Magneto-Rheological Fluid Assisted Abrasive Nanofinishing of ?-Phase Ti-Nb-Ta-Zr Alloy: Parametric Appraisal and Corrosion Analysis
Abstract
:1. Introduction
2. Materials and Methods
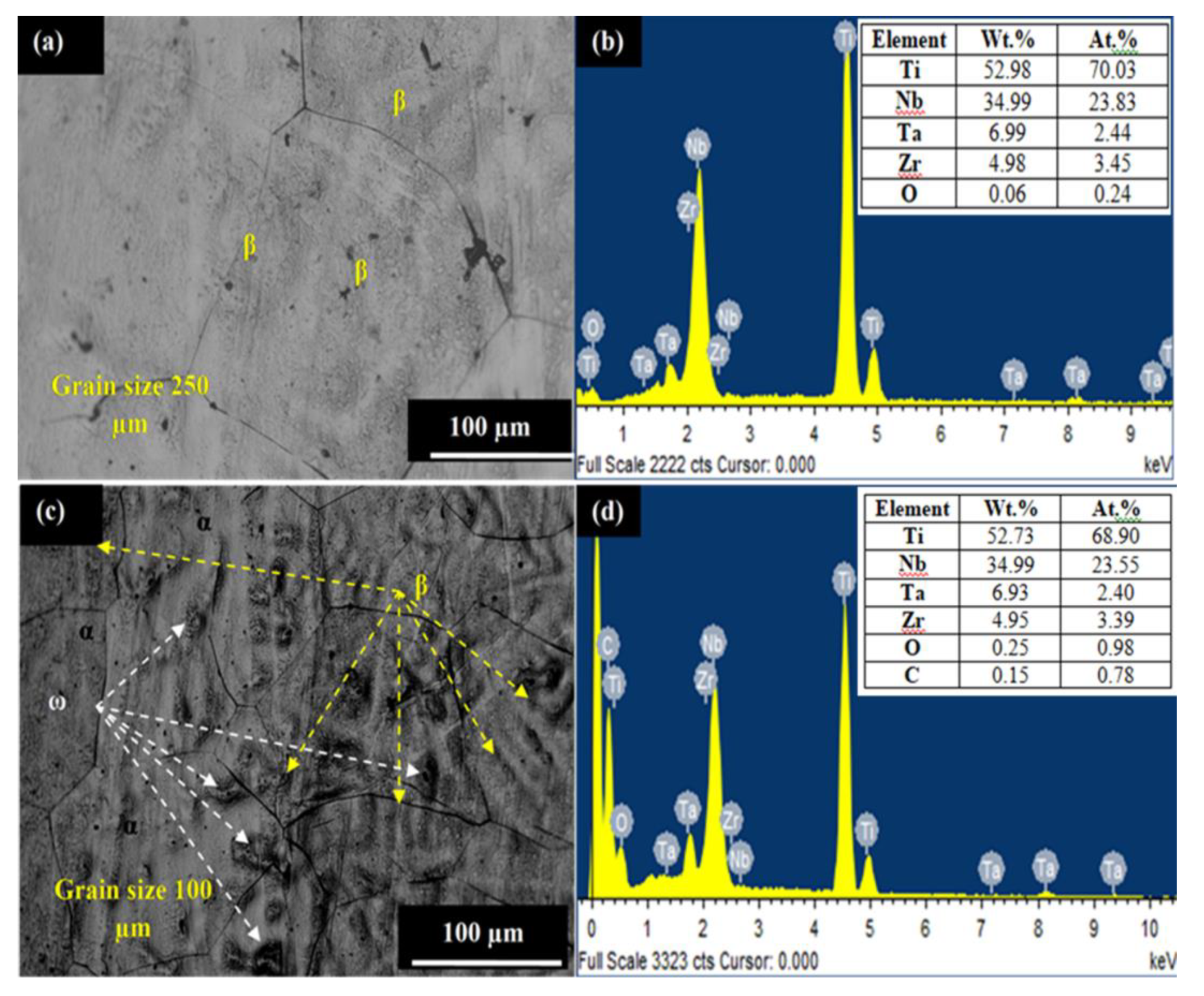
3. Results and Discussion
3.1. Parametric Optimization
3.2. Corrosion Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, M. Titanium-based biomaterials for preventing stress shielding between implant devices and bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, T.; Hu, L.; Ding, Q. Effective solution for the tribological problems of Ti-6Al-4V: Combination of laser surface texturing and solid lubricant film. Surf. Coat. Technol. 2012, 206, 5060–5066. [Google Scholar] [CrossRef]
- Collipriest, J.E., Jr. An experimentalist’s view of the surface flaw problem. In The Surface Crack—Physical Problems and Computational Solutions; ASME: New York, NY, USA, 1972; pp. 43–61. [Google Scholar]
- Lee, T.M.; Chang, E.; Yang, C.Y. A comparison of the surface characteristics and ion release of Ti6Al4V and heat-treated Ti6Al4V. J. Biomed. Mater. Res. 2000, 50, 499–511. [Google Scholar] [CrossRef]
- Zaffe, D.; Bertoldi, C.; Consolo, U. Accumulation of aluminium in lamellar bone after implantation of titanium plates, Ti–6Al–4V screws, hydroxyapatite granules. Biomaterials 2004, 25, 3837–3844. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, L.; Fu, Y.; Qin, J.; Lu, W.; Zhang, D. Influence of oxygen content on microstructure and mechanical properties of Ti–Nb–Ta–Zr alloy. Mater. Des. 2011, 32, 2934–2939. [Google Scholar] [CrossRef]
- Stráský, J.; Harcuba, P.; Václavová, K.; Horváth, K.; Landa, M.; Srba, O.; Janeček, M. Increasing strength of a biomedical Ti-Nb-Ta-Zr alloy by alloying with Fe, Si and O. J. Mech. Behav. Biomed. Mater. 2017, 71, 329–336. [Google Scholar] [CrossRef]
- Prakash, C.; Kansal, H.K.; Pabla, B.S.; Puri, S. Experimental investigations in powder mixed electric discharge machining of Ti–35Nb–7Ta–5Zrβ-titanium alloy. Mater. Manuf. Process. 2017, 32, 274–285. [Google Scholar] [CrossRef]
- Prakash, C.; Kansal, H.K.; Pabla, B.S.; Puri, S. Processing and characterization of novel biomimetic nanoporous bioceramic surface on β-Ti implant by powder mixed electric discharge machining. J. Mater. Eng. Perform. 2015, 24, 3622–3633. [Google Scholar] [CrossRef]
- Prakash, C.; Kansal, H.K.; Pabla, B.S.; Puri, S. Multi-objective optimization of powder mixed electric discharge machining parameters for fabrication of biocompatible layer on β-Ti alloy using NSGA-II coupled with Taguchi based response surface methodology. J. Mech. Sci. Technol. 2016, 30, 4195–4204. [Google Scholar] [CrossRef]
- Prakash, C.; Uddin, M.S. Surface modification of β-phase Ti implant by hydroaxyapatite mixed electric discharge machining to enhance the corrosion resistance and in-vitro bioactivity. Surf. Coat. Technol. 2017, 326, 134–145. [Google Scholar] [CrossRef]
