Comparison of Different Thermo-Chemical Treatments Methods of Ti-6Al-4V Alloy in Terms of Tribological and Corrosion Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Thermochemical Treatment
2.3. Characterization Methods
3. Results and Discussion
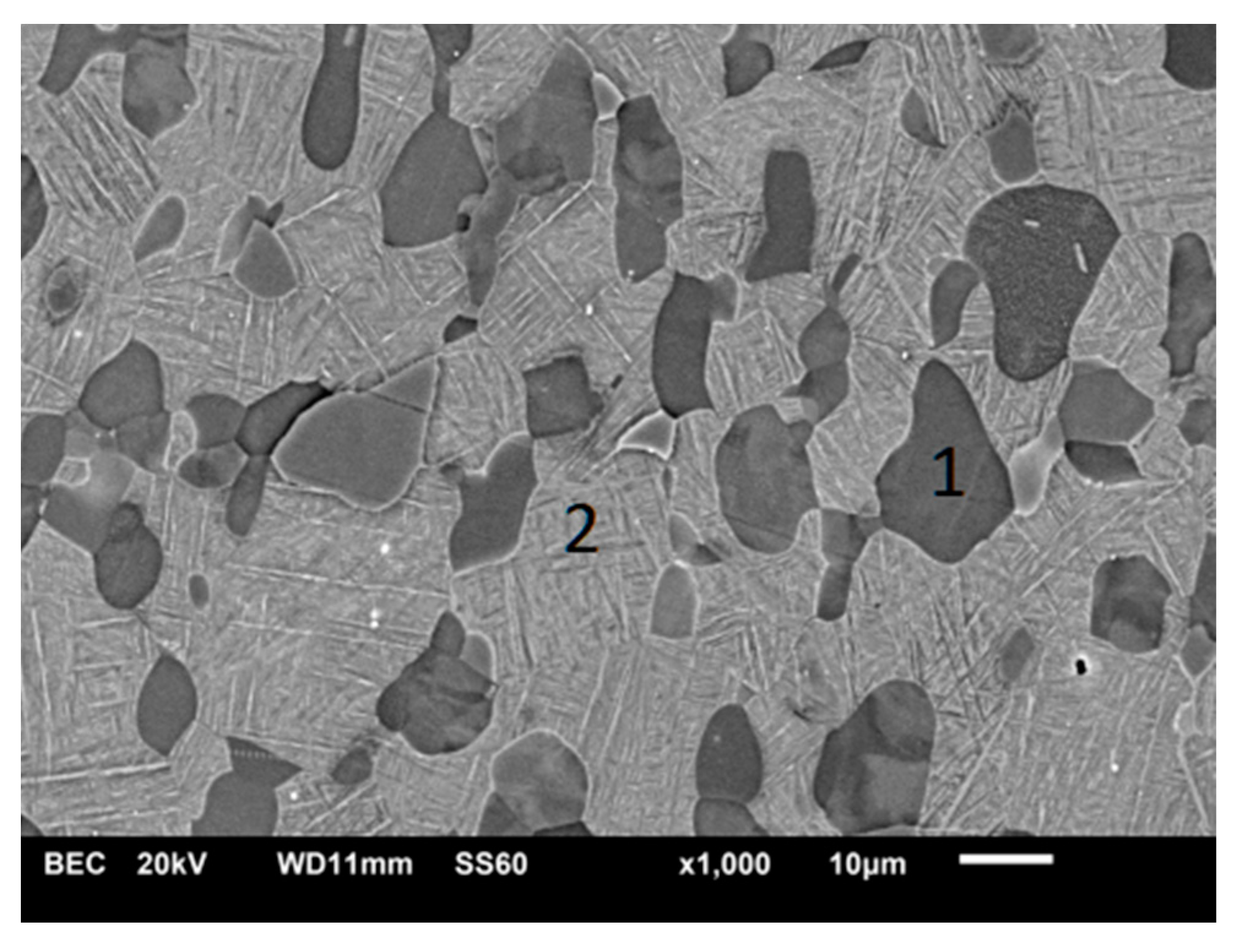
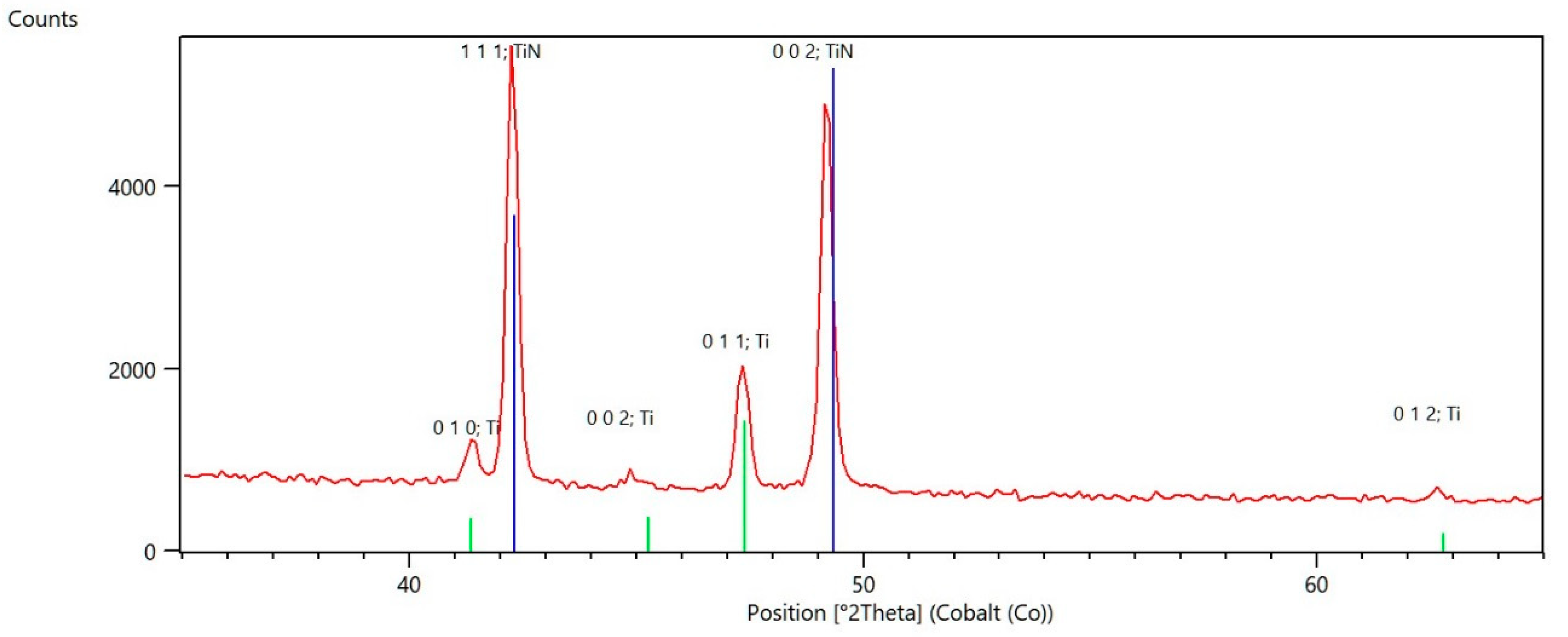
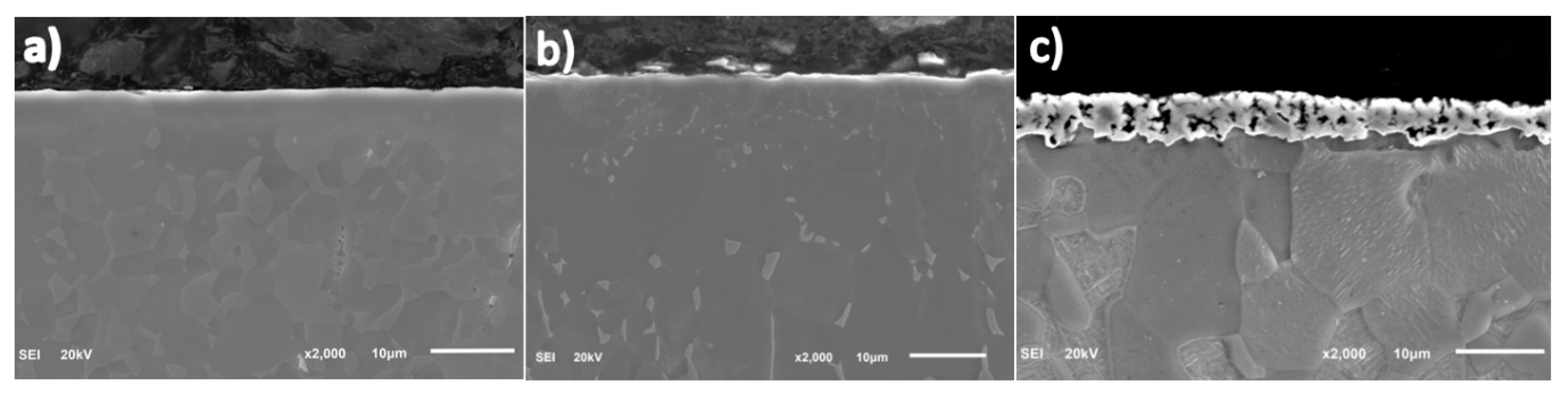
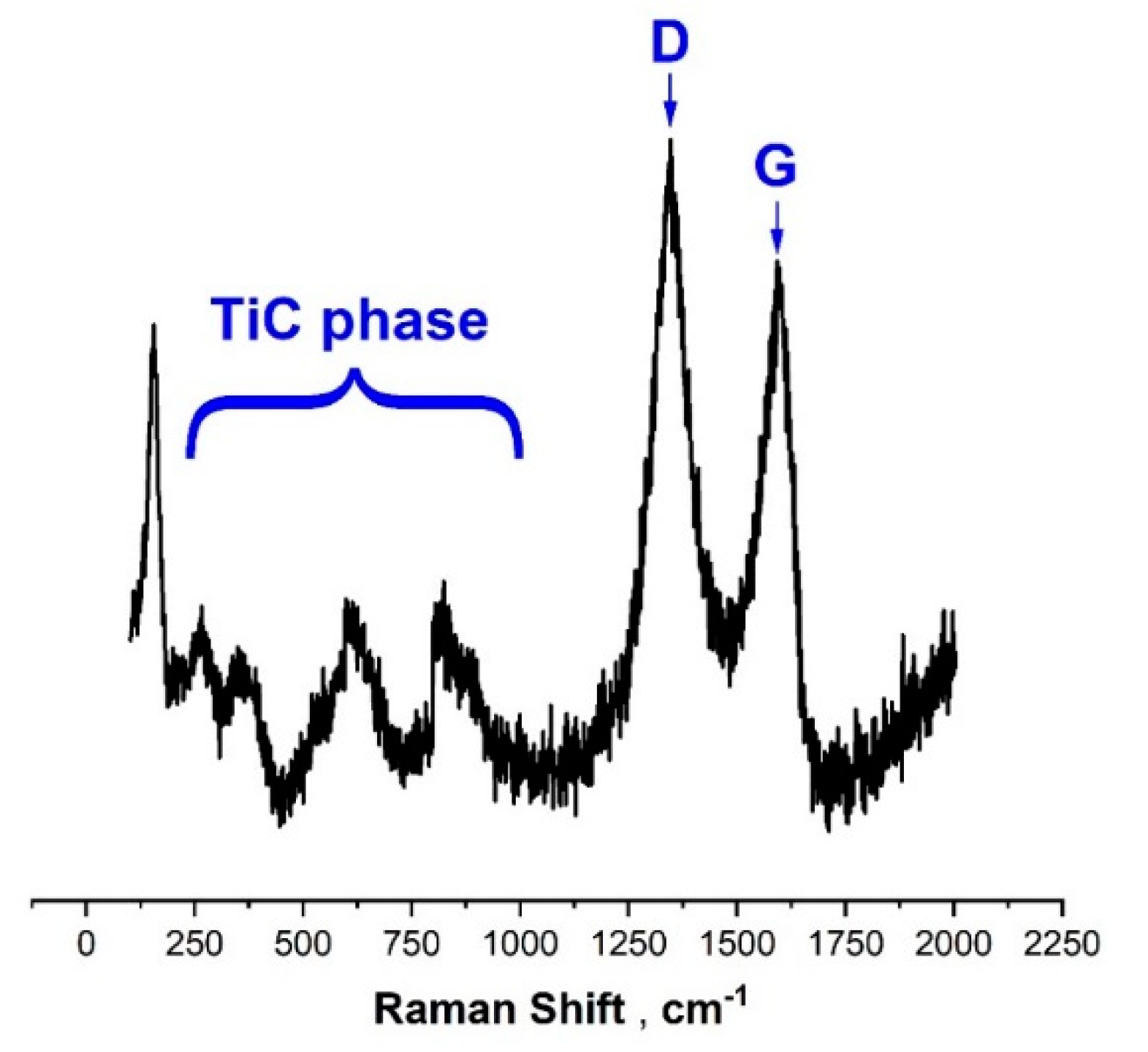
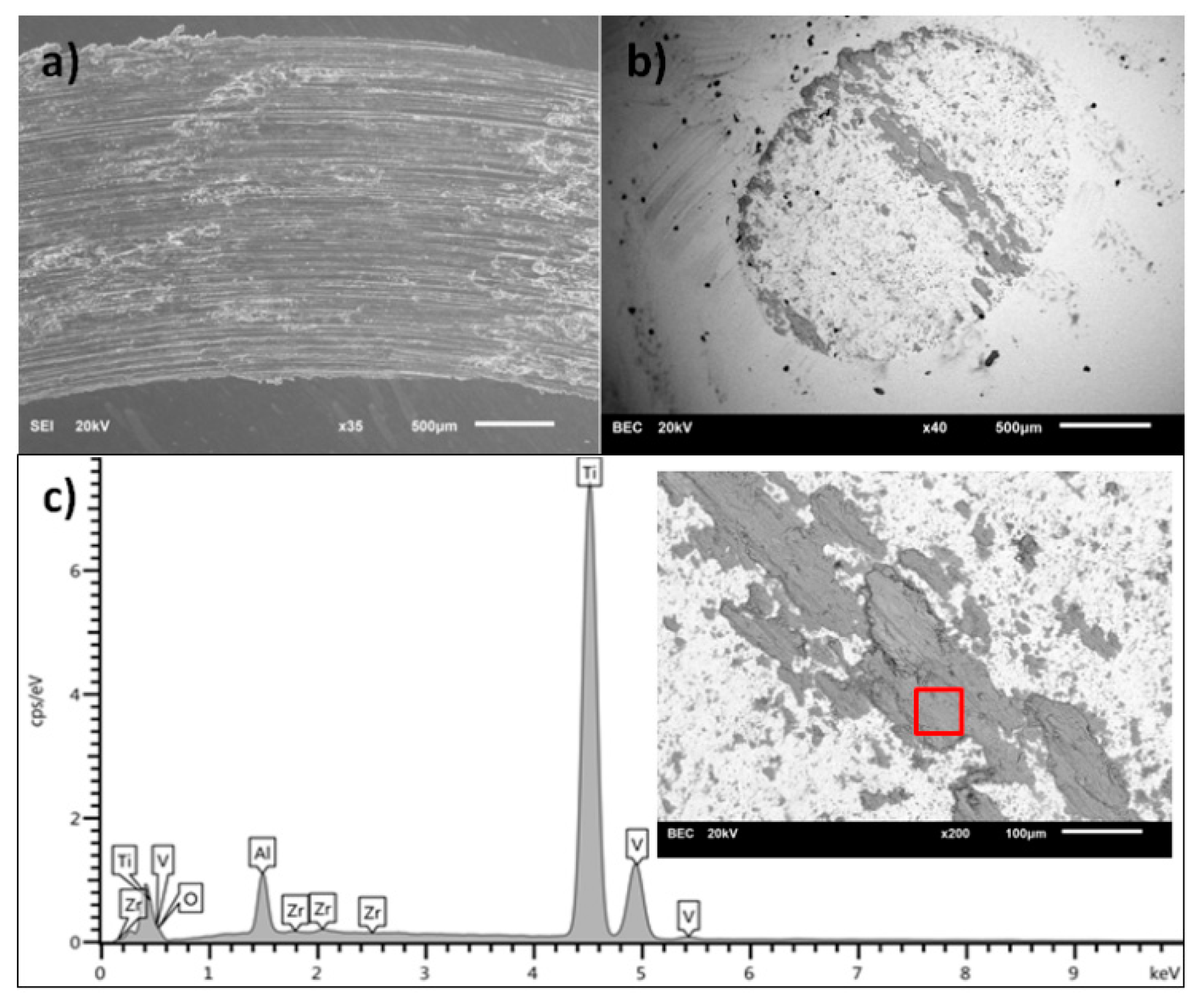
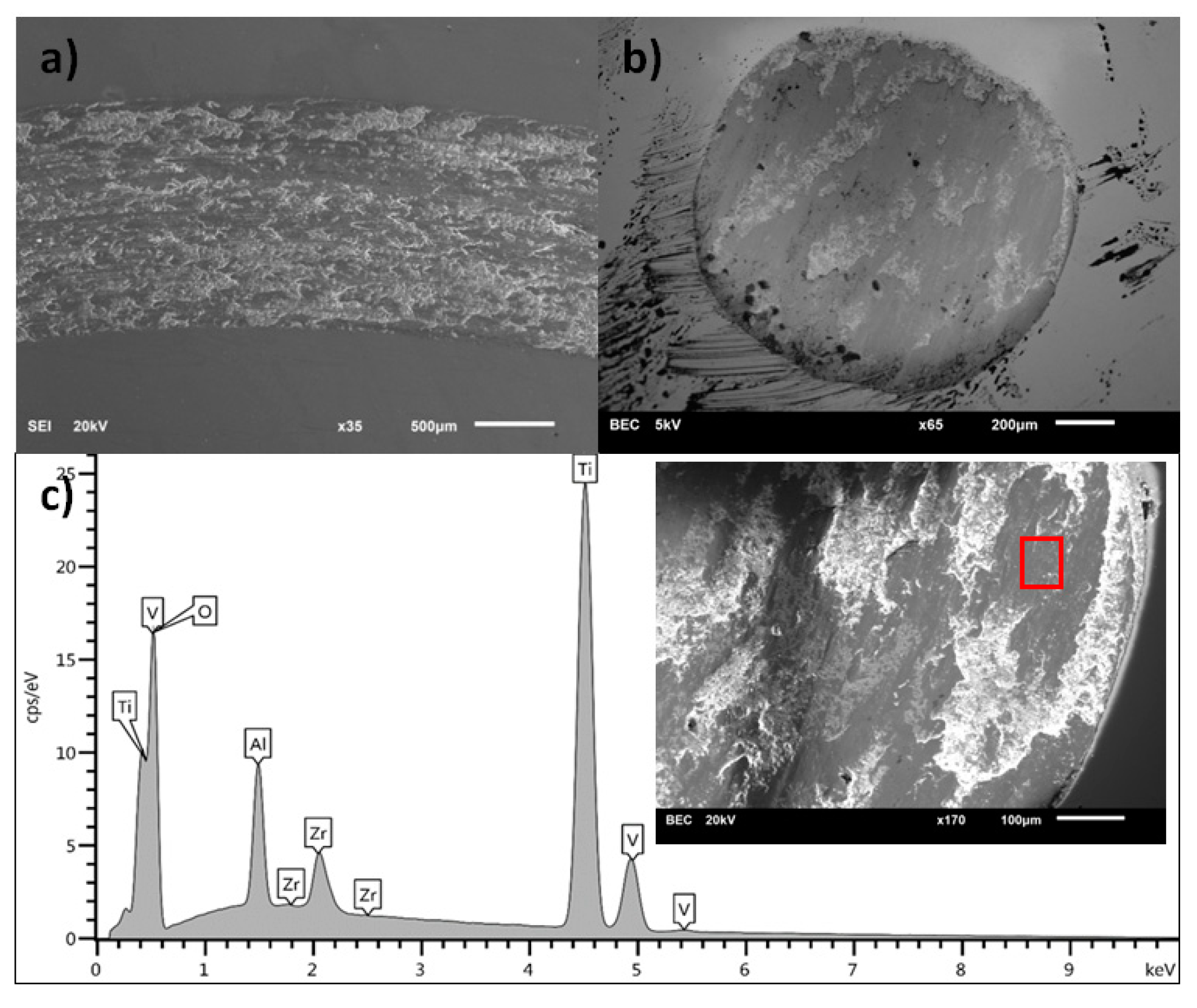
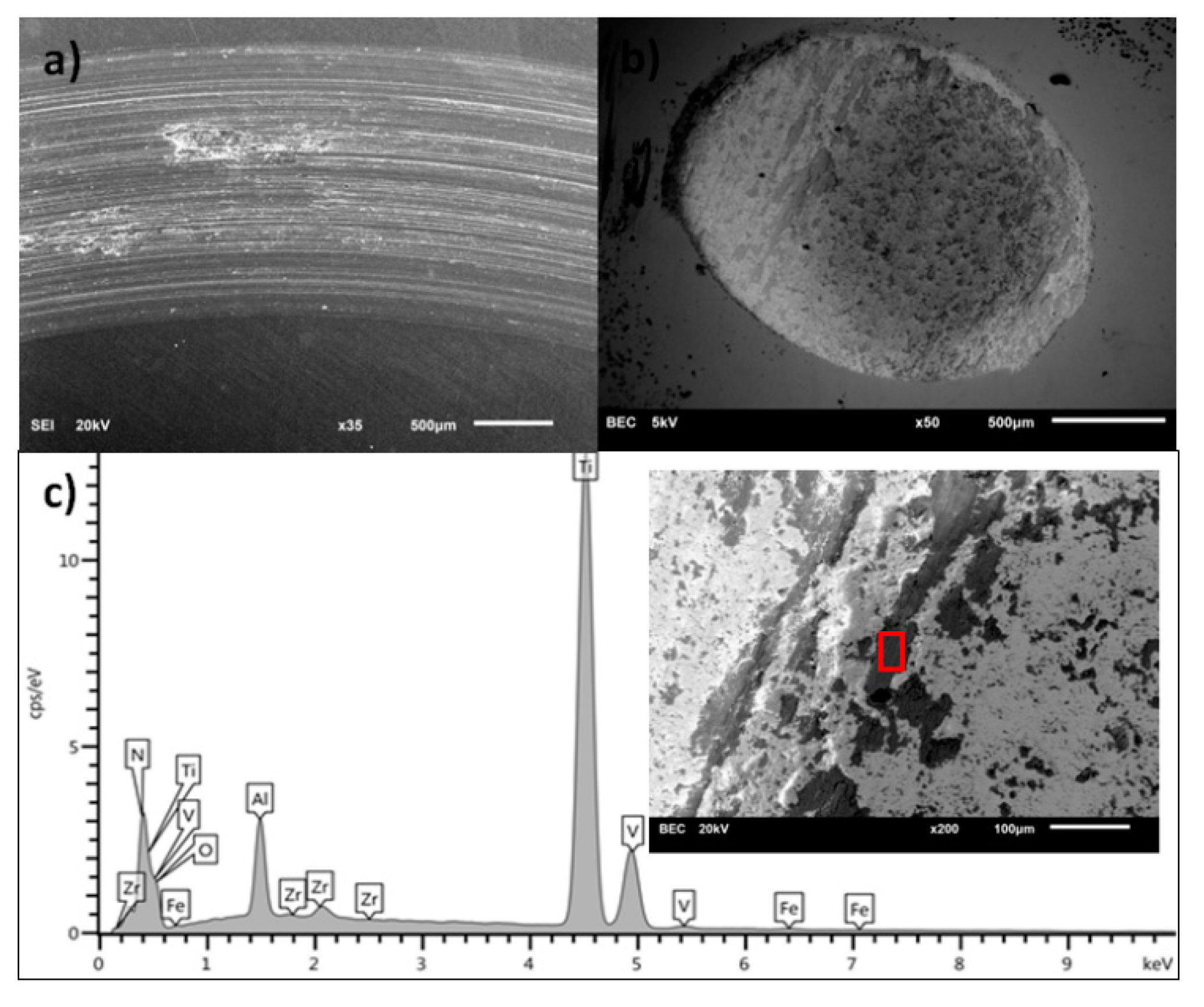
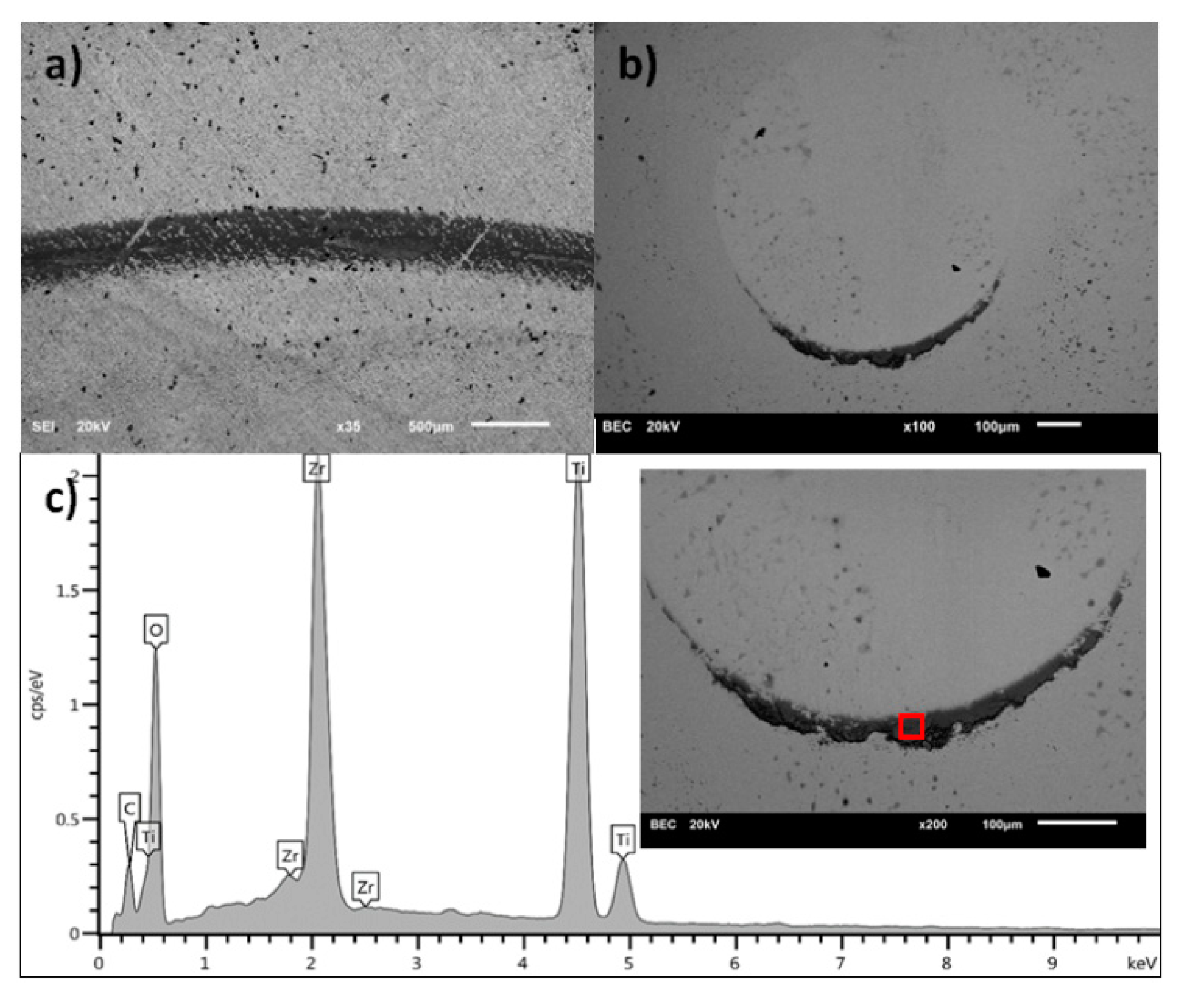
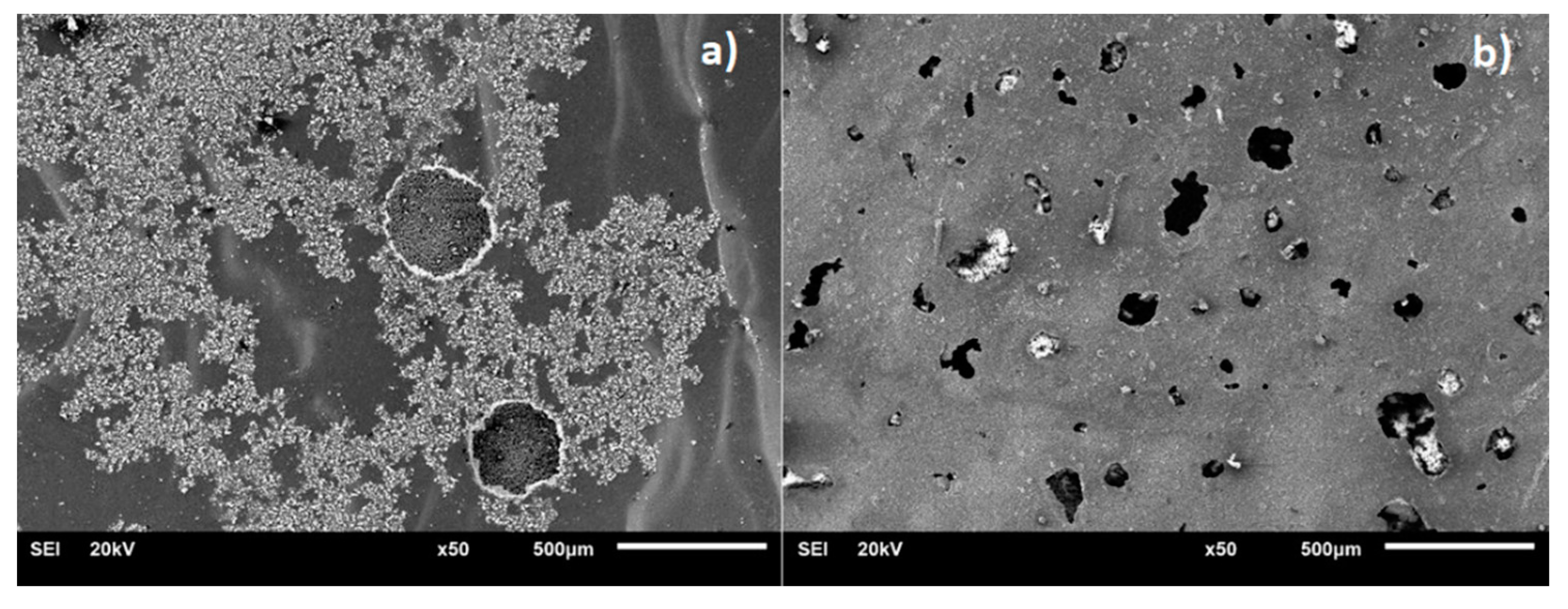
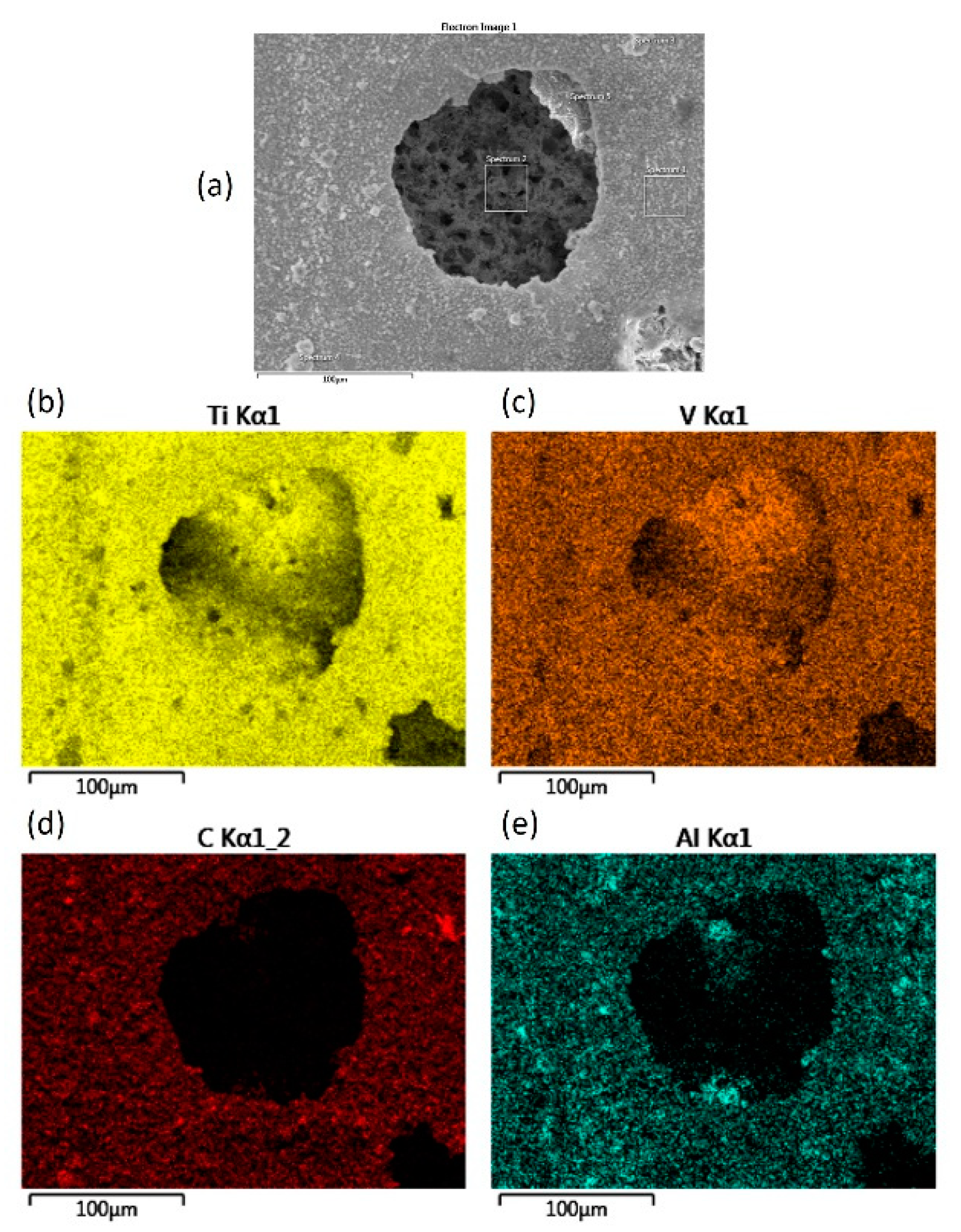
3.1. Scanning Electron Microscopy
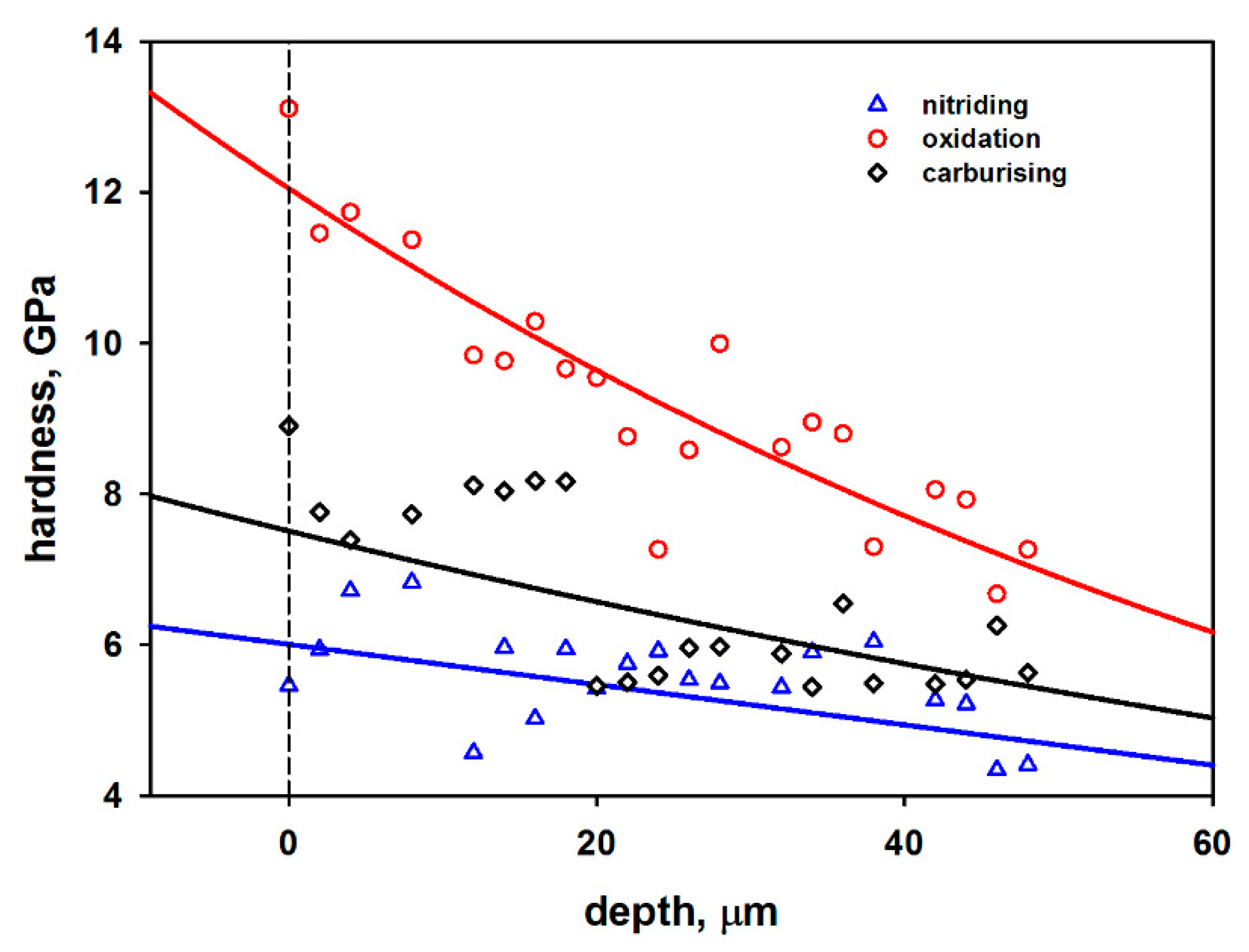
3.2. Hardness
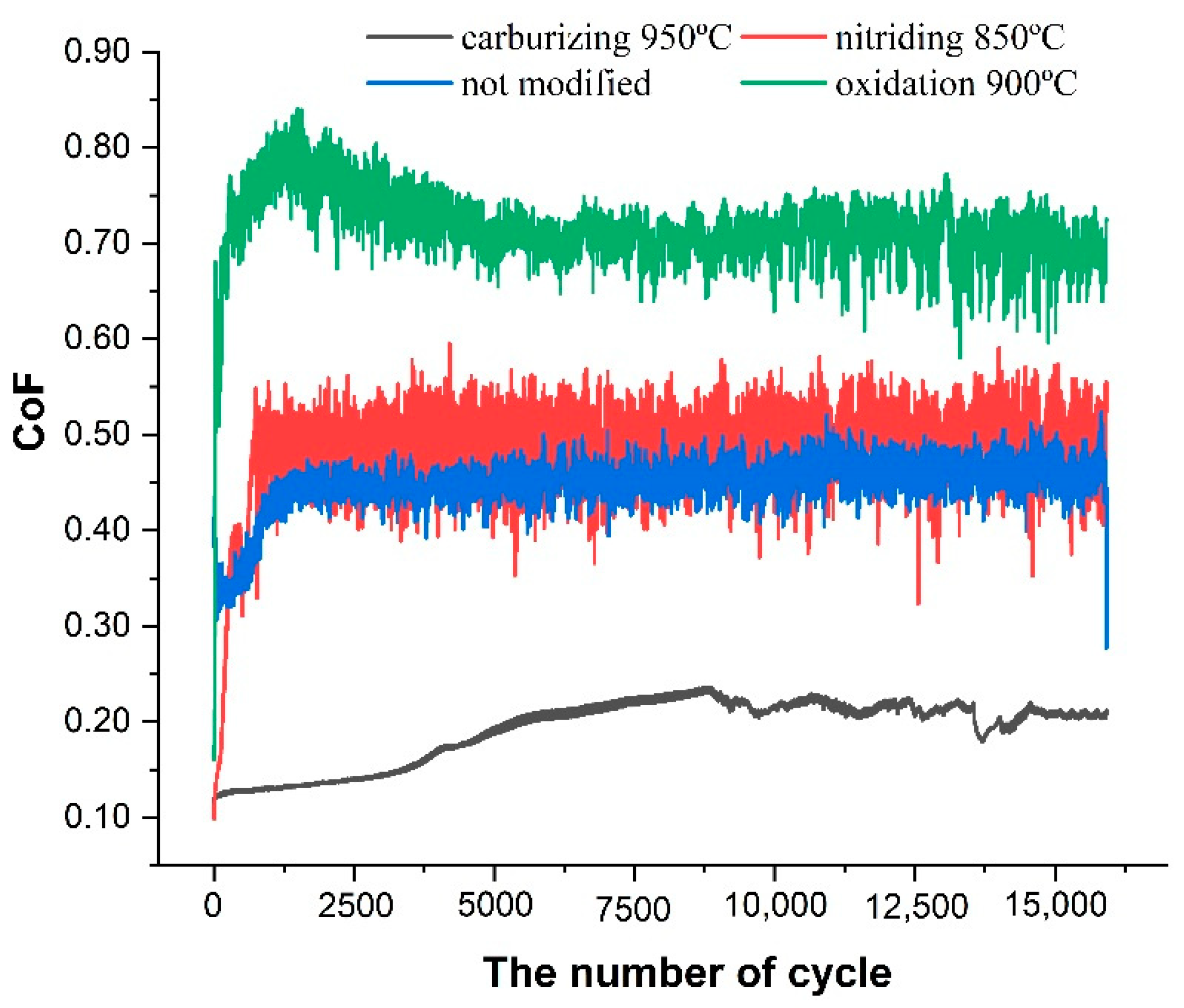
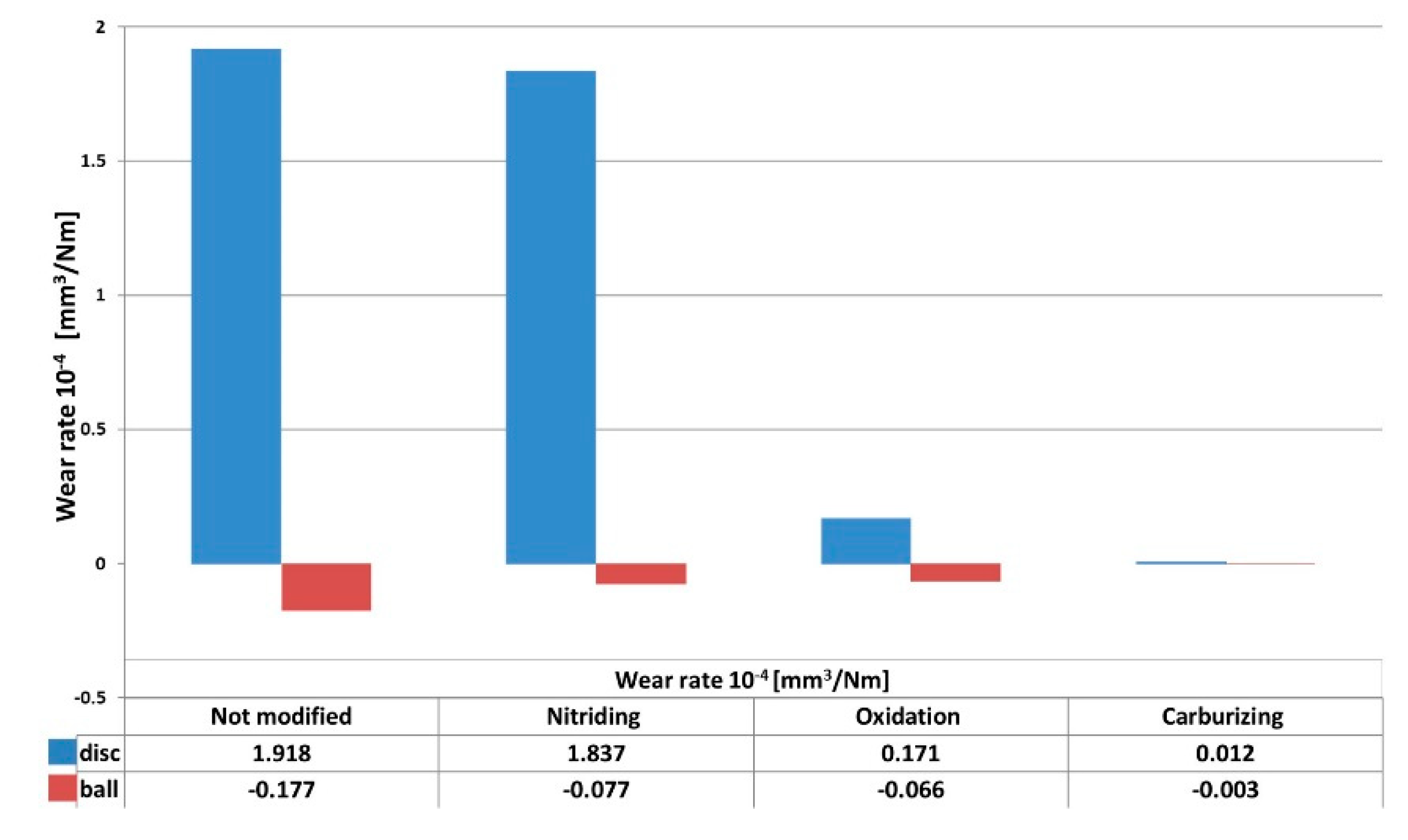
3.3. Coefficient of Friction and Wear Rate
3.4. Corrosion Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cimenoglu, H.; Gunyuz, M.; Torun Kose, G.; Baydogan, M.; Uğurlu, F.; Sener, C. Micro-arc oxidation of Ti6Al4V and Ti6Al7Nb alloys for biomedical applications. Mater. Charact. 2011, 62, 304–311. [Google Scholar] [CrossRef]
- Niinomi, M. Titanium Alloys. In Encyclopedia of Biomedical Engineering; Narayan, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 213–224. [Google Scholar]
- Kao, W.H.; Su, Y.L.; Horng, J.H.; Huang, H.C.; Yang, S.E. Improved tribological, electrochemical and biocompatibility properties of Ti6Al4V alloy by gas-nitriding and Ti–C:H coating. Surf. Coat. Technol. 2015, 283, 70–79. [Google Scholar] [CrossRef]
