An A- and B-Site Substitutional Study of SrFeO3−δ Perovskites for Solar Thermochemical Air Separation
Abstract
:1. Introduction
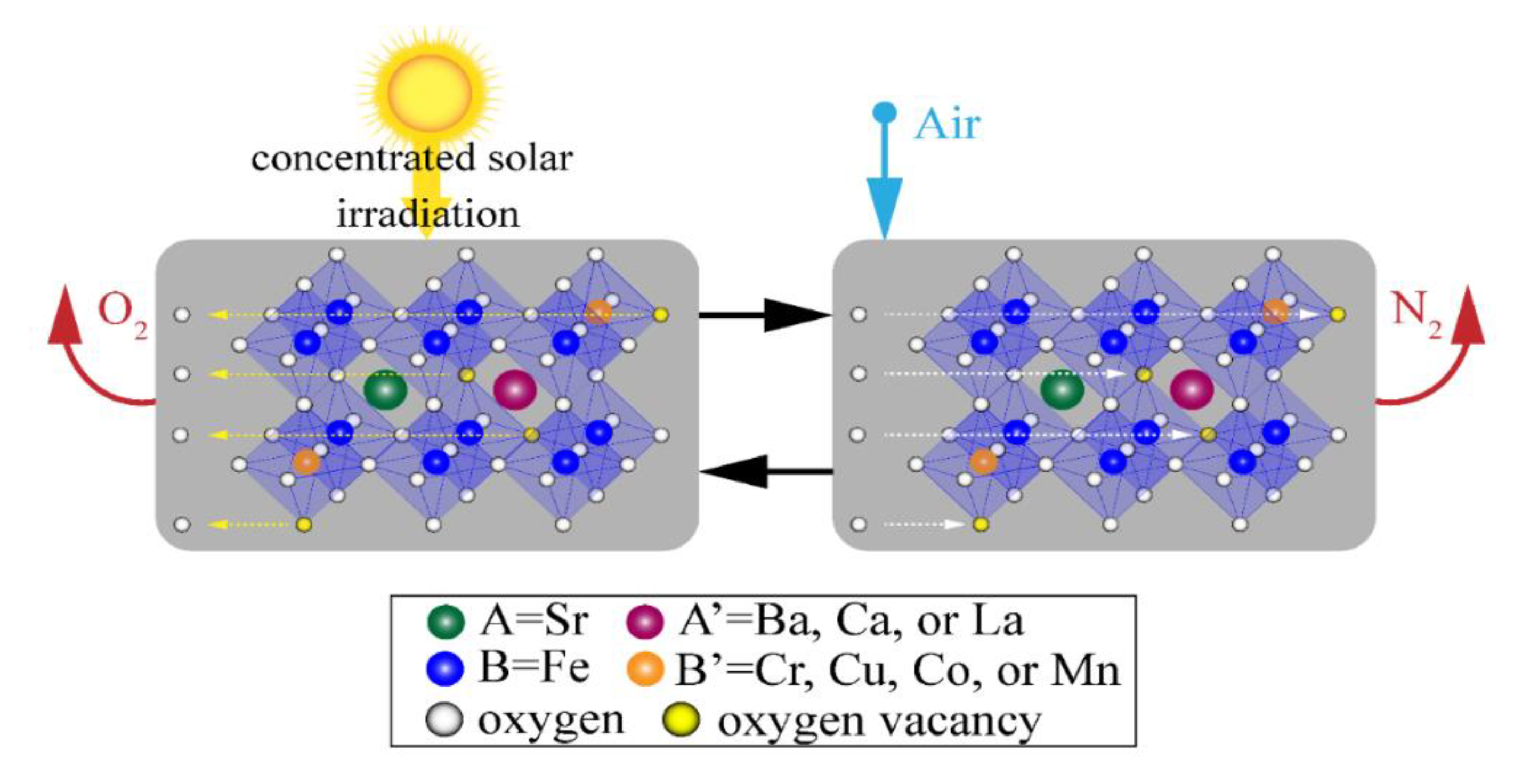
- The thermal reduction of Fe4+ → Fe3+ on the B-site using concentrated solar irradiation as process heat at elevated temperatures and low O2 partial pressures, where VÖ are formed as 2O2− → O2(g) to maintain electroneutrality.
- The oxidation in air at lower temperatures and elevated O2 partial pressure to produce N2 as Fe3+ → Fe4+, VÖ sites are filled, and the SrFeO3−δ is recycled back to the first step to complete the cycle.