- Prakash, C.; Kansal, H.K.; Pabla, B.S.; Puri, S. Powder mixed electric discharge machining: An innovative surface modification technique to enhance fatigue performance and bioactivity of β-Ti implant for orthopedics application. J. Comput. Inf. Sci. Eng. 2016, 16, 041006. [Google Scholar] [CrossRef]
- Prakash, C.; Kansal, H.K.; Pabla, B.S.; Puri, S. Effect of surface nano-porosities fabricated by powder mixed electric discharge machining on bone-implant interface: An experimental and finite element study. Nanosci. Nanotechnol. Lett. 2016, 8, 815–826. [Google Scholar] [CrossRef]
- Singh, S.; Prakash, C.; Singh, H. Deposition of HA-TiO2 by plasma spray on β-phase Ti-35Nb-7Ta-5Zr alloy for hip stem: Characterization, mechanical properties, corrosion, and in-vitro bioactivity. Surf. Coat. Technol. 2020, 398, 126072. [Google Scholar] [CrossRef]
- Singh, H.; Prakash, C.; Singh, S. Plasma Spray Deposition of HA-TiO2 on β-phase Ti-35Nb-7Ta-5Zr Alloy for Hip Stem: Characterization of Bio-mechanical Properties, Wettability, and Wear Resistance. J. Bionic Eng. 2020, 17, 1029–1044. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef]
- Bain, C.A.; Moy, P.K. The association between the failure of dental implants and cigarette smoking. Int. J. Oral Maxillofac. Implant. 1993, 8, 609–615. [Google Scholar]
- Brocard, D.; Barthet, P.; Baysse, E.; Duffort, J.F.; Eller, P.; Justumus, P. A multicenter report on 1.022 consecutively placed ITI implants: A 7-year longitudinal study. Int. J. Oral Maxillofac. Implant. 2000, 15, 691–700. [Google Scholar]
- Karoussis, I.K.; Kotsovilis, S.; Fourmousis, I. A comprehensive and critical review of dental implant prognosis in periodontally compromised partially edentulous patients. Clin. Oral Implant. Res. 2007, 18, 669–679. [Google Scholar] [CrossRef]
- Balshe, A.A.; Eckert, S.E.; Koka, S.; Assad, D.A.; Weaver, A.L. The effects of smoking on the survival of smooth- and rough-surface dental implants. Int. J. Oral Maxillofac. Implant. 2008, 23, 1117–1122. [Google Scholar]
- Aaraj Khodaii, S.J.; Barazandeh, F.; Adibi, H.; Sarhan, A. Optimization of Grinding partially stabilized zirconia (PSZ) for dental Implant application. Modares Mech. Eng. 2018, 18, 187–194. [Google Scholar]
- Rao, P.S.; Jain, P.K.; Dwivedi, D.K. Optimization of key process parameters on electro chemical honing (ECH) of external cylindrical surfaces of titanium alloy Ti 6Al 4V. Mater. Today Proc. 2017, 4, 2279–2289. [Google Scholar] [CrossRef]
- Affatato, S.; Ruggiero, A. Surface analysis on revised hip implants with stem taper for wear and failure incidence evaluation: A first investigation. Measurement 2019, 145, 38–44. [Google Scholar] [CrossRef]
- Tian, Y.; Shi, C.; Fan, Z.; Zhou, Q. Experimental investigations on magnetic abrasive finishing of Ti-6Al-4V using a multiple pole-tip finishing tool. Int. J. Adv. Manuf. Technol. 2020, 106, 3071–3080. [Google Scholar] [CrossRef]
- Singh, H.; Singh, S.; Prakash, C. Current Trends in Biomaterials and Bio-manufacturing; Springer: Berlin, Germany, 2019. [Google Scholar]
