Bismuth/Porous Graphene Heterostructures for Ultrasensitive Detection of Cd (II)
Abstract
:1. Introduction
2. Result and Discussion
2.1. Synthesis and Characterization of Bi@PG
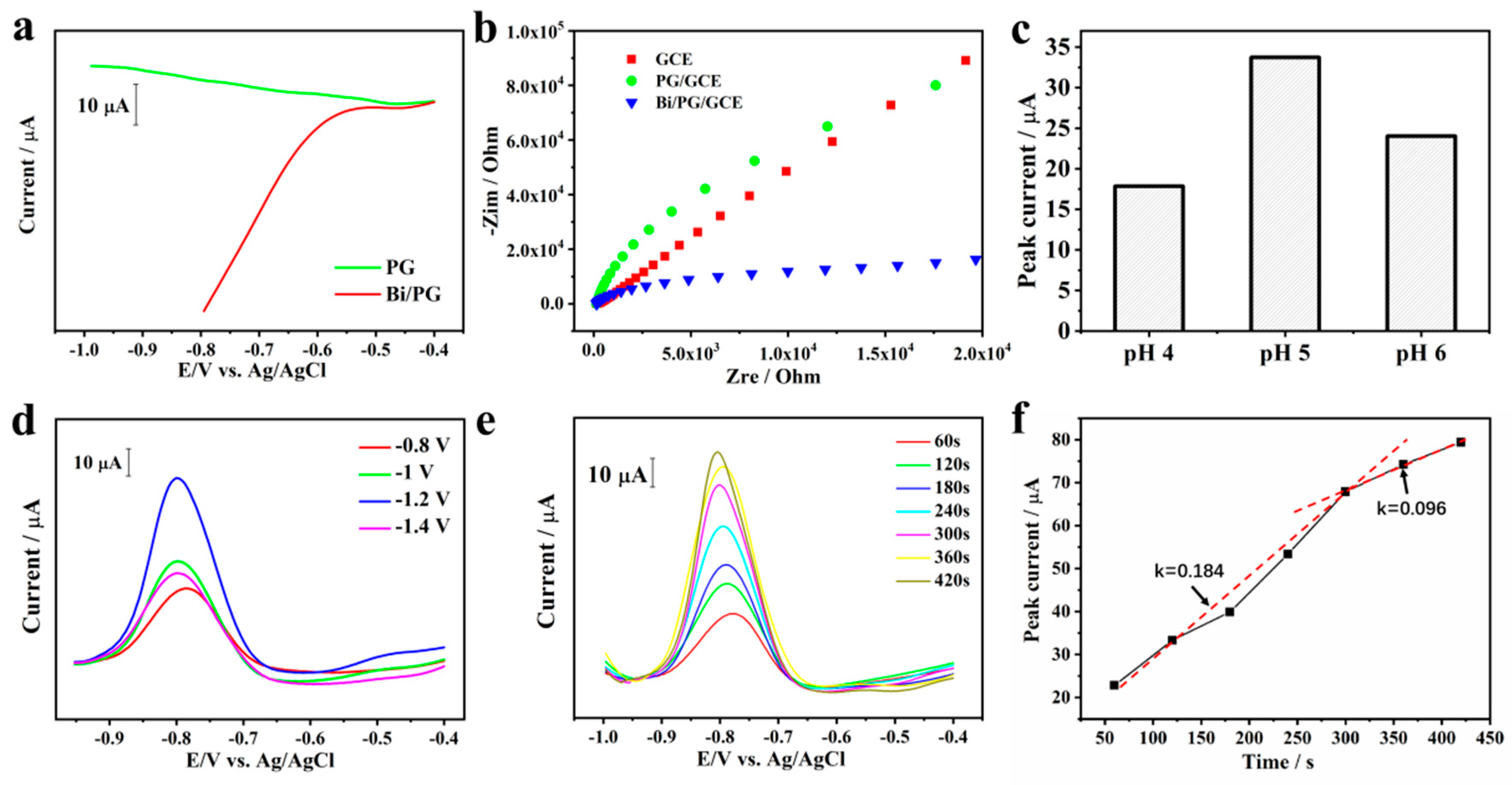
2.2. Optimization of Sensing Conditions
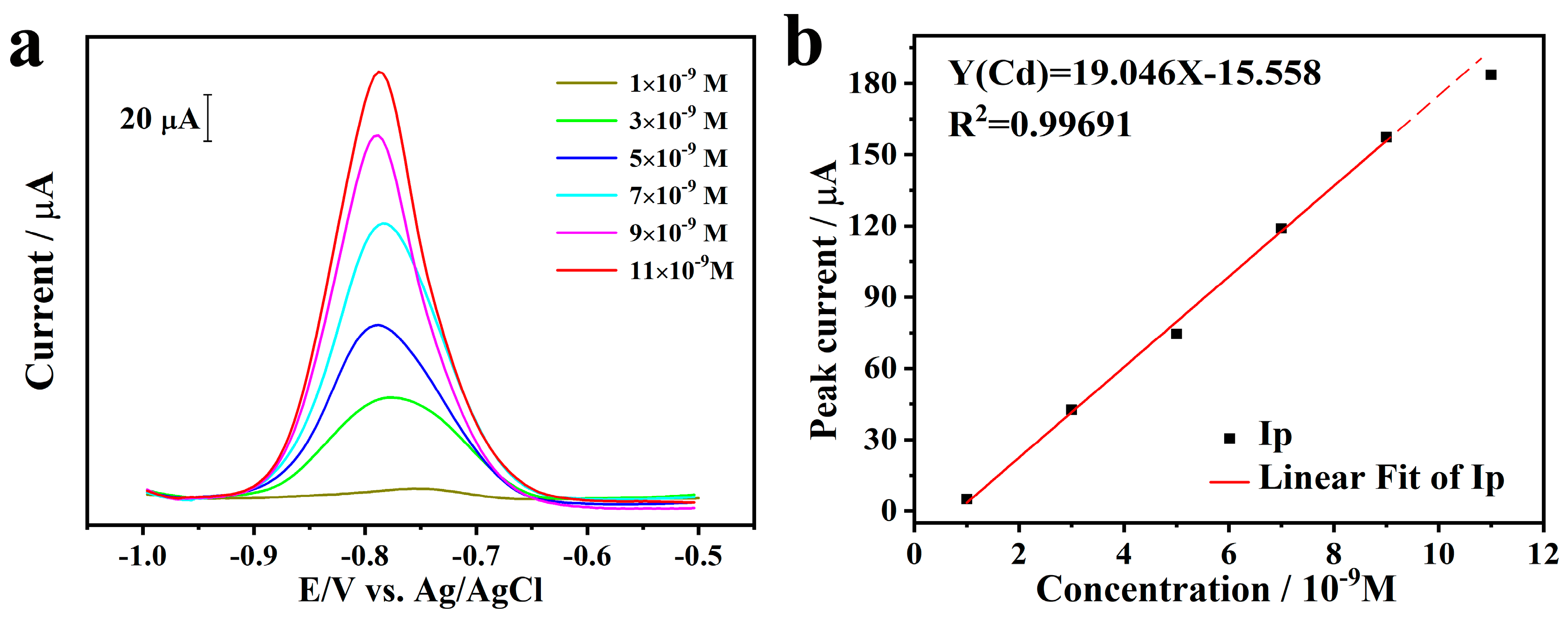
2.3. Stripping Behavior toward Cd2+
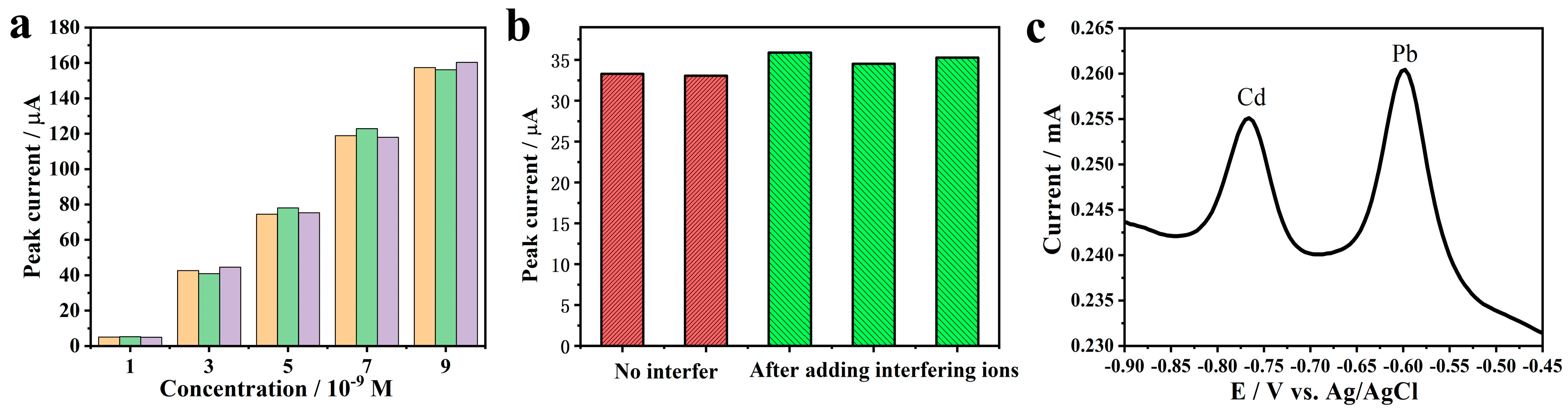
2.4. Test of Repeatability and Anti-Interference Ability
3. Conclusions
4. Experimental Sections
4.1. Material
4.2. Instruments
4.3. Electrodes Preparation and Modification
4.4. Electrochemical Detection of Heavy Ions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olafisoye, O.B.; Adefioye, T.; Osibote, O.A. Heavy metals contamination of water, soil, and plants around an electronic waste dumpsite. Pol. J. Environ. Stud. 2013, 22, 1431–1439. [Google Scholar]
- Malassa, H.; Al-Qutob, M.; Al-Khatib, M.; Al-Rimawi, F. Determination of different trace heavy metals in ground water of South West Bank/Palestine by ICP/MS. J. Environ. Prot. 2013, 4, 818. [Google Scholar] [CrossRef] [Green Version]
- Morton, J.; Havens, N.; Mugweru, A.; Wanekaya, A.K. Detection of trace heavy metal ions using carbon nanotube-modified electrodes. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2009, 21, 1597–1603. [Google Scholar] [CrossRef]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Ganjali, M.R.; Norouzi, P.; Khani, H.; Nayak, A.; Agarwal, S. Electrochemical Analysis of Some Toxic Metals by Ion–Selective Electrodes. Crit. Rev. Anal. Chem. 2011, 41, 282–313. [Google Scholar] [CrossRef] [PubMed]
- Economou, A. Bismuth-film electrodes: Recent developments and potentialities for electroanalysis. Trends Anal. Chem. 2005, 24, 334–340. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J.; Tian, G. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2017, 178, 324–338. [Google Scholar] [CrossRef]
