The Correlation of Temperature-Mineral Phase Transformation as a Controlling Factor of Thermal and Mechanical Performance of Fly Ash-Based Alkali-Activated Binders
Abstract
1. Introduction
- What processes occur during structure and phase formation and transformation considering differences in chemical and mineral composition of alumosilicates?
- How are these processes correlated with the physical and mechanical performances of alkali-activated composites?
2. Materials and Methods
3. Results and Discussions
3.1. Structural Characterization of Fly Ash Products
3.2. Mix Design and Specimens Preparation
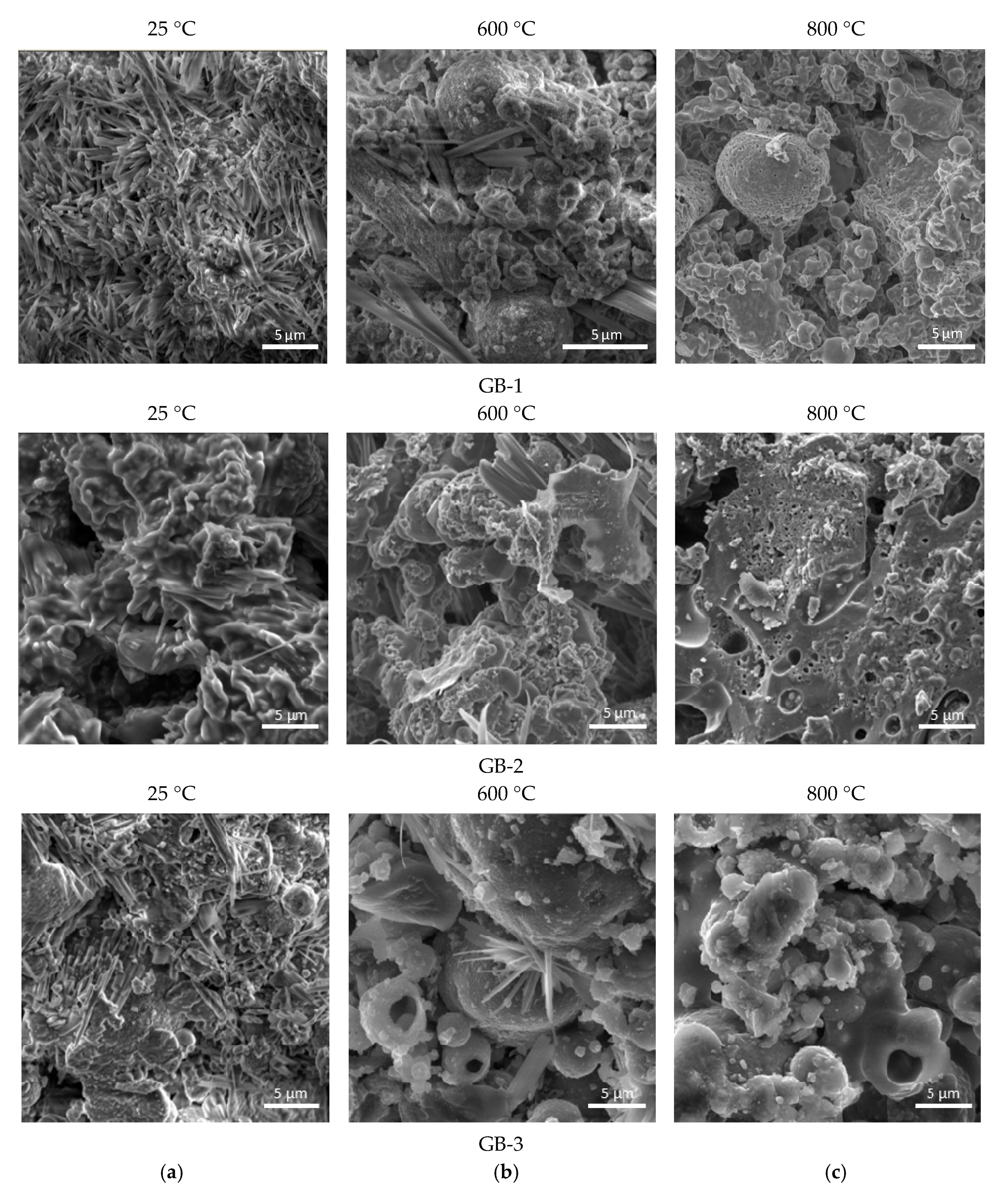
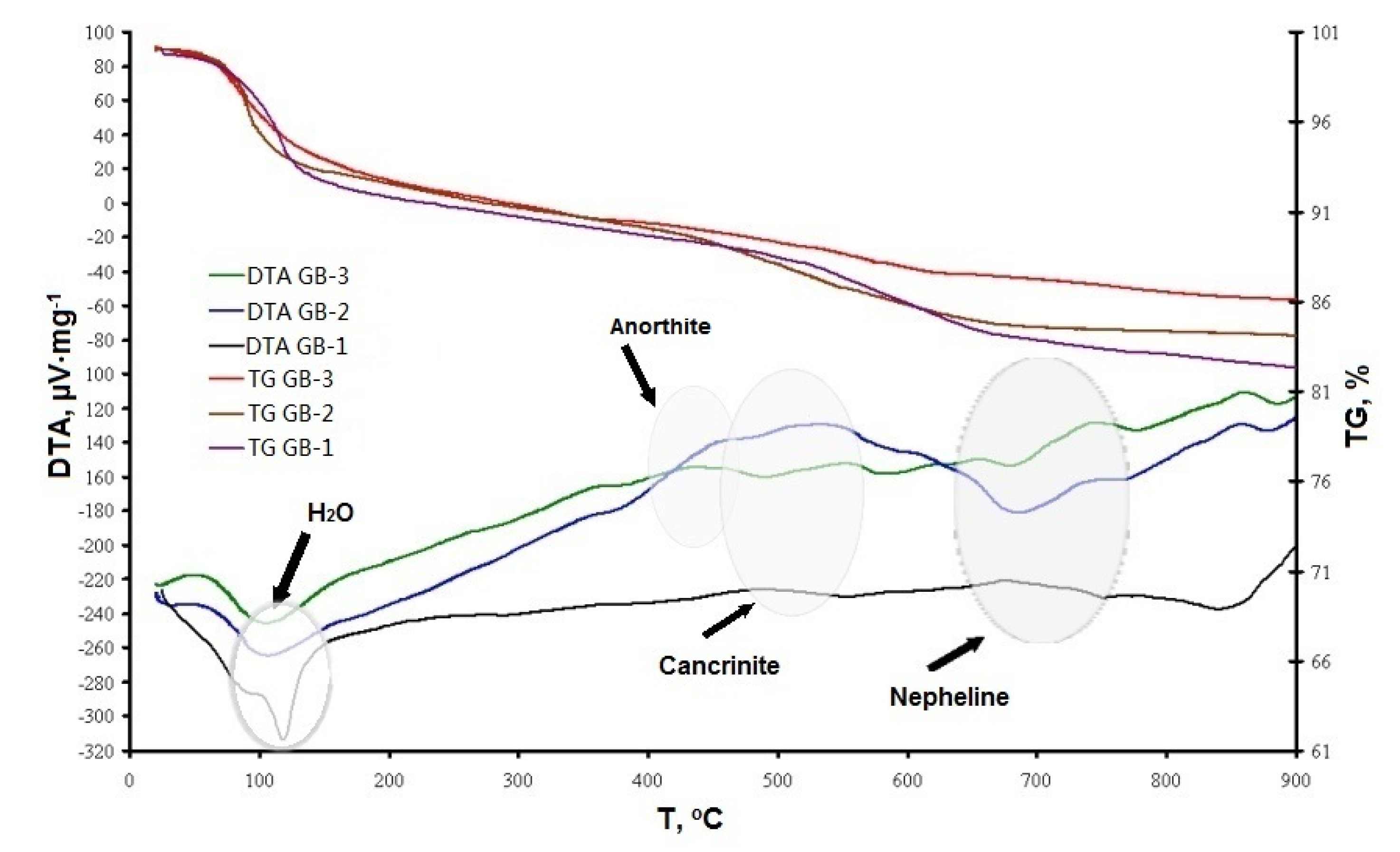
3.3. Thermal Effect on the Composition and Structural Changes
3.4. Effect on Structural Integrity, Compressive Strength, and Surface Appearance
4. Conclusions
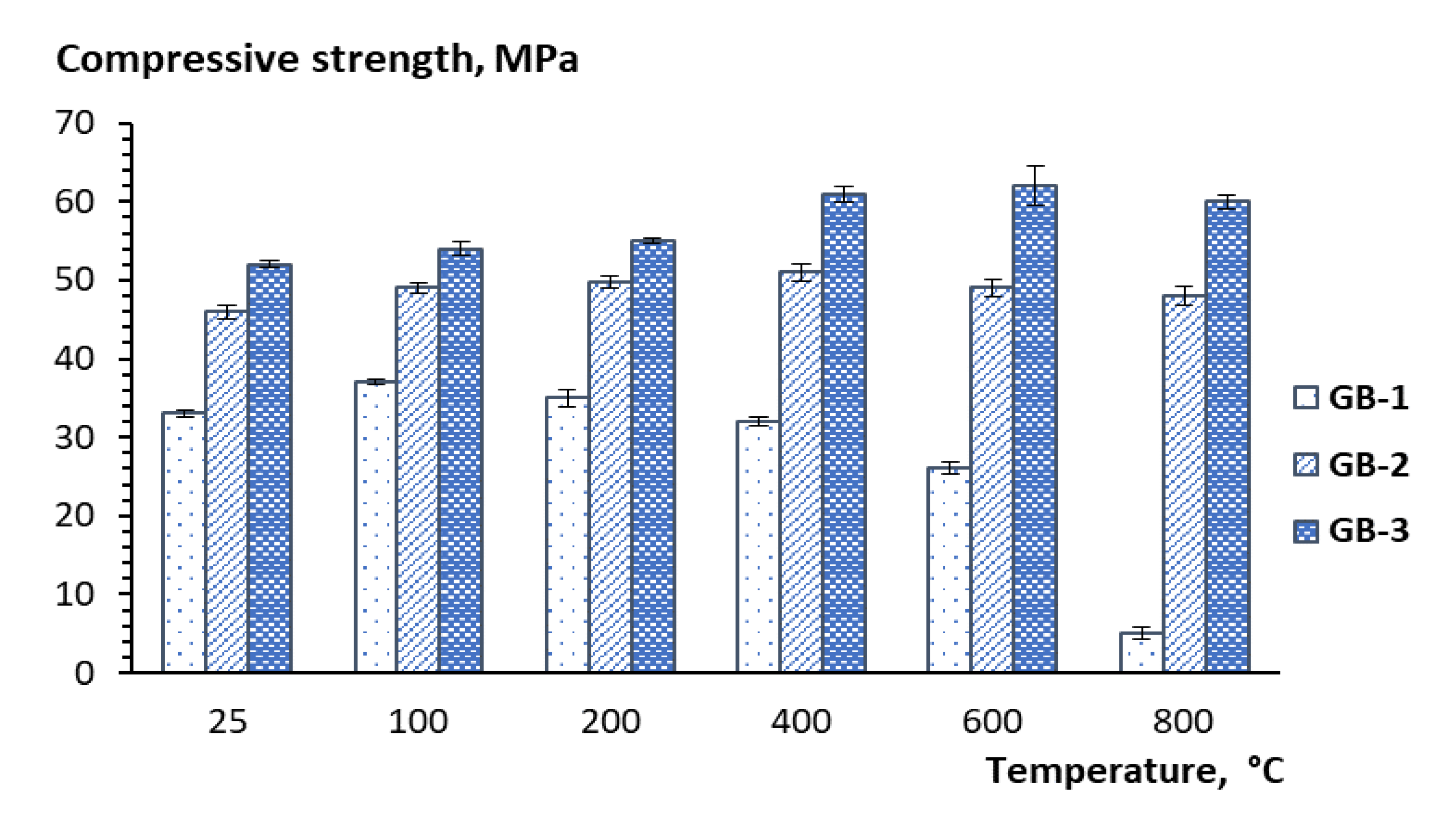
- The XRD, SEM, and DTA/TG analyses showed that the studied geopolymers mixes exposed to high temperatures within 450–550 °C underwent a partial transformation of amorphous alumosilicate substance to the crystalline phase of anorthite Ca(Al2Si2O8) and cancrinite Na7(Al6Si7O26)·5H2O. Furthermore, in the temperature interval 600–800 °C, the rest amorphous alumosilicate substance, mullite, and quartz, as well as low-temperature phases sodium hydrocarbonate (Na3(HCO3)(CO3)·2H2O) or trona in the case of GB-1 transforms into anhydrous crystals with network structure such as Na-alumosilicates nepheline.