2. Materials and Methods
3. Results
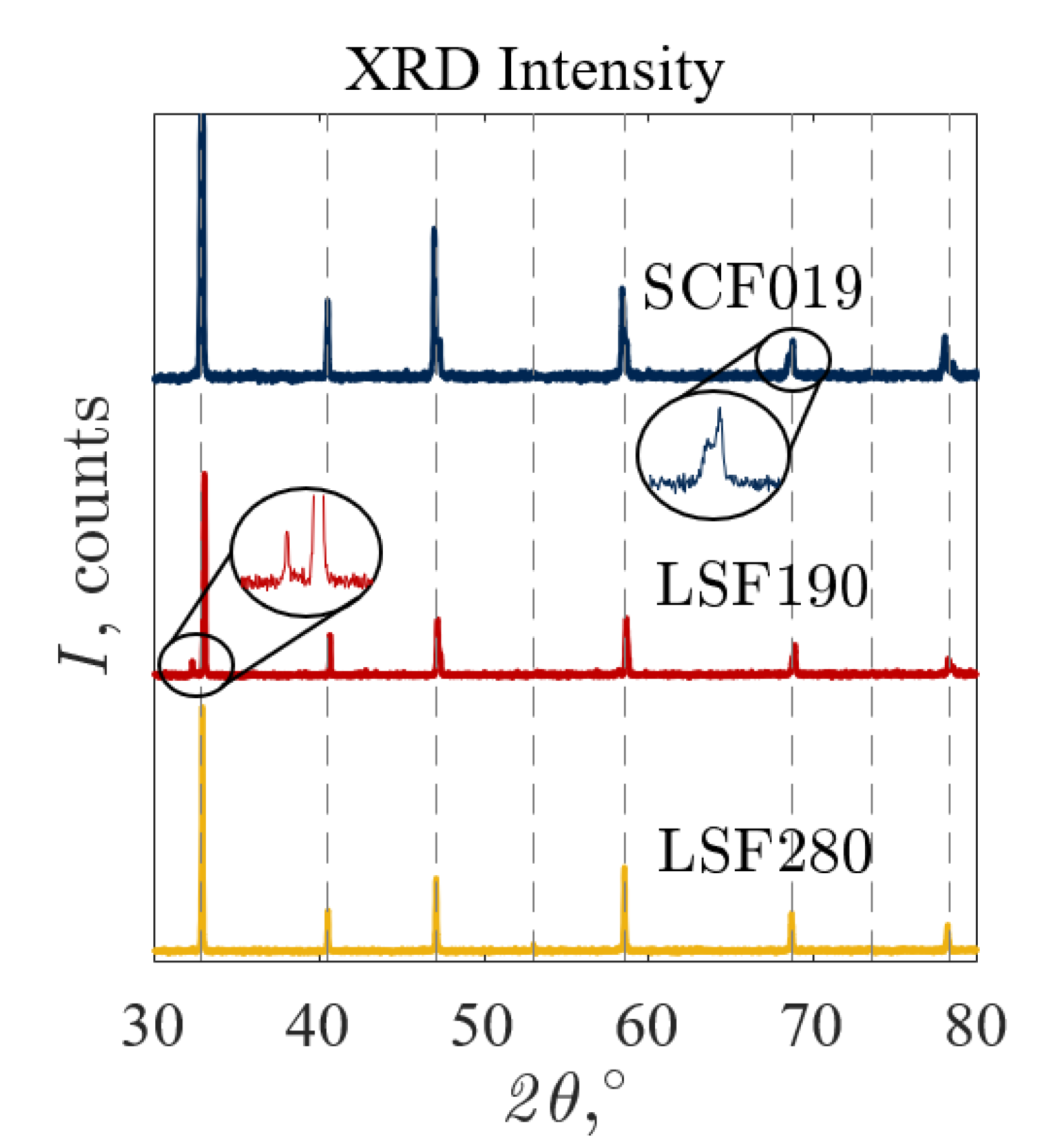
3.1. Characterization
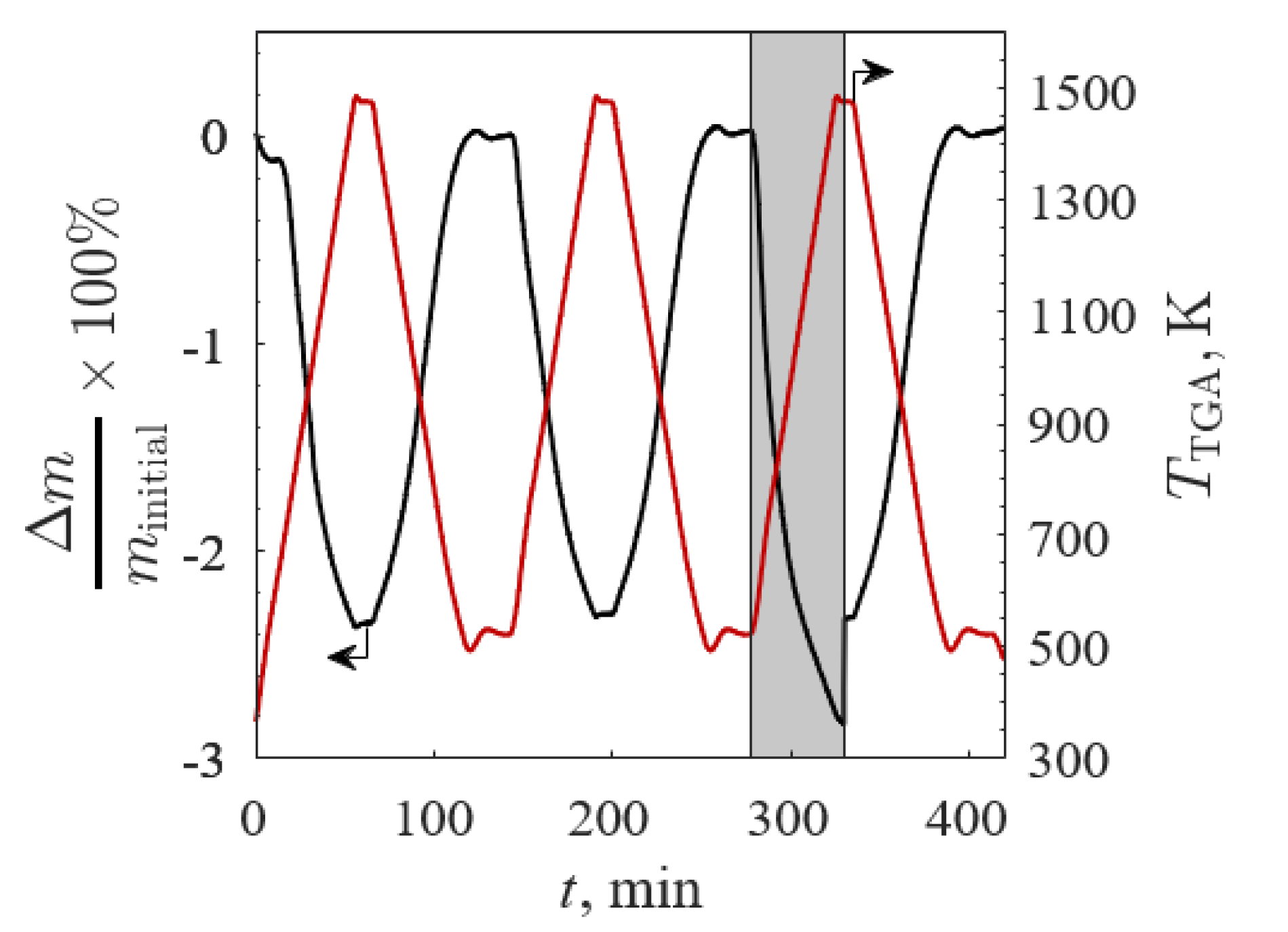
3.2. Thermogravimetry
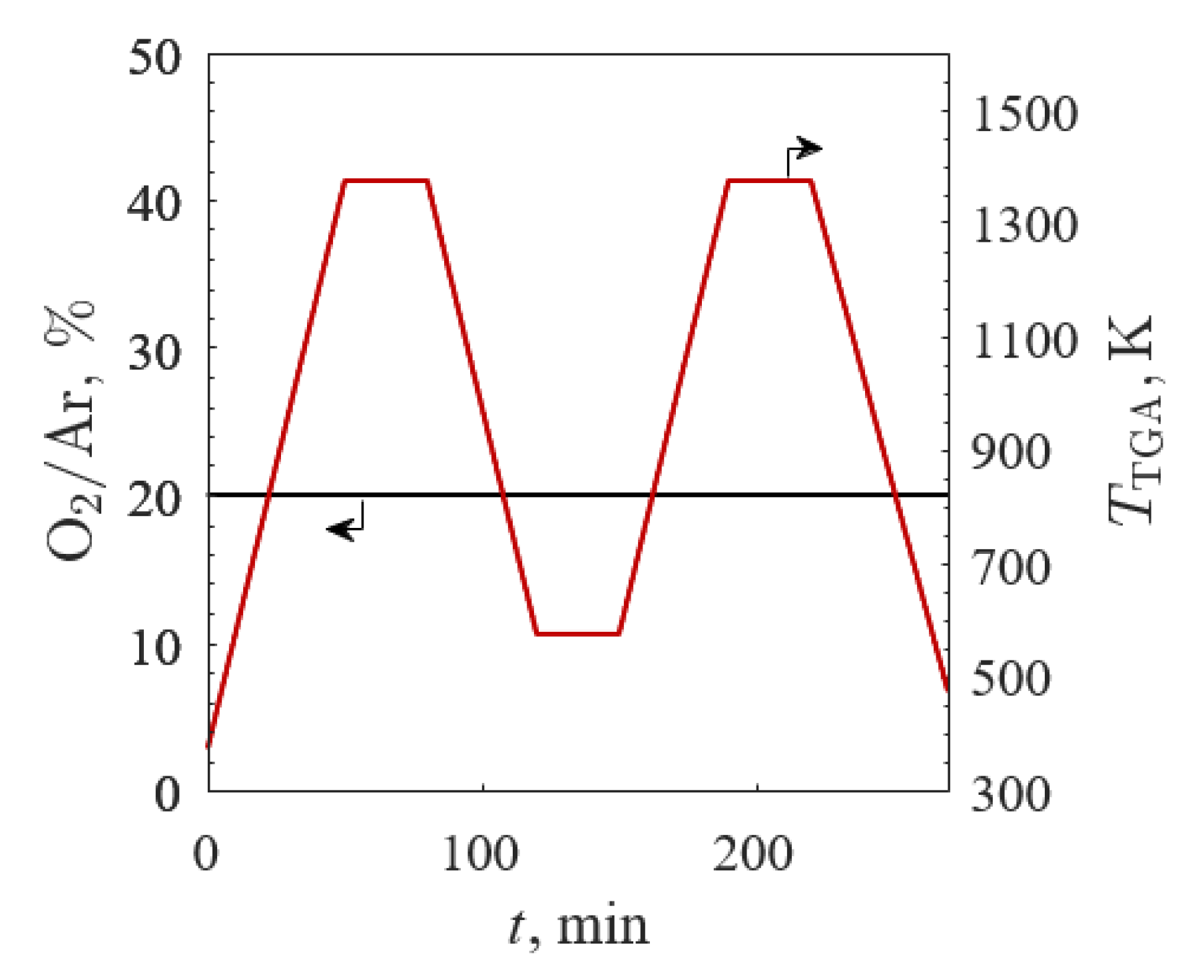
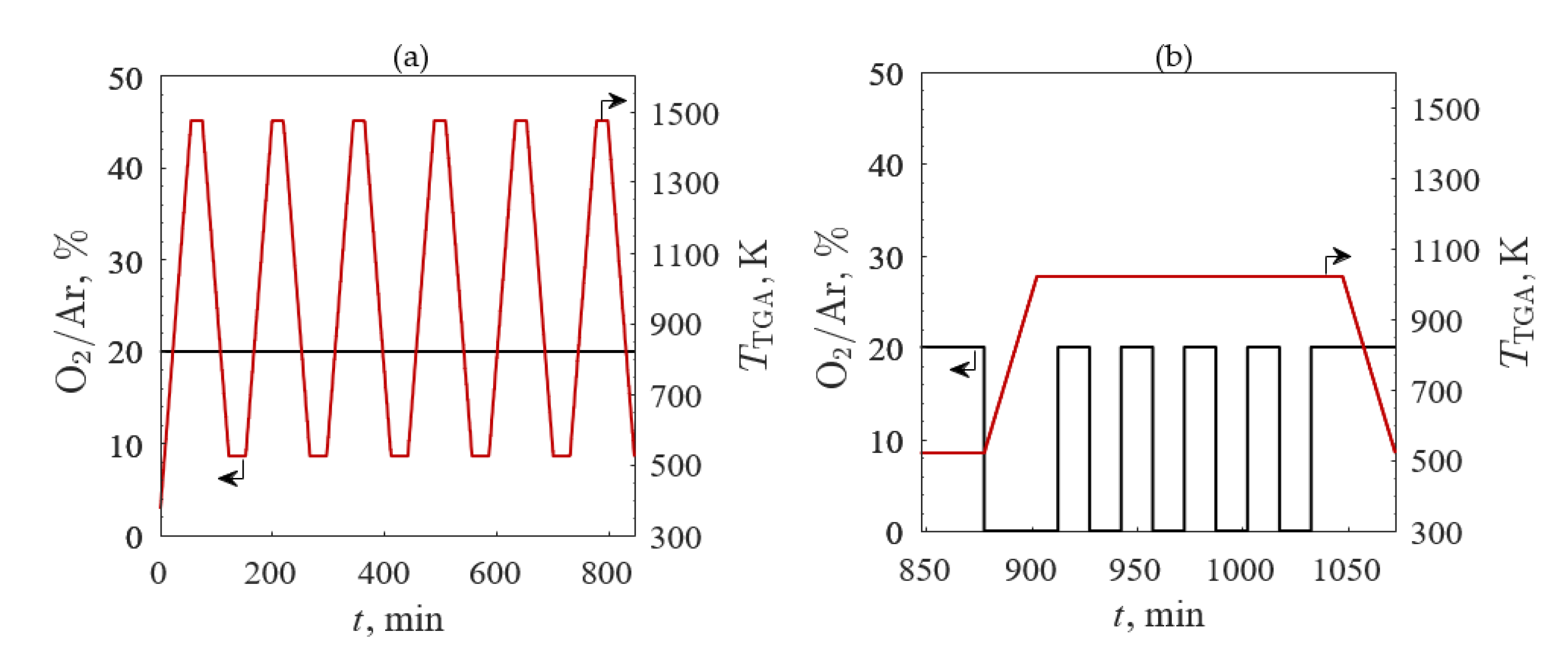
3.2.1. TGA Screening 1
3.2.2. TGA Screening 2
3.2.3. TGA Screening 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| δ | deviation from stoichiometry |
| σΔδ | standard deviation of the change in deviation from stoichiometry |
| 2θ | X-ray diffraction angle |
| A | A-site cation |
| A’ | A-site cation substitution |
| B | B-site cation |
| B’ | B-site cation substitution |
| C | cubic lattice structure |
| neutral charged Fe ion | |
| negatively charged Fe ion | |
| I | X-ray diffraction peak intensity |
| M | monoclinic lattice structure |
| Minitial | stochiometric molar mass of sample |
| minitial | initial mass |
| Δm | change in mass |
| molar mass of O2 | |
| O | orthorhombic lattice structure |
| neutral charged O ion | |
| T | tetragonal lattice structure |
| t | time |
| TTGA | measured temperature of the TGA |
| VÖ | O2− vacancy |
| x | A-site substitution fraction |
| y | B-site substitution fraction |
References
- Modak, J.M. Haber process for ammonia synthesis. Resonance 2002, 7, 69–77. [Google Scholar] [CrossRef]
- Banaszkiewicz, T.; Chorowski, M.; Gizicki, W. Comparative analysis of cryogenic and PTSA technologies for systems of oxygen production. In Proceedings of the AIP Conference Proceedings, Surakarta, Indonesia, 12 May 2018; pp. 1373–1378. [Google Scholar]