- Brusa, E.; Sesana, R.; Osola, E. Numerical Modelling and testing of mechanical behavior of AM Titanium alloy bracket for aerospace applications. Procedia Struct. Integr. 2017, 5, 753–760. [Google Scholar] [CrossRef]
- Manam, N.S.; Harun, W.S.; Shri, D.N.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatybile metals for implants: A review. J. Alloys Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef]
- Dong, H. Tribological properties of titanium-based alloys. In Surface Engineering of Light Alloys; Woodhead Publishing Series in Metals and Surface Engineering: Cambridge, UK, 2010; pp. 58–80. [Google Scholar]
- Garbacz, H.; Wieciński, P.; Ossowski, M.; Ortore, G.; Wierzchoń, T.; Kurzydłowski, K.J. Surface engineering techniques used for improving the mechanical and tribological properties of the Ti6A14V alloy. Surf. Coat. Technol. 2008, 202, 2453–2457. [Google Scholar] [CrossRef]
- Song, W.; Zhenhua, L.; Yuhong, L.; Weiqiang, L. Influence of thermal oxidation temperature on the microstructural and tribological behavior of Ti6Al4V alloy. Surf. Coat. Technol. 2014, 240, 470–477. [Google Scholar]
- Yildiz, F.; Yetim, A.F.; Alsaran, A.; Çelik, A. Plasma nitriding behavior of Ti6Al4V orthopedic alloy. Surf. Coat. Technol. 2008, 202, 2471–2476. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Vaz, F.; Ariza, E.; Rocha, L.A.; Ribeiro, A.R.L.; Vieira, A.C.; Rivière, J.P.; Pichon, L. Tribocorrosion behaviour of plasma nitrided and plasma nitrided+oxidised Ti6Al4V alloy. Surf. Coat. Technol. 2006, 200, 6218–6224. [Google Scholar] [CrossRef]