- The presence of 15% of trona in the GB-1 specimens demonstrates a considerable detrimental effect and leads to up to 86% of strength loss within the temperature range of 600–800 °C when trona undergoes the dehydration process. The other two specimens GB-2 and GB-3, which did not contain trona in the system were able to develop strength with a temperature increase up to 400 °C and 600 °C, followed by a strength loss within the temperature ranges of 400–800 °C and 600–800 °C, respectively.
- A strength loss up to 15% in the GB-2 and GB-3 within the 400–800 °C temperature range is the result of volume change, which occurs during the transformation of mullite and quartz of fly ash to crystalline phases such as anorthite, cancrinite, and nepheline, leading to inner pressure buildup and further matrix disintegration.
- High temperatures facilitate solubility of alumosilicate components in alkaline media, resulting in synthesis of additional vitreous phase and formation of nanosized crystals of high-temperature nepheline. These concurrent processes both contribute to formation of a high-temperature stable nepheline phase, densification of the alkali activated matrix, and an increase in strength response of alkali activated binders.
- The presence of an unfavorable carbonate mineral phase trona in the alkali activated binder system is the result of the reaction between CO2 and “free” alkaline component which did not react with alumosilicates.
- Low solubility of alumosilicates in alkaline media, leaving a big quantity of unreacted components, leads to a poor cross-linking of -Si-Al- chains, creating a low degree of geopolymerization that results in an incoherent matrix.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; El-Tawil, S.; Hansen, W.; Wang, F. Effect of slag cement on the properties of ultra-high-performance concrete. Constr. Build. Mater. 2018, 190, 830–837. [Google Scholar] [CrossRef]
- Wang, D.; Shi, C.; Wu, Z.; Xiao, J.; Huang, Z.; Fang, Z. A review on ultra-high performance concrete: Part II. Hydration, microstructure and properties. Constr. Build. Mater. 2015, 96, 368–377. [Google Scholar] [CrossRef]
- Andrew, R.M. Global CO2 emissions from cement production, 1928–2018. Earth Syst. Sci. Data 2019, 11, 1675–1710. [Google Scholar] [CrossRef]