- Hassan, M.; Ruthven, D.; Raghavan, N. Air separation by pressure swing adsorption on a carbon molecular sieve. Chem. Eng. Sci. 1986, 41, 1333–1343. [Google Scholar] [CrossRef]
- Grande, C.A. Advances in pressure swing adsorption for gas separation. ISRN Chem. Eng. 2012, 2012, 982934. [Google Scholar] [CrossRef] [Green Version]
- Kosinov, N.; Gascon, J.; Kapteijn, F.; Hensen, E.J.M. Recent developments in zeolite membranes for gas separation. J. Membr. Sci. 2016, 499, 65–79. [Google Scholar] [CrossRef]
- Bulfin, B.; Lapp, J.; Richter, S.; Gubàn, D.; Vieten, J.; Brendelberger, S.; Roeb, M.; Sattler, C. Air separation and selective oxygen pumping via temperature and pressure swing oxygen adsorption using a redox cycle of SrFeO3 perovskite. Chem. Eng. Sci. 2019, 203, 68–75. [Google Scholar] [CrossRef]
- Marek, E.; Hu, W.; Gaultois, M.; Grey, C.P.; Scott, S.A. The use of strontium ferrite in chemical looping systems. Appl. Energy 2018, 223, 369–382. [Google Scholar] [CrossRef]
- Vieten, J.; Bulfin, B.; Call, F.; Lange, M.; Schmücker, M.; Francke, A.; Roeb, M.; Sattler, C. Perovskite oxides for application in thermochemical air separation and oxygen storage. J. Mater. Chem. A 2016, 4, 13652–13659. [Google Scholar] [CrossRef]
- Bush, H.E.; Datta, R.; Loutzenhiser, P.G. Aluminum-doped strontium ferrites for a two-step solar thermochemical air separation cycle: Thermodynamic characterization and cycle analysis. Sol. Energy 2019, 188, 775–786. [Google Scholar] [CrossRef]