- Wang, Z.X.; Wu, H.R.; Shan, X.L.; Lin, N.M.; He, Z.Y.; Liu, X.P. Microstructure and erosive wear behaviors of Ti6Al4V alloy treated by plasma Ni alloying. Appl. Surf. Sci. 2016, 388, 510–516. [Google Scholar] [CrossRef]
- Batory, D.; Szymanski, W.; Panjan, M.; Zabeida, O.; Klemberg-Sapieha, J.E. Plasma Nitriding of Ti6Al4V alloy for improved water erosion resistance. Wear 2017, 374-375, 120–127. [Google Scholar] [CrossRef]
- Bloyce, A.; Qi, P.Y.; Dong, H.; Bell, T. Surface modification of titanium alloys for combined improvements in corrosion and wear resistance. Surf. Coat. Technol. 1998, 107, 125–132. [Google Scholar] [CrossRef]
- Aniołek, K.; Kupka, M.; Barylski, A. Sliding wear resistance of oxide layers formed on a titanium surface during thermal oxidation. Wear 2016, 356-357, 23–29. [Google Scholar] [CrossRef]
- Zhecheva, A.; Sha, W.; Malinov, S.; Long, A. Enhancing the microstructure and properties of titanium alloys through nitriding and other surface engineering methods. Surf. Coat. Technol. 2005, 200, 2192–2207. [Google Scholar] [CrossRef]
- Pązik, B.; Grabarczyk, J.; Batory, D.; Kaczorowski, W.; Burnat, B.; Czerniak-Reczulska, M.; Makówka, M.; Niedzielski, P. Plasma oxidized Ti6Al4V and Ti6Al7Nb alloys for biomedical applications. Eng. Biomater. 2016, 19, 8–12. [Google Scholar]
- Moskalewicz, T.; Wendler, B.; Makówka, M.; Czyrska-Filemonowicz, A. Microstructural Characterization of Low-friction Carbon-based Nanocomposite Coatings. In Proceedings of the 10th Multinational Congress on Microscopy, Urbino, Italy, 4–7 September 2011; pp. 579–580. [Google Scholar]