- Malhotra, V.M.; Mehta, P.K. Pozzolanic Cementitious Materials; Advances in concrete technology; Gordon and Breach: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Atis, C.D. High-Volume Fly Ash Concrete with High-Strength and Low Drying Shrinkage. J. Mater. Civ. Eng. 2003, 15, 153–156. [Google Scholar] [CrossRef]
- Kozhukhova, M.; Wittenberg, R.; Sobolev, K. Green Cementitious Systems Based on Off-spec Fly Ash. In Proceedings of the International RILEM Workshop on Concrete Durability and Service Life Planning (ConcreteLife), Haifa, Israel, 14–16 January 2020; pp. 104–108. [Google Scholar]
- Metin, A.; Konstantin, S.; Tomris, E.; Asim, Y.; Turker, P. Properties of blended cements with thermally activated kaolin. Constr. Build. Mater. 2009, 23, 62–70. [Google Scholar]
- Glukhovsky, V.D. Soil Silicates; Gostroiizdat Publish: Kiev, Ukraine, 1959. [Google Scholar]
- Erofeev, V.T.; Rodin, A.I.; Yakunin, V.V.; Bogatov, A.D.; Bochkin, V.S.; Chegodajkin, A.M. Alkali-activated slag binders from rock-wool production wastes. Magazine Civ. Eng. 2018, 82, 219–227. [Google Scholar]
- Feret, R. Slags for the manufacture of cement. Rev. Matriaux Deconstr. Trav. Publics 1939, 352, 121–126. [Google Scholar]
- Chao, L.; Henghu, S.; Longtu, L. A review: The comparison between alkali-activated slag (Si+Ca) and metakaolin (Si+Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar]
- Palomo, A.; Grutzek, M.W.; Blanco, M.T. Alkali-activated fly ashes. A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Composition and microstructure of alkali activated fly ash binder: Effect of the activator. Cem. Concr. Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 4th ed.; Geopolymer Institute: Saint-Quentin, France, 2015. [Google Scholar]
- Kumar, S.; Kumar, R. Mechanical activation of fly ash: Effect of reaction, Structure and properties of resulting geopolymer. Ceram. Int. 2010, 37, 533–541. [Google Scholar] [CrossRef]
- Fansuri, H.; Pritchard, D.; Zhang, D. Manufacture of low-grade zeolites from fly ash for fertilizer application. Res. Rep. 2008, 91, 98. [Google Scholar]
- Davidovits, J. Review. Geopolymers: Ceramic-Like Inorganic Polymers. J. Ceram. Sci. Technol. 2017, 8, 335–350. [Google Scholar]
- Hemra, K.; Aungkavattana, P. Effect of cordierite addition on compressive strength and thermal stability of metakaolin based geopolymer. Adv. Powder Technol. 2016, 27, 1021–1026. [Google Scholar] [CrossRef]