- Babiniec, S.M.; Coker, E.N.; Miller, J.E.; Ambrosini, A. Doped calcium manganites for advanced high-temperature thermochemical energy storage. Int. J. Energy Res. 2016, 40, 280–284. [Google Scholar] [CrossRef]
- Schrader, A.J.; Bush, H.E.; Ranjan, D.; Loutzenhiser, P.G. Aluminum-doped calcium manganite particles for solar thermochemical energy storage: Reactor design, particle characterization, and heat and mass transfer modeling. Int. J. Heat Mass Transf. 2020, 152, 119461. [Google Scholar] [CrossRef]
- Schrader, A.J.; Schieber, G.L.; Ambrosini, A.; Loutzenhiser, P.G. Experimental demonstration of a 5 kWth granular-flow reactor for solar thermochemical energy storage with aluminum-doped calcium manganite particles. Appl. Therm. Eng. 2020, 173, 115257. [Google Scholar] [CrossRef]
- Curnan, M.T.; Kitchin, J.R. Effects of concentration, crystal structure, magnetism, and electronic structure method on first-principles oxygen vacancy formation energy trends in perovskites. J. Phys. Chem. C 2014, 118, 28776–28790. [Google Scholar] [CrossRef]
- Pena, M.A.; Fierro, J.L. Chemical structures and performance of perovskite oxides. Chem. Rev. 2001, 101, 1981–2017. [Google Scholar] [CrossRef] [PubMed]
- Varadaraju, V.B.U.V. Effect of La3+ substitution on the structure and superconductivity in TlBa2−xLaxCaCu2O7 (x = 0.0 − 1.0). Solid State Commun. 1995, 93, 1003–1007. [Google Scholar]
- Babiniec, S.M.; Coker, E.N.; Miller, J.E.; Ambrosini, A. Investigation of LaxSr1−xCoyM1−yO3−δ (M = Mn, Fe) perovskite materials as thermochemical energy storage media. Sol. Energy 2015, 118, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Gokon, N.; Yawata, T.; Bellan, S.; Kodama, T.; Cho, H.-S. Thermochemical behavior of perovskite oxides based on LaxSr1−x(Mn, Fe, Co)O3−δ and BaySr1−yCoO3−δ. Energy Procedia 2019, 171, 971–980. [Google Scholar] [CrossRef]