- Luo, Y.; Jiang, H.; Cheng, G.; Liu, H. Effect of Carburization on the Mechanical Properties of Biomedical Grade Titanium Alloys. J. Bionic. Eng. 2011, 8, 86–89. [Google Scholar] [CrossRef]
- Januszewicz, B.; Kaczmarek, Ł.; Siniarski, D. Wear and fatigue resistance of vacuum carburized titanium substrates. Mater. Eng. 2007, 3–4, 640–642. [Google Scholar]
- Kaczmarek, Ł.; Januszewicz, B.; Siniarski, D.; Kula, P. Influence of Carburizing Gas Mixture Composition Atmosphere on the Surface Properties of Titanium Alloy-Ti6Al4V. Mater. Eng. 2007, 3–4, 647–649. [Google Scholar]
- Gwam, C.U.; Mistry, J.B.; Mohamed, N.S.; Thomas, M.; Bigart, K.C.; Mont, M.A.; Delanois, R.E. Current epidemiology of revision total hip arthroplasty in the United States: National inpatient sample 2009 to 2013. J. Arthroplast. 2017, 32, 2088–2092. [Google Scholar] [CrossRef]
- Love, C.A.; Cook, R.B.; Harvey, I.L.; Dearnley, P.A.; Wood, R.J.K. Diamond like carbon coatings for potential application in biological implants—A review. Tribol. Int. 2013, 63, 141–150. [Google Scholar] [CrossRef]
- Singh, R.K.; Tilbrook, M.T.; Xie, Z.H.; Bendavid, A.; Martin, P.J.; Munroe, P. Contact damage evolution in diamond like carbon coatings on ductile substrates. J. Mater. Res. 2008, 23, 27–36. [Google Scholar] [CrossRef]
- Grabarczyk, J.; Gaj, J.; Pazik, B.; Kaczorowski, W.; Januszewicz, B. Tribocorrosion behavior of Ti6Al4V alloy after thermo-chemical treatment and DLC deposition for biomedical applications. Tribol. Int. 2020, 153, 106560. [Google Scholar] [CrossRef]
- Pharr, G.M.; Oliver, W.C. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 613–617. [Google Scholar] [CrossRef]