- Bajare, D.; Vitola, L.; Dembovska, L.; Bumanis, G. Waste Stream Porous Alkali Activated Materials for High Temperature Application. Front. Mater. 2019, 6, 92. [Google Scholar] [CrossRef]
- Bumanis, G.; Vitola, L.; Bajare, D.; Dembovska, L.; Pundiene, I. Impact of reactive SiO2/Al2O3 ratio in precursor on durability of porous alkali activated materials. Ceram. Int. 2017, 43, 5471–5477. [Google Scholar] [CrossRef]
- Kuenzel, C.; Grover, L.M.; Vandeperre, L.; Boccaccini, A.R.; Cheeseman, C.R. Production of nepheline/quartz ceramics from geopolymer mortars. J. Eur. Ceram. Soc. 2013, 33, 251–258. [Google Scholar] [CrossRef]
- Promakhov, V.V.; Zhukov, A.S.; Vorozhtsov, A.B.; Schults, N.A.; Kovalchuk, S.V.; Kozhevnikov, S.V.; Olisov, A.V.; Klimenko, V.A. Structure and Mechanical Properties of 3D-Printed Ceramic Specimens. Russ. Phys. J. 2019, 62, 876–881. [Google Scholar] [CrossRef]
- Pivinsky, Y.E. Ceramic Binders and Ceramic Concretes; Metallurgy: Moscow, Russia, 1990. [Google Scholar]
- Cherevatova, A.V.; Strokova, V.V.; Zhernovsky, I.V. Mineral Nanostructured Binders. Nature, Technology and Applying Prospective; Monography; BSTU named after V.G. Shukhov: Belgorod, Russia, 2010. [Google Scholar]
- Cherevatova, A.V.; Zhernovskaya, I.V.; Alehin, D.A.; Kozhukhova, M.I.; Kozhukhova, N.I.; Yakovlev, E.A. Theoretical aspects of development of composite nanostructured gypsum binder characterized by increased heat resistance. Constr. Mater. Prod. 2019, 2, 5–13. [Google Scholar] [CrossRef]
- Gernovskii, I.V.; Pavlenko, N.V.; Strokova, V.V.; Cherevatova, A.V.; Nelubova, V.V.; Sobolev, K.G. The application of Nanostructured Zero-cement Binder in Fire-Resistant Cellular Concrete. In Proceedings of the XXII International Material Research Congress, Cancun, Mexico, 16–20 August 2015. [Google Scholar]
- Nergis, D.D.B.; Abdullah, M.M.A.; Sandu, A.V.; Vizureanu, P. XRD and TG-DTA Study of New Alkali Activated Materials Based on Fly Ash with Sand and Glass Powder. Materials 2020, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- Winnefeld, F.; Leemann, A.; Lucuk, M.; Svoboda, P.; Neuroth, M. Assessment of phase formation in alkali activated low and high calcium fly ashes in building materials. Constr. Build. Mater. 2010, 24, 1086–1093. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G.; Sagoe-Crentsil, K. Comparative performance of geopolymers made with metakaolin and fly ash after exposure to elevated temperatures. Cem. Concr. Res. 2007, 37, 1583–1589. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G. Damage behavior of geopolymer composites exposed to elevated temperatures. Cem. Concr. Res. 2008, 30, 986–991. [Google Scholar] [CrossRef]