- Bulfin, B.; Vieten, J.; Richter, S.; Naik, J.M.; Patzke, G.R.; Roeb, M.; Sattler, C.; Steinfeld, A. Isothermal relaxation kinetics for the reduction and oxidation of SrFeO3 based perovskites. Phys. Chem. Chem. Phys. 2020, 22, 2466–2474. [Google Scholar] [CrossRef] [Green Version]
- Dou, J.; Krzystowczyk, E.; Wang, X.; Robbins, T.; Ma, L.; Liu, X.; Li, F. A- and B-site codoped SrFeO3 oxygen sorbents for enhanced chemical looping air separation. ChemSusChem 2020, 13, 385–393. [Google Scholar] [CrossRef]
- Ezbiri, M.; Allen, K.M.; Galvez, M.E.; Michalsky, R.; Steinfeld, A. Design principles of perovskites for thermochemical oxygen separation. ChemSusChem 2015, 8, 1966–1971. [Google Scholar] [CrossRef] [Green Version]
- Pechini, M.P. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to Form a Capacitor. U.S. Patent 3330697, 11 July 1967. [Google Scholar]
- Gates-Rector, S.; Blanton, T. The powder diffraction file: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Emery, A.A.; Saal, J.E.; Kirklin, S.; Hegde, V.I.; Wolverton, C. High-throughput computational screening of perovskites for thermochemical water splitting applications. Chem. Mater. 2016, 28, 5621–5634. [Google Scholar] [CrossRef]