- Basim, G.B.; Ozdemir, Z.; Mutlu, O. Biomaterials applications of chemical mechanical polishing. In Proceedings of the Planarization/CMP Technology (ICPT 2012), International Conference VDE 2012, Grenoble, France, 15–17 October 2012; pp. 1–5. [Google Scholar]
- Okawa, S.; Watanabe, K. Chemical mechanical polishing of titanium with colloidal silica containing hydrogen peroxide—Mirror polishing and surface properties. Dent. Mater. J. 2009, 28, 68–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zur Rahman Pompa, L.; Haider, W. Influence of electropolishing and magnetoelectropolishing on corrosion and biocompatibility of titanium implants. J. Mater. Eng. Perform. 2014, 23, 3907–3915. [Google Scholar]
- Okada, A.; Uno, Y.; Yabushita, N.; Uemura, K.; Raharjo, P. High efficient surface finishing of bio-titanium alloy by large-area electron beam irradiation. J. Mater. Process. Technol. 2004, 149, 506–511. [Google Scholar] [CrossRef]
- Sidpara, A.; Jain, V.K. Analysis of forces on the freeform surface in magnetorheological fluid based finishing process. Int. J. Mach. Tools Manuf. 2013, 69, 1–10. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, V.K.; Sidpara, A. Nanofinishing of freeform surfaces (knee joint implant) by rotational-magnetorheological abrasive flow finishing (R-MRAFF) process. Precis. Eng. 2015, 42, 165–178. [Google Scholar] [CrossRef]
- Barman, A.; Das, M. Force analysis during spot finishing of titanium alloy using novel tool in magnetic field assisted finishing process. Int. J. Precis. Technol. 2019, 8, 190–200. [Google Scholar]
- Barman, A.; Das, M. Nano-finishing of bio-titanium alloy to generate different surface morphologies by changing magnetorheological polishing fluid compositions. Precis. Eng. 2018, 51, 145–152. [Google Scholar] [CrossRef]
- Barman, A.; Das, M. Toolpath generation and finishing of bio-titanium alloy using novel polishing tool in MFAF process. Int. J. Adv. Manuf. Technol. 2019, 100, 1123–1135. [Google Scholar] [CrossRef]
- Parameswari, G.; Jain, V.K.; Ramkumar, J.; Nagdeve, L. Experimental investigations into nanofinishing of Ti6Al4V flat disc using magnetorheological finishing process. Int. J. Adv. Manuf. Technol. 2019, 100, 1055–1065. [Google Scholar] [CrossRef]
- Nagdeve, L.; Jain, V.K.; Ramkumar, J. Preliminary investigations into nano-finishing of freeform surface (femoral) using inverse replica fixture. Int. J. Adv. Manuf. Technol. 2019, 100, 1081–1092. [Google Scholar] [CrossRef]
- Barman, A.; Das, M. Magnetic field assisted finishing process for super-finished Ti alloy implant and its 3D surface characterization. J. Micromanufacturing 2018, 1, 154–169. [Google Scholar] [CrossRef]
- Singh, S.; Prakash, C. Effect of cryogenic treatment on the microstructure, mechanical properties and finishability of β-TNTZ alloy for orthopedic applications. Mater. Lett. 2020, 278, 128461. [Google Scholar] [CrossRef]