- Barbosa, V.F.; MacKenzie, K.J. Synthesis and thermal behaviour of potassium sialate geopolymers. Mater. Lett. 2003, 57, 1477–1482. [Google Scholar] [CrossRef]
- Russian Standard GOST 2263. Sodium Hydroxide for Industrial Use Specifications; Izdatelstvo Standartov: Moscow, Russia, 2001; p. 18. [Google Scholar]
- Solovyov, L.A. Full-profile refinement by derivative difference minimization. J. Appl. Crystallogr. 2004, 37, 743–749. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; De La Torre, A.; Palomo, A.; López-Olmo, G.; Alonso, M.M.; Aranda, M.A.G. Quantitative determination of phases in the alkali activation of fly ash. Part I. Potential ash reactivity. Fuel 2006, 85, 625–634. [Google Scholar] [CrossRef]
- Kozhukhova, N.I.; Zhernovsky, I.V.; Lebedev, M.S.; Sobolev, K. Influence of Fe component from milling yield on characteristics of perlite based geopolymers. IOP Conf. Ser. Mater. Sci. Eng. 2019, 560, 012148. [Google Scholar] [CrossRef]
- Kozhukhova, N.I.; Zhernovskaya, I.V.; Cherevatova, A.V.; Sobolev, K. Role of Fe-bearing component in perlite-based geopolymer when structural and phase transformations. Bull. Belgorod State Technol. Univ. Named V.G. Shukhov 2020, 2, 126–133. [Google Scholar]
- Azimi, E.A.; Abdullah, M.M.A.; Vizureanu, P.; Salleh, M.A.A.M.; Sandu, A.V.; Chaiprapa, J.; Yoriya, S.; Hussin, K.; Aziz, I.H. Strength Development and Elemental Distribution of Dolomite/Fly Ash Geopolymer Composite under Elevated Temperature. Materials 2020, 13, 1015. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Kodur, V.; Wu, B.; Cao, L.; Wang, F. Thermal behavior and mechanical properties of geopolymer mortar after exposure to elevated temperatures. Constr. Build. Mater. 2016, 109, 17–24. [Google Scholar] [CrossRef]
- Wons, W.; Rzepa, K.; Reben, M.; Murzyn, P.; Sitarz, M.; Olejniczak, Z. Effect of thermal processing on the structural characteristics of fly ashes. J. Mol. Struct. 2018, 1165, 299–304. [Google Scholar] [CrossRef]
- William, D.A.R.; Kealley, C.S.; van Riessen, A. Thermally Induced Microstructural Changes in Fly Ash Geopolymers: Experimental Results and Proposed Mode. J. Am. Ceram. Soc. 2015, 98, 929–939. [Google Scholar] [CrossRef]
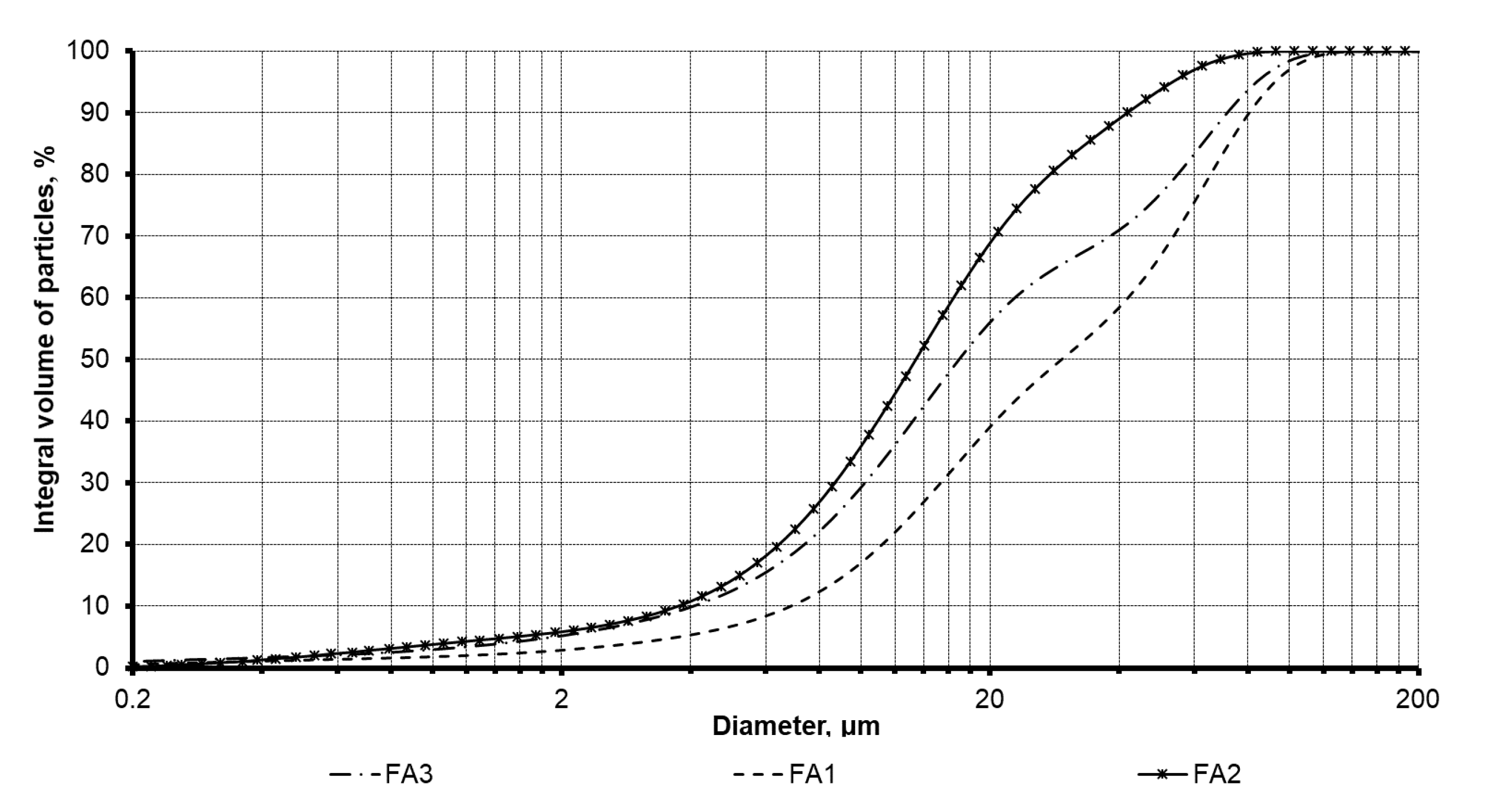