- Sastre, D.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Chemical insights on the activity of La1−xSrxFeO3 perovskites for chemical looping reforming of methane coupled with CO2-splitting. J. CO2 Util. 2019, 31, 16–26. [Google Scholar] [CrossRef]
- Vieten, J.; Bulfin, B.; Senholdt, M.; Roeb, M.; Sattler, C.; Schmücker, M. Redox thermodynamics and phase composition in the system SrFeO3−δ—SrMnO3−δ. Solid State Ion. 2017, 308, 149–155. [Google Scholar] [CrossRef]
- Vieten, J.; Bulfin, B.; Starr, D.E.; Hariki, A.; de Groot, F.M.F.; Azarpira, A.; Zachäus, C.; Hävecker, M.; Skorupska, K.; Knoblauch, N.; et al. Redox behavior of solid solutions in the SrFe1−xCuxO3−δ system for application in thermochemical oxygen storage and air separation. Energy Technol. 2019, 7, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Muhich, C.L.; Evanko, B.W.; Weston, K.C.; Lichty, P.; Liang, X.; Martinek, J.; Musgrave, C.B.; Weimer, A.W. Efficient generation of H2 by splitting water with an isothermal redox cycle. Science 2013, 341, 540–542. [Google Scholar] [CrossRef]

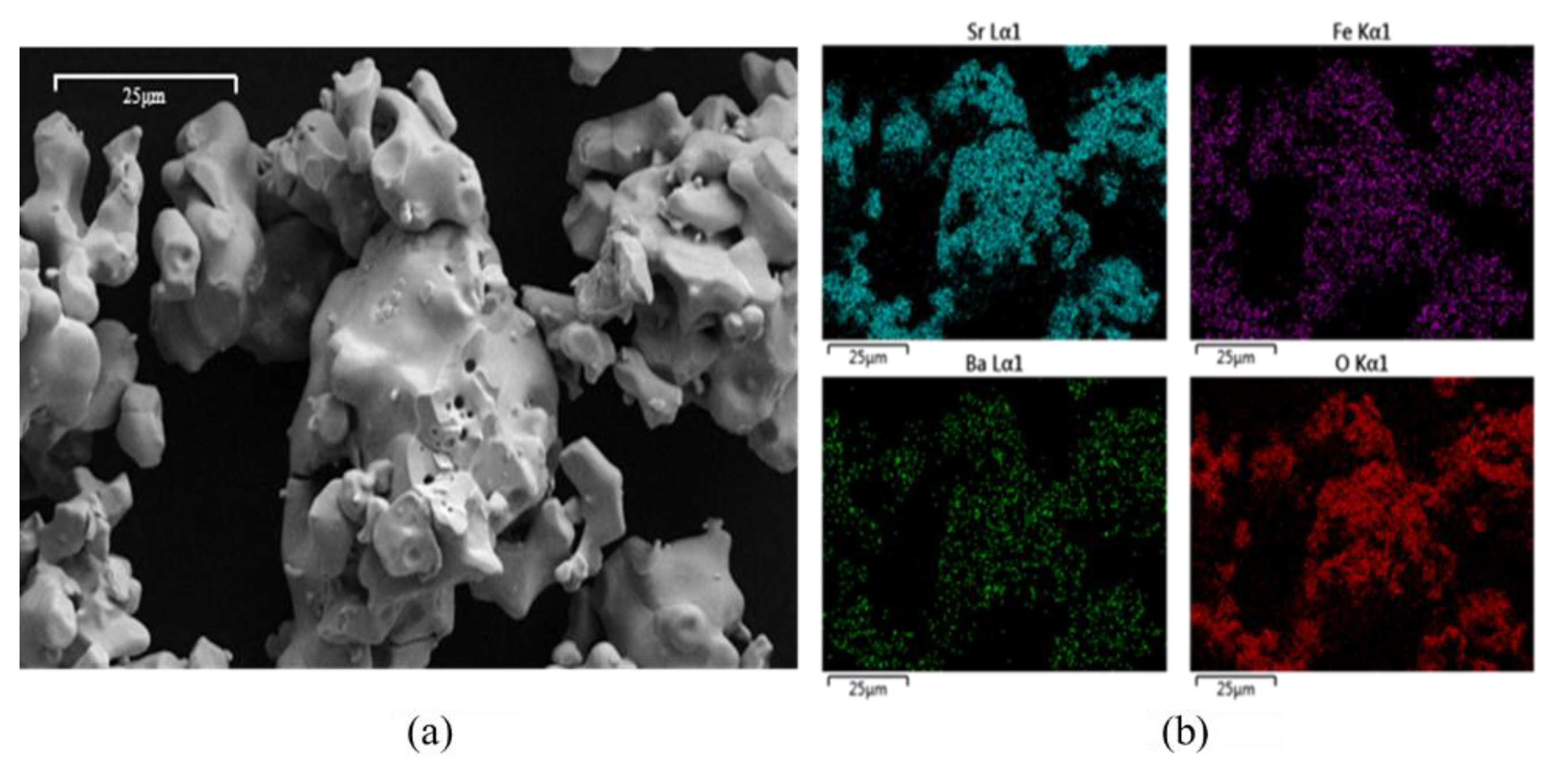


| Sample | Crystal Structure | PDF-4+ Card # | Secondary Phase (s) |
|---|---|---|---|
| SrFeO3−δ | T | 04-023-5157 | N |
| BSF190 | C | 00-059-0658 | N |
| BSF280 | C | 00-059-0658 | N |
| CaSF190 | T | 04-011-5465 | N |
| CaSF280 | O | 01-083-3533 | N |
| LSF190 | C | 04-023-5157 | Y |
| LSF280 | C | 04-023-5157 | Y |
| SCF019 | O | 04-011-5466 | N |
| SCF028 | T | 04-002-0279 | N |
| SCrF019 | O | 04-020-6506 | N |
| SCrF028 | T | 04-006-9226 | N |
| SCuF019 | T | 04-018-8743 | N |
| SCuF028 | T | 04-021-6591 | Y |
| SMF019 | C | 04-007-9930 | N |