- Hammood, A.S.; Thair, T.; Altawaly, H.D.; Parvin, N. Tribocorrosion Behaviour of Ti–6Al–4V Alloy in Biomedical Implants: Effects of Applied Load and Surface Roughness on Material Degradation. J. Bio-Tribo-Corros. 2019, 5, 85. [Google Scholar] [CrossRef]
- Raaif, M.F.; El-Hossary, F.M.; Negm, N.Z.; Khalil, S.M.; Schaaf, P. Surface treatment of Ti–6Al–4V alloy by rf plasma nitriding. J. Phys. Condens. Matter 2007, 19, 396003–396015. [Google Scholar] [CrossRef]
- Mahdipoor, M.S.; Kevorkov, D.; Jedrzejowski, P.; Medraj, M. Water droplet erosion behavior of gas nitrided Ti6Al4V. Surf. Coat. Technol. 2016, 292, 78–89. [Google Scholar] [CrossRef]
- Tamaki, M. The role of hydrogen in plasma nitriding: Hydrogen behavior in the titanium nitride layer. Plasmas Ions 2000, 3, 33–39. [Google Scholar] [CrossRef]
- Januszewicz, B.; Klimek, L. Nitriding of titanium and Ti6Al4V alloy in ammonia gas under low pressure. J. Mater. Sci. Technol. 2010, 26, 586–590. [Google Scholar] [CrossRef]
- Kang, J.; Wang, M.; Yue, W.; Fu, Z.; Zhu, L.; She, D.; Wang, C. Tribological Behavior of Titanium Alloy Treated by Nitriding and Surface Texturing Composite Technology. Materials 2019, 12, 301. [Google Scholar] [CrossRef]
- She, D.; Yue, W.; Fu, Z.; Wang, C.; Yang, X.; Liu, J. Effects of nitriding temperature on microstructures and vacuum tribological properties of plasma-nitrided titanium. Surf. Coat. Technol. 2015, 264, 32–40. [Google Scholar] [CrossRef]
- Jedrzejczak, A.; Kolodziejczyk, L.; Szymanski, W.; Piwonski, I.; Cichomski, M.; Kisielewska, A.; Dudek, M.; Batory, D. Friction and wear of a-C:H:SiOx coatings in combination with AISI 316L and ZrO2 counterbodies. Tribol. Int. 2017, 112, 155–162. [Google Scholar] [CrossRef]