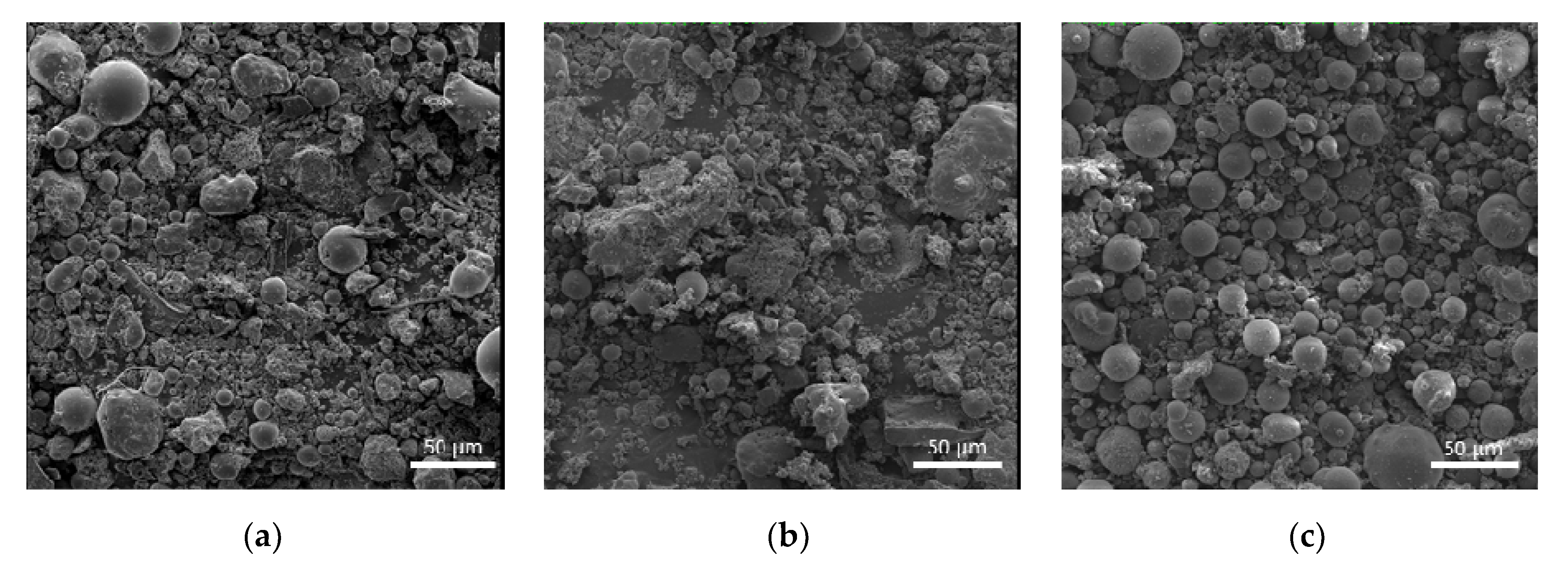


| Oxide Content (WT. %) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fly Ash ID | LOI | SiO2 | Al2O3 | Fe2O3 | CaO | TiO2 | K2O | Na2O | MgO | SO3 | P2O5 | Si/Al Ratio |
| FA1 | 6.1 | 58. 6 | 24.3 | 5.3 | 2.3 | 0.9 | 0.7 | 0.5 | 0.5 | 0.3 | 0.1 | 2.41 |
| FA2 | 5.2 | 57.4 | 32.7 | 0.5 | 0.4 | 0.1 | 0.4 | 1.2 | 2.2 | - | 0.6 | 1.64 |
| FA3 | 1.3 | 45.6 | 27.8 | 15.6 | 3.6 | 1.2 | 1.7 | 1.0 | 1.3 | 1.3 | 0.4 | 1.76 |
| Fly Ash ID | Parameter | Mineral Composition, % | ||||||
|---|---|---|---|---|---|---|---|---|
| Spec. Gravity | Specific Surface Area, m2/kg | Quartz | Mullite | Anorthite | Magnetite | Hematite | Vitreous Phase | |
| FA1 | 1.87 | 290 | 10.7 | 23.5 | 4.3 | 1.0 | - | 60.5 |
| FA2 | 1.80 | 435 | 9.3 | 18.7 | - | 1.9 | - | 70.1 |
| FA3 | 1.69 | 257 | 6.4 | 13.5 | - | 7.2 | 4.5 | 68.4 |
| Specimen | FA, % | NaOH, % | Water, % | Na2O/Al2O3 Molar Ratio | Compressive Strength, MPa |
|---|---|---|---|---|---|
| GB-1 | 66.5 | 10.5 | 23.0 | 0.75 | 34.1 |
| GB-2 | 63.3 | 8.0 | 28.7 | 0.75 | 45.2 |
| GB-3 | 68.8 | 12.6 | 18.5 | 0.75 | 50.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozhukhova, N.; Kozhukhova, M.; Zhernovskaya, I.; Promakhov, V. The Correlation of Temperature-Mineral Phase Transformation as a Controlling Factor of Thermal and Mechanical Performance of Fly Ash-Based Alkali-Activated Binders. Materials 2020, 13, 5181. https://doi.org/10.3390/ma13225181
Kozhukhova N, Kozhukhova M, Zhernovskaya I, Promakhov V. The Correlation of Temperature-Mineral Phase Transformation as a Controlling Factor of Thermal and Mechanical Performance of Fly Ash-Based Alkali-Activated Binders. Materials. 2020; 13(22):5181. https://doi.org/10.3390/ma13225181
Chicago/Turabian StyleKozhukhova, Natalia, Marina Kozhukhova, Irina Zhernovskaya, and Vladimir Promakhov. 2020. "The Correlation of Temperature-Mineral Phase Transformation as a Controlling Factor of Thermal and Mechanical Performance of Fly Ash-Based Alkali-Activated Binders" Materials 13, no. 22: 5181. https://doi.org/10.3390/ma13225181
APA StyleKozhukhova, N., Kozhukhova, M., Zhernovskaya, I., & Promakhov, V. (2020). The Correlation of Temperature-Mineral Phase Transformation as a Controlling Factor of Thermal and Mechanical Performance of Fly Ash-Based Alkali-Activated Binders. Materials, 13(22), 5181. https://doi.org/10.3390/ma13225181