| SMF028 | C | 04-006-6180 | Y |
| BSCF1919 | C | 04-013-0029 | Y |
| BSCF2828 | C | 04-013-0029 | N |
| CaSCF1919 | C | 04-008-4374 | Y |
| CaSCF2828 | C | 04-008-3172 | Y |
| LSCF1919 | T | 04-002-3182 | N |
| LSCF2828 | C | 04-016-4840 | Y |
| Sample | Δm/minitial × 100% | Δδ | Sample | Δm/minitial × 100% | Δδ |
|---|---|---|---|---|---|
| SrFeO3-δ | −2.33 | 0.28 | - | - | - |
| BSF190 | −2.36 | 0.29 | BSF280 | −2.23 | 0.28 |
| LSF190 | −2.61 | 0.32 | LSF280 | −2.54 | 0.32 |
| CaSF190 | −2.34 | 0.27 | CaSF280 | −2.37 | 0.27 |
| SCF019 | −2.31 | 0.28 | SCF028 | −2.28 | 0.28 |
| SCrF019 | −1.78 | 0.21 | SCrF028 | −1.37 | 0.16 |
| SCuF019 | −1.79 | 0.22 | SCuF028 | −1.85 | 0.23 |
| SMnF019 | −1.85 | 0.22 | SMnF028 | −1.99 | 0.24 |
| Sample | Δm/minitial × 100% | Δδ | Sample | Δm/minitial × 100% | Δδ | ||||
|---|---|---|---|---|---|---|---|---|---|
| 20% O2/Ar | 100% Ar | 20% O2/Ar | 100% Ar | 20% O2/Ar | 100% Ar | 20% O2/Ar | 100% Ar | ||
| SrFeO3-δ | −2.34 | −2.71 | 0.28 | 0.32 | - | - | - | - | - |
| BSF190 | −2.90 | −3.25 | 0.36 | 0.40 | BSF280 | −2.32 | −2.57 | 0.29 | 0.32 |
| BSCF1919 | −2.59 | −3.00 | 0.32 | 0.37 | BSCF2828 | −2.33 | −2.84 | 0.29 | 0.36 |
| LSF190 | −2.71 | −3.16 | 0.33 | 0.39 | LSF280 | −2.55 | −2.96 | 0.32 | 0.37 |
| LSCF1919 | −2.91 | −3.37 | 0.36 | 0.42 | LSCF2828 | −2.72 | −3.31 | 0.34 | 0.42 |
| CaSF190 | −2.50 | −2.87 | 0.29 | 0.34 | CaSF280 | −2.53 | −2.88 | 0.29 | 0.33 |
| CaSCF1919 | −2.66 | −3.14 | 0.31 | 0.37 | CaSCF2828 | −2.31 | −2.94 | 0.26 | 0.34 |