- Wang, S.; Liao, Z.; Liu, Y.; Liua, W. Different tribological behaviors of titanium alloys modified by thermal oxidation and spraying diamond like carbon. Surf. Coat. Technol. 2014, 252, 64–73. [Google Scholar] [CrossRef]
- Pawlak, W.; Kubiak, K.J.; Wendler, B.; Mathia, T.G. Wear resistant multilayer nanocomposite WC1x/C coating on Ti–6Al–4V titanium alloy. Tribol. Int. 2015, 82, 400–406. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Wendler, B.; Zimowski, S.; Dubiel, B.; Czyrska-Filemonowicz, A. Microstructure, micro-mechanical and tribological properties of the nc-WC/a-C nanocomposite coatings magnetron sputtered on non-hardened and oxygen hardened Ti–6Al–4V alloy. Surf. Coat. Technol. 2010, 205, 2668–2677. [Google Scholar] [CrossRef]
- Feng, X.; Sun, M.; Ma, X.; Tang, G. Structure and tribological performance by nitrogen and oxygen plasma based ion implantation on Ti6Al4V alloy. Appl. Surf. Sci. 2011, 257, 9904–9908. [Google Scholar] [CrossRef]
- Ferrari, C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Kaczorowski, W.; Gajewski, K.; Szymanski, W.; Batory, D.; Wojciechowska, A.; Swiatek, L.; Gotszalk, T.; Niedzielski, P. Evaluation of mechanical properties of carbon coatings synthesised in radio frequency plasma on PDMS. Surf. Coat. Technol. 2018, 333, 220–228. [Google Scholar] [CrossRef]
- Zouina Ait Djafer, A.; Saoula, N.; Madaoui, N.; Zerizer, A. Deposition and characterization of titanium carbide thin films by magnetron sputtering using Ti and TiC targets. Appl. Surf. Sci. 2014, 312, 57–62. [Google Scholar] [CrossRef]