- Cadevall, M.; Ros, J.; Merkoçi, A. Bismuth nanoparticles integration into heavy metal electrochemical stripping sensor. Electrophoresis 2015, 36, 1872–1879. [Google Scholar] [CrossRef]
- Mafa, P.J.; Idris, A.O.; Mabuba, N.; Arotiba, O.A. Electrochemical co-detection of As (III), Hg (II) and Pb (II) on a bismuth modified exfoliated graphite electrode. Talanta 2016, 153, 99–106. [Google Scholar] [CrossRef]
- Pandey, S.K.; Singh, P.; Singh, J.; Sachan, S.; Srivastava, S.; Singh, S.K. Nanocarbon-based Electrochemical Detection of Heavy Metals. Electroanalysis 2016, 28, 2472–2488. [Google Scholar] [CrossRef]
- Aragay, G.; Pons, J.; Merkoçi, A. Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, S.; Luque, R.; Han, S.; Hu, L.; Xu, G. Recent development of carbon electrode materials and their bioanalytical and environmental applications. Chem. Soc. Rev. 2016, 45, 715–752. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, X.; Chen, P. Biological and chemical sensors based on graphene materials. Chem. Soc. Rev. 2012, 41, 2283–2307. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Tanabe, Y.; Sugawara, K.; Koshino, M.; Takahashi, T.; Tanigaki, K.; Aoki, H.; Chen, M. Three-dimensional porous graphene networks expand graphene-based electronic device applications. Phys. Chem. Chem. Phys. 2018, 20, 6024–6033. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, Y.; Ito, Y.; Sugawara, K.; Hojo, D.; Koshino, M.; Fujita, T.; Aida, T.; Xu, X.; Huynh, K.K.; Shimotani, H.; et al. Electric Properties of Dirac Fermions Captured into 3D Nanoporous Graphene Networks. Adv. Mater. 2016, 28, 10304–10310. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Tanabe, Y.; Qiu, H.J.; Sugawara, K.; Heguri, S.; Tu, N.H.; Huynh, K.K.; Fujita, T.; Takahashi, T.; Tanigaki, K.; et al. High-Quality Three-Dimensional Nanoporous Graphene. Angew. Chem. Int. Ed. 2014, 53, 4822–4826. [Google Scholar] [CrossRef]
