Alloys Eng. 2005, 4, 646–650. [Google Scholar]
- Donald, I.W. Preparation, properties and chemistry of glass- and glass-ceramic-to-metal seals and coatings. J. Mater. Sci. 1993, 28, 2841–2886. [Google Scholar] [CrossRef]
- Gomez-Vega, J.M.; Saiz, E.; Tomsia, A.P. Glass-based coatings for titanium implant alloys. J. Biomed. Mater. Res. 1999, 46, 549–559. [Google Scholar] [CrossRef]
- Bloyer, D.; Gomez-Vega, J.; Saiz, E.; McNaney, J.; Cannon, R.; Tomsia, A. Fabrication and characterization of a bioactive glass coating on titanium implant alloys. Acta Mater. 1999, 47, 4221–4224. [Google Scholar] [CrossRef] [Green Version]
- Pazo, A.; Saiz, E.; Tomsia, A. Silicate glass coatings on Ti-based implants. Acta Mater. 1998, 46, 2551–2558. [Google Scholar] [CrossRef]
- Rone, A.P.; Valbusa, G.; Biagini, E. The titaniummolten glass system: Interactions and wetting. J. Mater. Sci. 1977, 12, 2465–2474. [Google Scholar] [CrossRef]
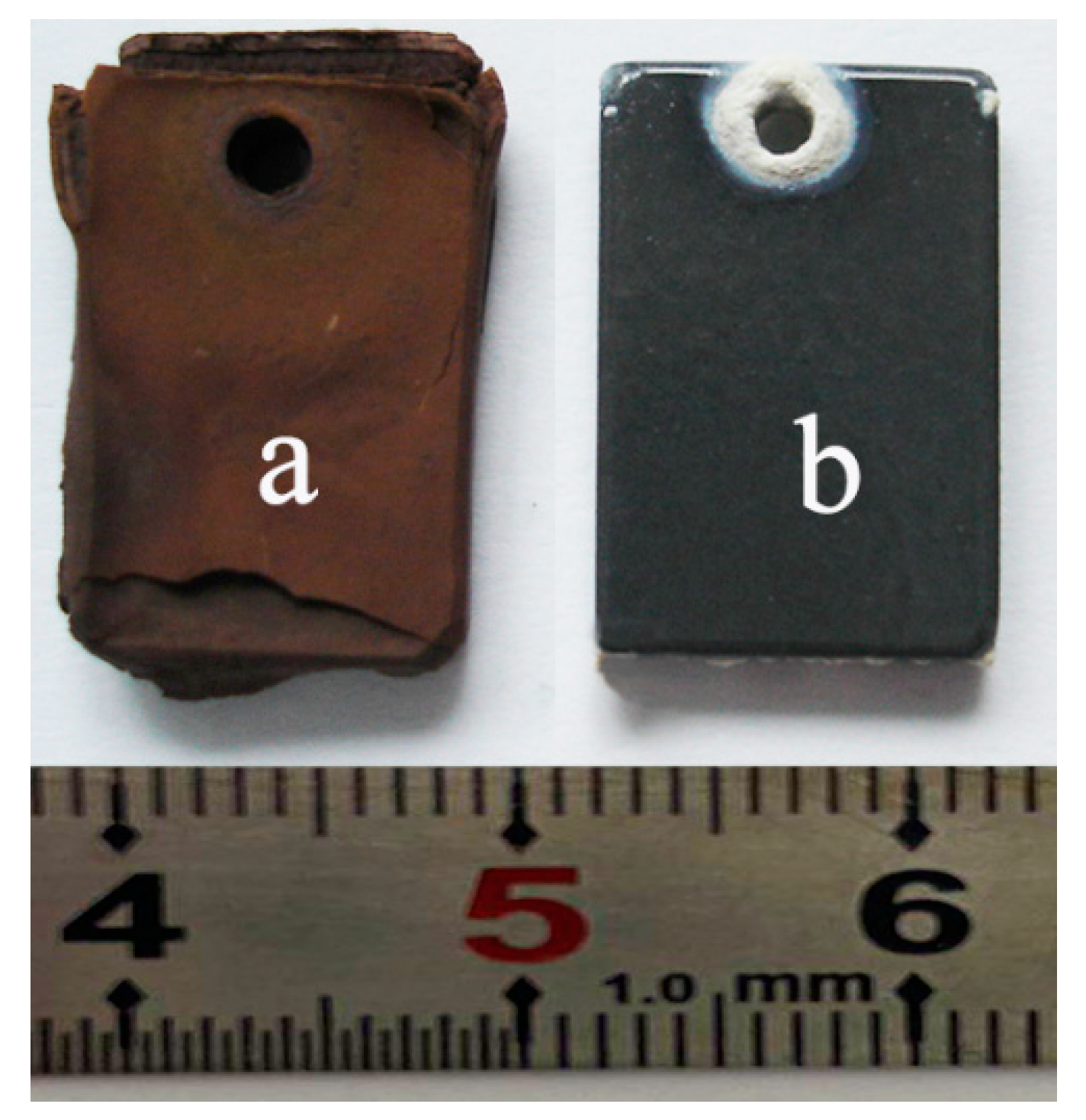

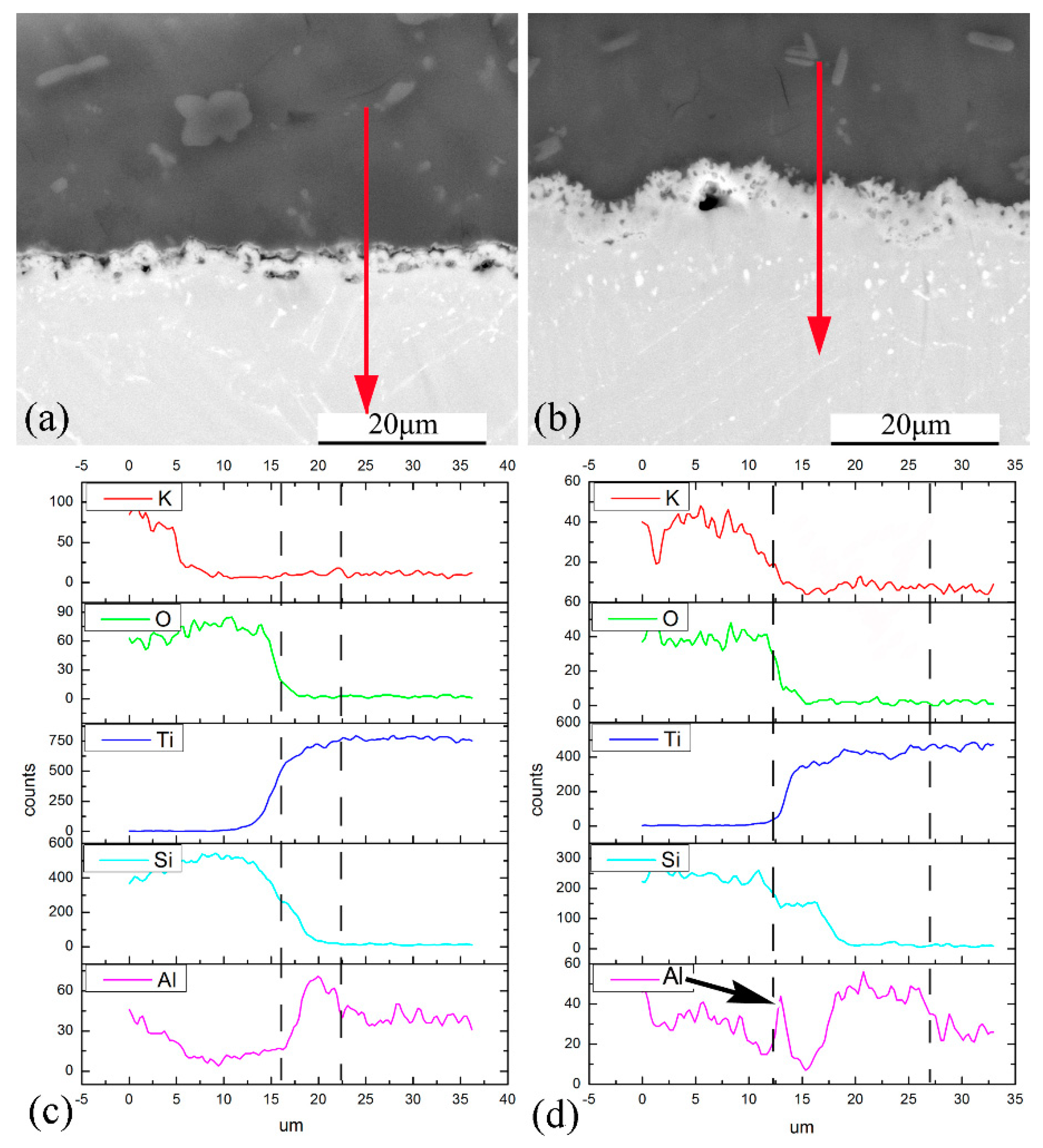
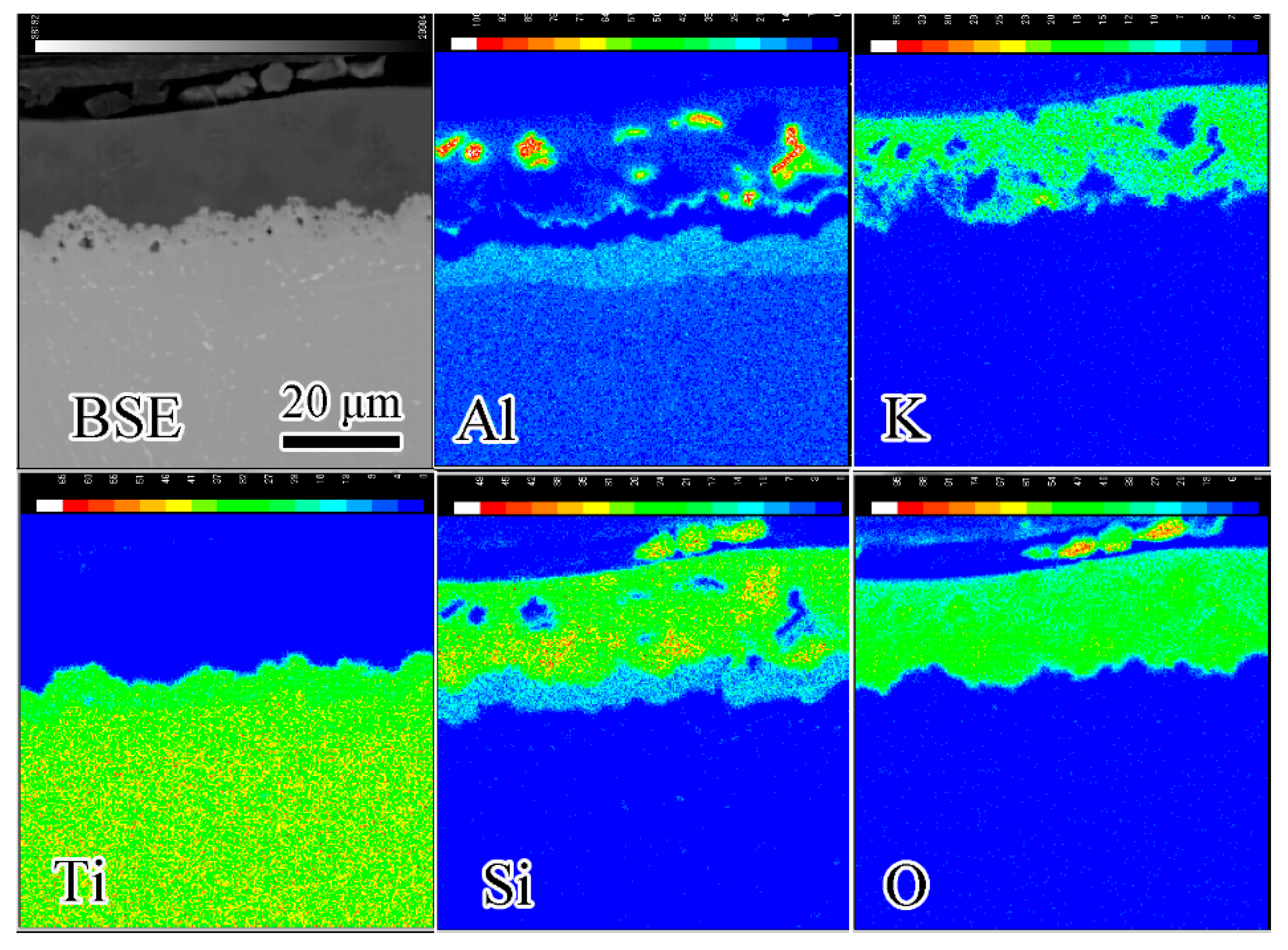