- Ajikumar, P.K.; Vijayakumar, M.; Kamruddin, M.; Kalavathi, S.; Kumar, N.; Ravindran, T.R.; Tyagi, A.K. Effect of reactive gas composition on the microstructure, growth mechanism and friction coefficient of TiC overlayers. Int. J. Refract. Met. Hard Mater. 2012, 31, 62–70. [Google Scholar] [CrossRef]
- Manhabosco, T.M.; Tamborim, S.M.; dos Santos, C.B.; Müller, I.L. Tribological, electrochemical and tribo-electrochemical characterization of bare and nitrided Ti6Al4V in simulated body fluid solution. Corros. Sci. 2011, 53, 1786–1793. [Google Scholar] [CrossRef]
- Batory, D.; Jedrzejczak, A.; Kaczorowski, W.; Kolodziejczyk, L.; Burnat, B. The effect of Si incorporation on the corrosion resistance of a-C:H:SiOx coatings. Diam. Relat. Mater. 2016, 67, 1–7. [Google Scholar] [CrossRef]
- Yi, Y.; Cho, P.; Al Zaabi, A.; Addad, Y.; Jang, C. Potentiodynamic polarization behaviour of AISI type 316 stainless steel in NaCl solution. Corros. Sci. 2013, 74, 92–97. [Google Scholar] [CrossRef]
- Al Ameri, M.; Yi, Y.; Cho, P.; Al Saadi, S.; Jang, C.; Beeley, P. Critical conditions for pit initiation and growth of austenitic stainless steels. Corros. Sci. 2015, 92, 209–216. [Google Scholar] [CrossRef]
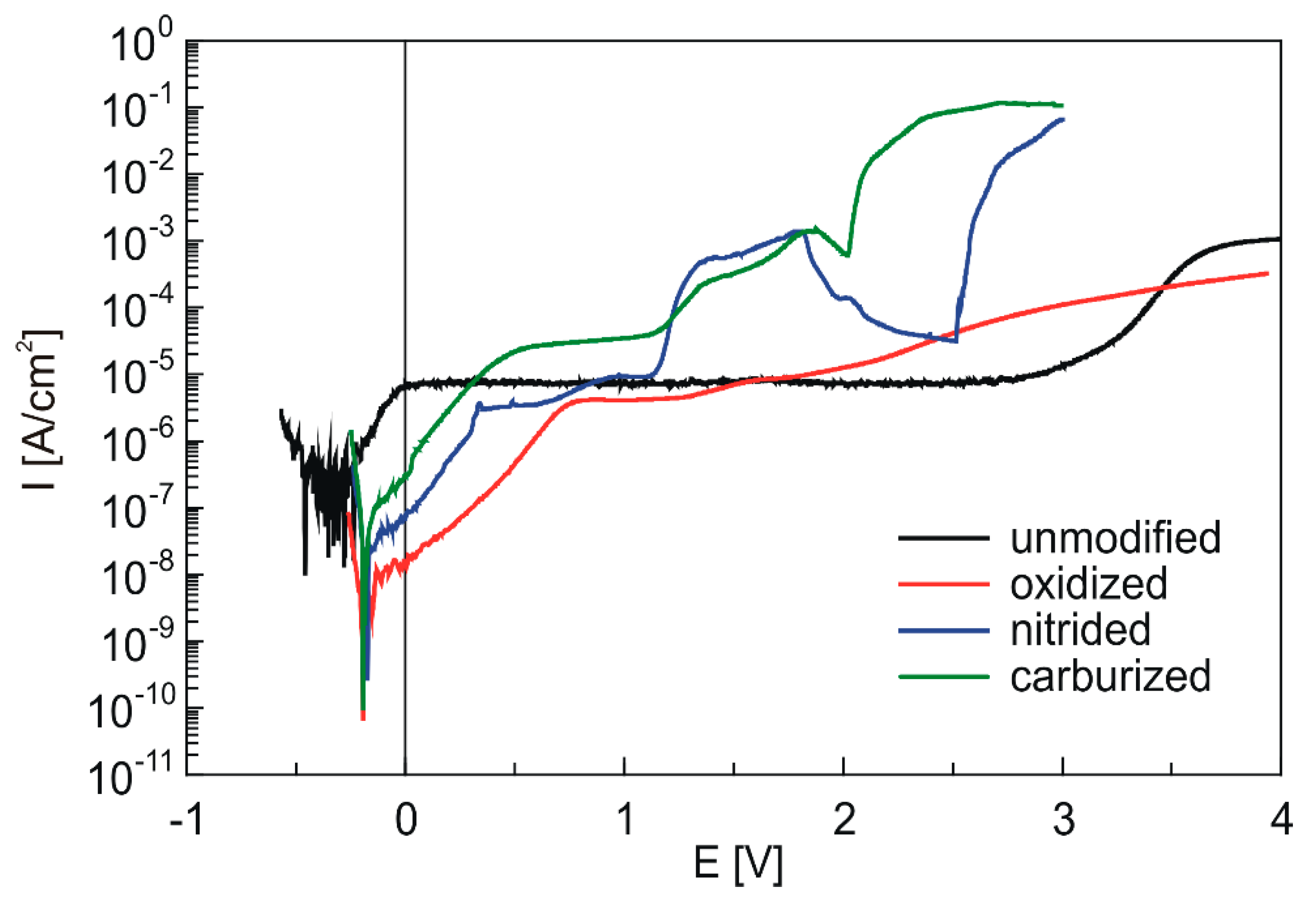
| Sample | Ecor [V] | Rp [MΩ·cm2] | icor [A/cm2] | CR [µm/year] |
|---|---|---|---|---|
| unmodified | −0.410 ± 0.039 | 0.46 ± 0.21 | (6.69 ± 3.22) × 10−8 | 0.59 ± 0.28 |
| oxidized | −0.049 ± 0.021 | 1.25 ± 0.20 | (2.11 ± 0.31) × 10−8 | 0.19 ± 0.03 |