- Ito, Y.; Cong, W.; Fujita, T.; Tang, Z.; Chen, M. High catalytic activity of nitrogen and sulfur co-doped nanoporous graphene in the hydrogen evolution reaction. Angew. Chem. 2015, 127, 2159–2164. [Google Scholar] [CrossRef]
- Trentelman, K. A note on the characterization of bismuth black by Raman microspectroscopy. J. Raman Spectrosc. 2009, 40, 585–589. [Google Scholar] [CrossRef]
- Królicka, A.; Bobrowski, A.; Kowal, A. Effects of electroplating variables on the voltammetric properties of bismuth deposits plated potentiostatically. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2006, 18, 1649–1657. [Google Scholar] [CrossRef]
- Afkhami, A.; Ghaedi, H.; Madrakian, T.; Rezaeivala, M. Highly sensitive simultaneous electrochemical determination of trace amounts of Pb (II) and Cd (II) using a carbon paste electrode modified with multi-walled carbon nanotubes and a newly synthesized Schiff base. Electrochim. Acta 2013, 89, 377–386. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, T.; Chou, K.C.; Chen, J.; Su, L.; Hou, X. The effective determination of Cd (ii) and Pb (ii) simultaneously based on an aluminum silicon carbide-reduced graphene oxide nanocomposite electrode. Analyst 2017, 142, 2741–2747. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.K.; Panigrahy, B.; Sahoo, S.; Satpati, A.K.; Li, D.; Bahadur, D. In situ synthesis and properties of reduced graphene oxide/Bi nanocomposites: As an electroactive material for analysis of heavy metals. Biosens. Bioelectron. 2013, 43, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.H.; Han, W.K.; Park, J.S.; Kang, S.G. Determination of trace metals by anodic stripping voltammetry using a bismuth-modified carbon nanotube electrode. Talanta 2008, 76, 301–308. [Google Scholar] [CrossRef]