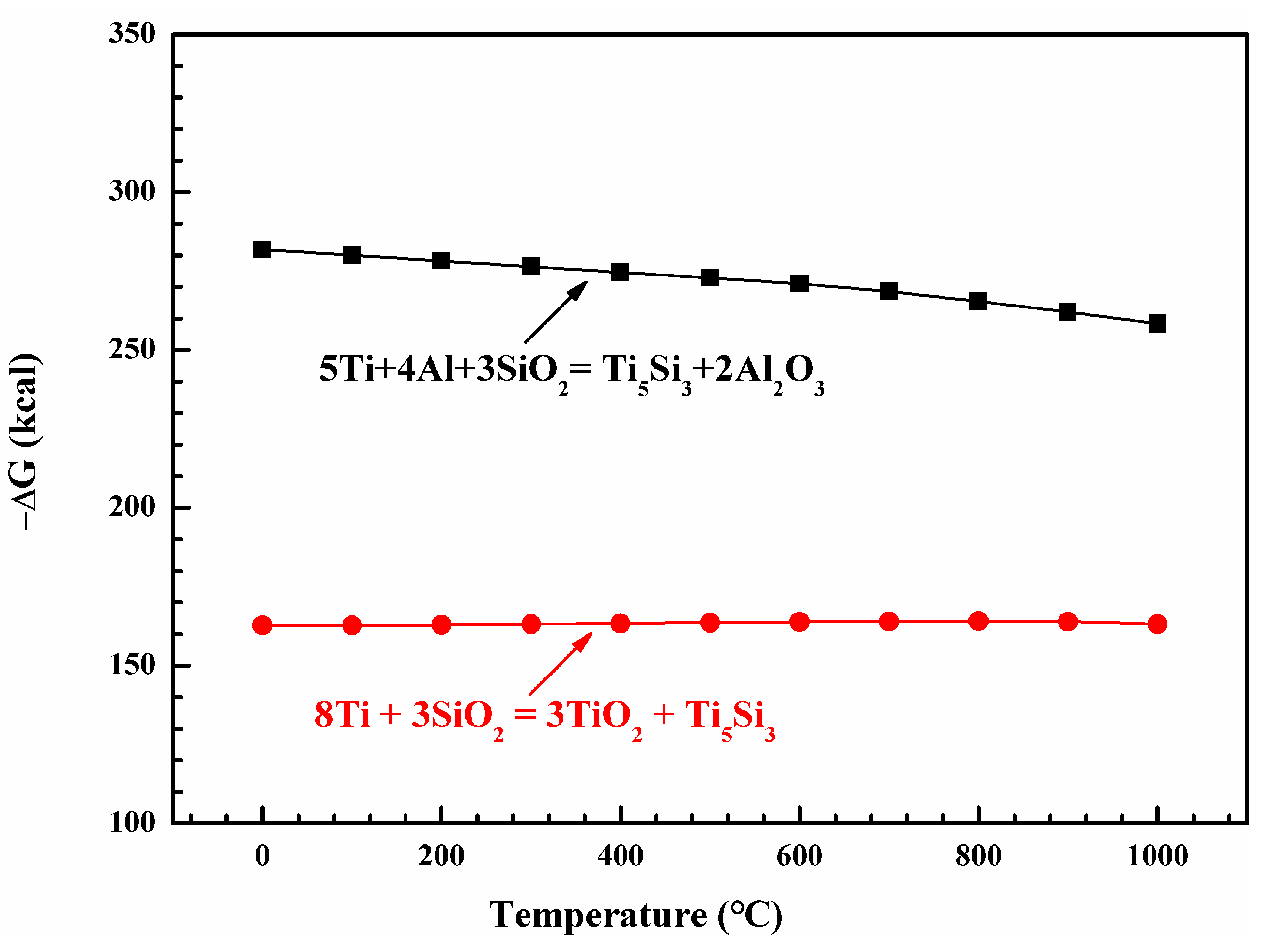

| Elements | Al | Sn | Zr | Mo | Nd | Si | Ti |
|---|---|---|---|---|---|---|---|
| Nominal Composition | 5.6 | 4.8 | 2.0 | 1.0 | 1.0 | 0.3 | Balance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Chen, K.; Liu, L.; Yang, Y.; Zhu, S. Effect of SiO2–Al2O3 Glass Composite Coating on the Oxidation Behavior of Ti60 Alloy. Materials 2020, 13, 5085. https://doi.org/10.3390/ma13225085
Li W, Chen K, Liu L, Yang Y, Zhu S. Effect of SiO2–Al2O3 Glass Composite Coating on the Oxidation Behavior of Ti60 Alloy. Materials. 2020; 13(22):5085. https://doi.org/10.3390/ma13225085
Chicago/Turabian StyleLi, Wenbo, Ken Chen, Lanlan Liu, Yingfei Yang, and Shenglong Zhu. 2020. "Effect of SiO2–Al2O3 Glass Composite Coating on the Oxidation Behavior of Ti60 Alloy" Materials 13, no. 22: 5085. https://doi.org/10.3390/ma13225085
APA StyleLi, W., Chen, K., Liu, L., Yang, Y., & Zhu, S. (2020). Effect of SiO2–Al2O3 Glass Composite Coating on the Oxidation Behavior of Ti60 Alloy. Materials, 13(22), 5085. https://doi.org/10.3390/ma13225085