| nitrided | −0.075 ± 0.063 | 0.41 ± 0.05 | (6.49 ± 0.87) × 10−8 | 0.57 ± 0.08 |
| carburized | −0.045 ± 0.006 | 0.14 ± 0.01 | (1.85 ± 0.03) × 10−7 | 1.62 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabarczyk, J.; Batory, D.; Kaczorowski, W.; Pązik, B.; Januszewicz, B.; Burnat, B.; Czerniak-Reczulska, M.; Makówka, M.; Niedzielski, P. Comparison of Different Thermo-Chemical Treatments Methods of Ti-6Al-4V Alloy in Terms of Tribological and Corrosion Properties. Materials 2020, 13, 5192. https://doi.org/10.3390/ma13225192
Grabarczyk J, Batory D, Kaczorowski W, Pązik B, Januszewicz B, Burnat B, Czerniak-Reczulska M, Makówka M, Niedzielski P. Comparison of Different Thermo-Chemical Treatments Methods of Ti-6Al-4V Alloy in Terms of Tribological and Corrosion Properties. Materials. 2020; 13(22):5192. https://doi.org/10.3390/ma13225192
Chicago/Turabian StyleGrabarczyk, Jacek, Damian Batory, Witold Kaczorowski, Bartosz Pązik, Bartłomiej Januszewicz, Barbara Burnat, Małgorzata Czerniak-Reczulska, Marcin Makówka, and Piotr Niedzielski. 2020. "Comparison of Different Thermo-Chemical Treatments Methods of Ti-6Al-4V Alloy in Terms of Tribological and Corrosion Properties" Materials 13, no. 22: 5192. https://doi.org/10.3390/ma13225192
APA StyleGrabarczyk, J., Batory, D., Kaczorowski, W., Pązik, B., Januszewicz, B., Burnat, B., Czerniak-Reczulska, M., Makówka, M., & Niedzielski, P. (2020). Comparison of Different Thermo-Chemical Treatments Methods of Ti-6Al-4V Alloy in Terms of Tribological and Corrosion Properties. Materials, 13(22), 5192. https://doi.org/10.3390/ma13225192