- Málek, J.; Hnilica, F.; Veselý, J.; Smola, B.; Bartáková, S.; Vaněk, J. The influence of chemical composition and thermo-mechanical treatment on Ti–Nb–Ta–Zr alloys. Mater. Des. 2012, 35, 731–740. [Google Scholar] [CrossRef]
- Majumdar, P. Microstructural Evaluation of Boron Free and Boron Containing Heat-Treated Ti-35Nb-7.2 Zr-5.7 Ta Alloy. Microsc. Microanal. 2012, 18, 295. [Google Scholar] [CrossRef]
- Tang, X.; Ahmed, T.; Rack, H.J. Phase transformations in Ti-Nb-Ta and Ti-Nb-Ta-Zr alloys. J. Mater. Sci. 2000, 35, 1805–1811. [Google Scholar] [CrossRef]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data aAnalysis for Biologists; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Rosalbino, F.; De Negri, S.; Scavino, G.; Saccone, A. Electrochemical corrosion behaviour of binary magnesium-heavy rare earth alloys. Metall. Ital. 2018, 2, 34–43. [Google Scholar]
- Babbar, A.; Prakash, C.; Singh, S.; Gupta, M.K.; Mia, M.; Pruncu, C.I. Application of hybrid nature-inspired algorithm: Single and bi-objective constrained optimization of magnetic abrasive finishing process parameters. J. Mater. Res. Technol. 2020, 4, 7961–7974. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Dao, T.P.; Prakash, C.; Singh, S.; Pramanik, A.; Krolczyk, G.; Pruncu, C.I. Machining parameter optimization in shear thickening polishing of gear surfaces. J. Mater. Res. Technol. 2020, 9, 5112–5126. [Google Scholar] [CrossRef]
- Abron, A.; Hopfensperger, M.; Thompson, J.; Cooper, L.F. Evaluation of a predictive model for implant surface topography effects on early osseointegration in the rat tibia model. J. Prosthet. Dent. 2001, 85, 40–46. [Google Scholar] [CrossRef]
- Ogawa, T.; Nishimura, I. Genes differentially expressed in titanium implant healing. J. Dent. Res. 2006, 85, 566–570. [Google Scholar] [CrossRef]
- Wang, G.; Wan, Y.; Wang, T.; Liu, Z. Corrosion behavior of titanium implant with different surface morphologies. Procedia Manuf. 2017, 10, 363–370. [Google Scholar] [CrossRef]
- Kubo, K.; Tsukimura, N.; Iwasa, F.; Ueno, T.; Saruwatari, L.; Aita, H.; Chiou, W.A.; Ogawa, T. Cellular behavior on TiO2 nanonodular structures in a micro-to-nanoscale hierarchy model. Biomaterials 2009, 30, 5319–5329. [Google Scholar] [CrossRef] [PubMed]






| Symbol | Process Parameters | Unit | Range |
|---|---|---|---|
| CIP | Iron (Fe); size~25 µm | % by vol. | 30, 35, 40 |
| Nt | Rotational speed of tool | rpm | 600, 900, 1200 |
| Gp | Work-gap | Mm | 1, 1.5, 2 |
| Experiment No. | CIP | Nt | Gp |
|---|---|---|---|
| 1 | 35 | 600 | 1.0 |
| 2 | 35 | 900 | 1.5 |
| 3 | 35 | 1200 | 2.0 |
| 4 | 40 | 600 | 1.5 |
| 5 | 40 | 900 | 2.0 |
| 6 | 40 | 1200 | 1.0 |
| 7 | 45 | 600 | 2.0 |
| 8 | 45 | 900 | 1.0 |
| 9 | 45 | 1200 | 1.5 |
| Experiment No. | MR | Ra | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Raw Value (g) | Mean | S/N | Raw Value (nm) | Mean | S/N | |||||
| 1 | 60 | 50 | 70 | 60 | 35.56 | 16 | 15 | 11 | 14 | −22.92 |
| 2 | 50 | 55 | 75 | 60 | 35.56 | 8 | 10 | 6 | 8 | −18.06 |