- Hočevar, S.B.; Švancara, I.; Vytřas, K.; Ogorevc, B. Novel electrode for electrochemical stripping analysis based on carbon paste modified with bismuth powder. Electrochim. Acta 2005, 51, 706–710. [Google Scholar] [CrossRef]
- Chaiyo, S.; Mehmeti, E.; Žagar, K.; Siangproh, W.; Chailapakul, O.; Kalcher, K. Electrochemical sensors for the simultaneous determination of zinc, cadmium and lead using a Nafion/ionic liquid/graphene composite modified screen-printed carbon electrode. Anal. Chim. Acta 2016, 918, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.P.; Trinh, X.G.; Uyen, D.T.T. Using Electrode Made of Carbon Nanotubes and Bismuth Oxide for the Determination of Metal Concentration by Anodic Stripping Voltammetry. J. Chem. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Hassan, K.M.; Gaber, S.E.; Altahan, M.F.; Azzem, M.A. Single and simultaneous voltammetric sensing of lead(II), cadmium(II) and zinc(II) using a bimetallic Hg-Bi supported on poly(1,2-diaminoanthraquinone)/glassy carbon modified electrode. Sens. Bio-Sens. Res. 2020, 29, 100369. [Google Scholar] [CrossRef]
- Muralikrishna, S.; Sureshkumar, K.; Varley, T.S.; Nagaraju, D.H.; Ramakrishnappa, T. In situ reduction and functionalization of graphene oxide with L-cysteine for simultaneous electrochemical determination of cadmium (II), lead (II), copper (II), and mercury (II) ions. Anal. Methods 2014, 6, 8698–8705. [Google Scholar] [CrossRef]
- Guo, X.; Yun, Y.; Shanov, V.N.; Halsall, H.B.; Heineman, W.R. Determination of trace metals by anodic stripping voltammetry using a carbon nanotube tower electrode. Electroanalysis 2011, 23, 1252–1259. [Google Scholar] [CrossRef]
- Fu, L.; Li, X.; Yu, J.; Ye, J. Facile and Simultaneous Stripping Determination of Zinc, Cadmium and Lead on Disposable Multiwalled Carbon Nanotubes Modified Screen-Printed Electrode. Electroanalysis 2013, 25, 567–572. [Google Scholar] [CrossRef]