| SCF019 | −2.21 | −2.58 | 0.27 | 0.31 | SCF028 | −2.13 | −2.64 | 0.26 | 0.32 |
| Sample | Temperature-Swing | O2 Pressure-Swing | ||||
|---|---|---|---|---|---|---|
| Δm/minitial × 100% | σΔδ | Δm/minitial × 100% | σΔδ | |||
| SrFeO3−δ | −2.60 | 0.31 | 0.0015 | −1.16 | 0.14 | 0.0006 |
| BSF190 | −2.75 | 0.34 | 0.0035 | −1.00 | 0.12 | 0.0039 |
| BSCF1919 | −2.62 | 0.33 | 0.0041 | −0.90 | 0.11 | 0.0033 |
| LSF190 | −2.74 | 0.34 | 0.0049 | −1.09 | 0.13 | 0.0042 |
| LSCF1919 | −2.89 | 0.37 | 0.0034 | −1.03 | 0.13 | 0.0045 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farr, T.P.; Nguyen, N.P.; Bush, H.E.; Ambrosini, A.; Loutzenhiser, P.G. An A- and B-Site Substitutional Study of SrFeO3−δ Perovskites for Solar Thermochemical Air Separation. Materials 2020, 13, 5123. https://doi.org/10.3390/ma13225123
Farr TP, Nguyen NP, Bush HE, Ambrosini A, Loutzenhiser PG. An A- and B-Site Substitutional Study of SrFeO3−δ Perovskites for Solar Thermochemical Air Separation. Materials. 2020; 13(22):5123. https://doi.org/10.3390/ma13225123
Chicago/Turabian StyleFarr, Tyler P., Nhu Pailes Nguyen, H. Evan Bush, Andrea Ambrosini, and Peter G. Loutzenhiser. 2020. "An A- and B-Site Substitutional Study of SrFeO3−δ Perovskites for Solar Thermochemical Air Separation" Materials 13, no. 22: 5123. https://doi.org/10.3390/ma13225123
APA StyleFarr, T. P., Nguyen, N. P., Bush, H. E., Ambrosini, A., & Loutzenhiser, P. G. (2020). An A- and B-Site Substitutional Study of SrFeO3−δ Perovskites for Solar Thermochemical Air Separation. Materials, 13(22), 5123. https://doi.org/10.3390/ma13225123