| 3 | 28 | 35 | 30 | 31 | 29.83 | 60 | 55 | 65 | 60 | −35.56 |
| 4 | 68 | 55 | 57 | 60 | 35.56 | 10 | 12 | 8 | 10 | −20.00 |
| 5 | 65 | 50 | 65 | 60 | 35.56 | 4 | 2 | 6 | 4 | −12.04 |
| 6 | 68 | 67 | 60 | 65 | 36.26 | 45 | 50 | 40 | 45 | −33.06 |
| 7 | 23 | 27 | 25 | 25 | 27.96 | 21 | 20 | 22 | 21 | −26.44 |
| 8 | 66 | 62 | 67 | 65 | 36.26 | 14 | 15 | 10 | 13 | −22.28 |
| 9 | 30 | 28 | 32 | 30 | 29.54 | 75 | 50 | 55 | 60 | −35.56 |
| Overall mean S/N ratio (mo) | 33.56 | 25.10 | ||||||||
| Source | Degree of Freedom | Sum of Square | Variance | F-Test | p-Value | Contribution (%) |
|---|---|---|---|---|---|---|
| MR | ||||||
| CIP | 2 | 30.9716 | 15.4858 | 53.19 | 0.018 * | 33.64 |
| Nt | 2 | 24.3382 | 12.1691 | 41.80 | 0.023 * | 26.44 |
| Gp | 2 | 36.1651 | 18.0825 | 62.11 | 0.016 * | 39.28 |
| Residual Error | 2 | 0.5823 | 0.2912 | 0.632 | ||
| Total | 8 | 92.0573 | 100 | |||
| Ra | ||||||
| CIP | 2 | 47.086 | 23.543 | 2.40 | 0.294 | 9.39 |
| Nt | 2 | 429.047 | 214.523 | 21.85 | 0.044 * | 85.57 |
| Gp | 2 | 5.630 | 2.815 | 0.29 | 0.777 | 1.12 |
| Residual Error | 2 | 19.639 | 9.819 | 3.91 | ||
| Total | 8 | 501.401 | 100 | |||
| Level | CIP | Nt | Gp |
|---|---|---|---|
| MR | |||
| 1 | 33.65 | 33.03 | 36.03 * |
| 2 | 35.79 * | 35.79 * | 33.56 |
| 3 | 31.25 | 31.88 | 31.12 |
| Delta | 4.54 | 3.92 | 4.91 |
| Rank | 2 | 3 | 1 |
| Ra | |||
| 1 | −25.52 | −23.40 | −26.09 |
| 2 | −21.98 * | −17.46 * | −24.23 * |
| 3 | −27.51 | −34.14 | −24.68 |
| Delta | 5.53 | 16.68 | 1.86 |
| Rank | 2 | 1 | 3 |
| Responses | Predicted Values | Confirmatory Values | Difference (±) |
|---|---|---|---|
| MR (g) | 105.8 | 103.7 | 2.1 |
| Ra (nm) | 4.63 | 4.67 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Prakash, C.; Pramanik, A.; Basak, A.; Shabadi, R.; Królczyk, G.; Bogdan-Chudy, M.; Babbar, A. Magneto-Rheological Fluid Assisted Abrasive Nanofinishing of ?-Phase Ti-Nb-Ta-Zr Alloy: Parametric Appraisal and Corrosion Analysis. Materials 2020, 13, 5156. https://doi.org/10.3390/ma13225156
Singh S, Prakash C, Pramanik A, Basak A, Shabadi R, Królczyk G, Bogdan-Chudy M, Babbar A. Magneto-Rheological Fluid Assisted Abrasive Nanofinishing of ?-Phase Ti-Nb-Ta-Zr Alloy: Parametric Appraisal and Corrosion Analysis. Materials. 2020; 13(22):5156. https://doi.org/10.3390/ma13225156
Chicago/Turabian StyleSingh, Sunpreet, Chander Prakash, Alokesh Pramanik, Animesh Basak, Rajasekhara Shabadi, Grzegorz Królczyk, Marta Bogdan-Chudy, and Atul Babbar. 2020. "Magneto-Rheological Fluid Assisted Abrasive Nanofinishing of ?-Phase Ti-Nb-Ta-Zr Alloy: Parametric Appraisal and Corrosion Analysis" Materials 13, no. 22: 5156. https://doi.org/10.3390/ma13225156
APA StyleSingh, S., Prakash, C., Pramanik, A., Basak, A., Shabadi, R., Królczyk, G., Bogdan-Chudy, M., & Babbar, A. (2020). Magneto-Rheological Fluid Assisted Abrasive Nanofinishing of ?-Phase Ti-Nb-Ta-Zr Alloy: Parametric Appraisal and Corrosion Analysis. Materials, 13(22), 5156. https://doi.org/10.3390/ma13225156