- Zhang, H.; Shuang, S.; Wang, G.; Guo, Y.; Tong, X.; Yang, P.; Chen, A.; Dong, C.; Qin, Y. TiO2–graphene hybrid nanostructures by atomic layer deposition with enhanced electrochemical performance for Pb(II) and Cd(II) detection. RSC Adv. 2014, 5, 4343–4349. [Google Scholar] [CrossRef]

| Electrode | Linear Range | Detection Limit | Sensitivity | Method | Ref. |
|---|---|---|---|---|---|
| RGO/Bi/GCE | 20–120 μg/L | 2.8 μg/L | 1.25 μA·μg−1·L | SWASV | [22] |
| CNTs/Bi | 20–100 μg/L | 0.7 μg/L | 1.45 μA·μg−1·L | SWASV | [23] |
| Bi/CPE | 10–100 μg/L | 1.2 μg/L | N.M. | ASV | [24] |
| GR/BiF/Nafion/IL/SPCE | 0.1–100 μg/L | 0.06 ng/L | 0.52 μA·μg−1·L | SWASV | [25] |
| Bi2O3/CNTs | 1.5–20 μg/L | 0.22 μg/L | 1.55 μA·μg−1·L | SWASV | [26] |
| Hg-Bi/PDAAQ/GC | 0–50 μg/L | 0.107 μg/L | 3.763 μA·μg−1·L | SWV | [27] |
| L-cystine-rGO/GCE | 44.9–225 μg/L | 0.366 μg/L | 24.5 nA·μg−1·L | DPASV | [28] |
| CNTs tower | 112–449 μg/L | 2.8 μg/L | 7.5 nA·μg−1·L | ASV | [29] |
| MWCNTs/NA/Bi/SPE | 0.5–80 μg/L | 0.1 μg/L | 13.42 mA·μg−1·L | DPASV | [30] |
| TiO2/GR | 67.2–3584 μg/L | 0.22 μg/L | 17.3 μA·μg−1·L | DPASV | [31] |
| Bi@PG | 0.11–1.12 μg/L | 0.011 μg/L | 0.17 mA·μg−1·L | SWASV | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Ito, Y.; Fujita, T.; Ge, X.; Zhang, L.; Zeng, H. Bismuth/Porous Graphene Heterostructures for Ultrasensitive Detection of Cd (II). Materials 2020, 13, 5102. https://doi.org/10.3390/ma13225102
Huang L, Ito Y, Fujita T, Ge X, Zhang L, Zeng H. Bismuth/Porous Graphene Heterostructures for Ultrasensitive Detection of Cd (II). Materials. 2020; 13(22):5102. https://doi.org/10.3390/ma13225102
Chicago/Turabian StyleHuang, Luyi, Yoshikazu Ito, Takeshi Fujita, Xingbo Ge, Ling Zhang, and Heping Zeng. 2020. "Bismuth/Porous Graphene Heterostructures for Ultrasensitive Detection of Cd (II)" Materials 13, no. 22: 5102. https://doi.org/10.3390/ma13225102





