Novel Aggregation-Induced Emission Materials/Cadmium Sulfide Composite Photocatalyst for Efficient Hydrogen Evolution in Absence of Sacrificial Reagent
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
2.1.1. Synthesis of CdS on Fluorine Doped tin Oxide (FTO) Glass
2.1.2. Synthesis of AIE Materials (TPE-Ca)
2.1.3. Preparation of CdS/TPE-Ca Electrode
2.2. Characterization
3. Results and Discussions
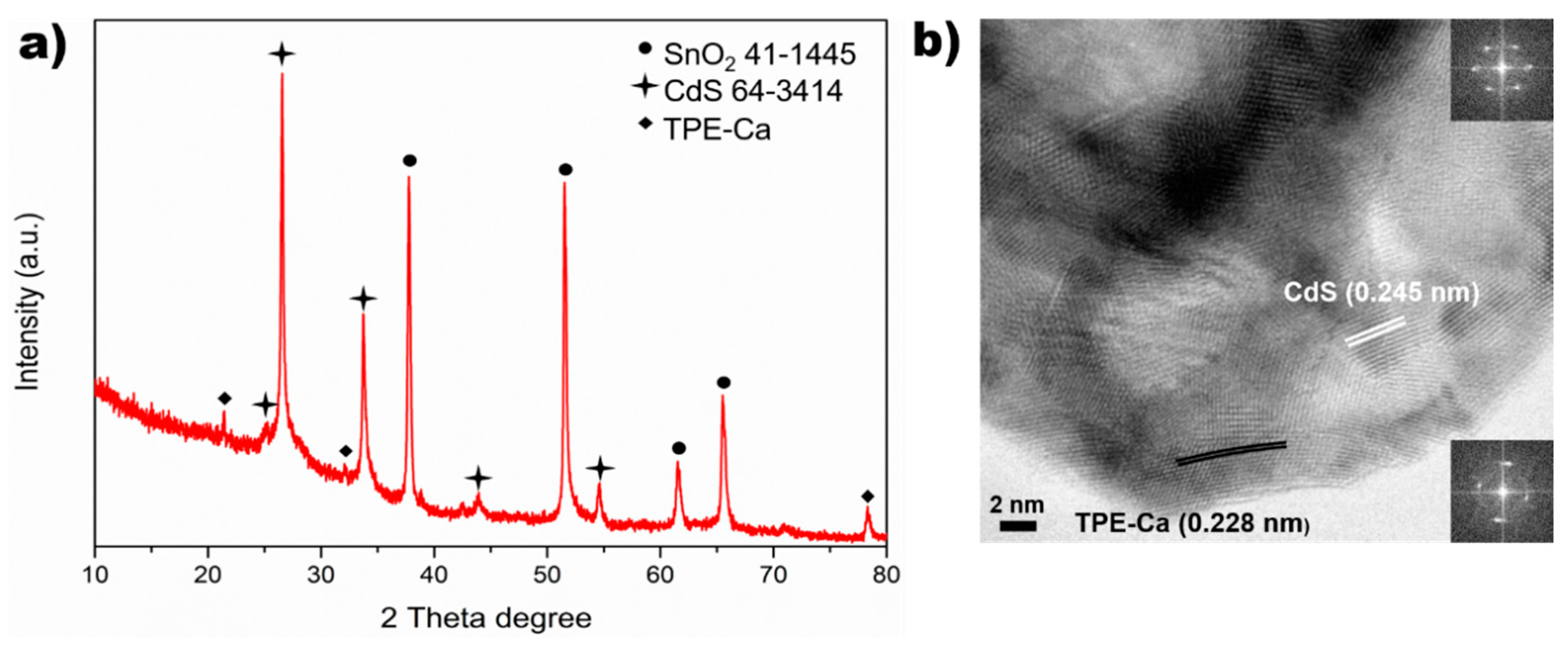
3.1. Crystal Structure of CdS/TPE-Ca
3.2. Optical Features
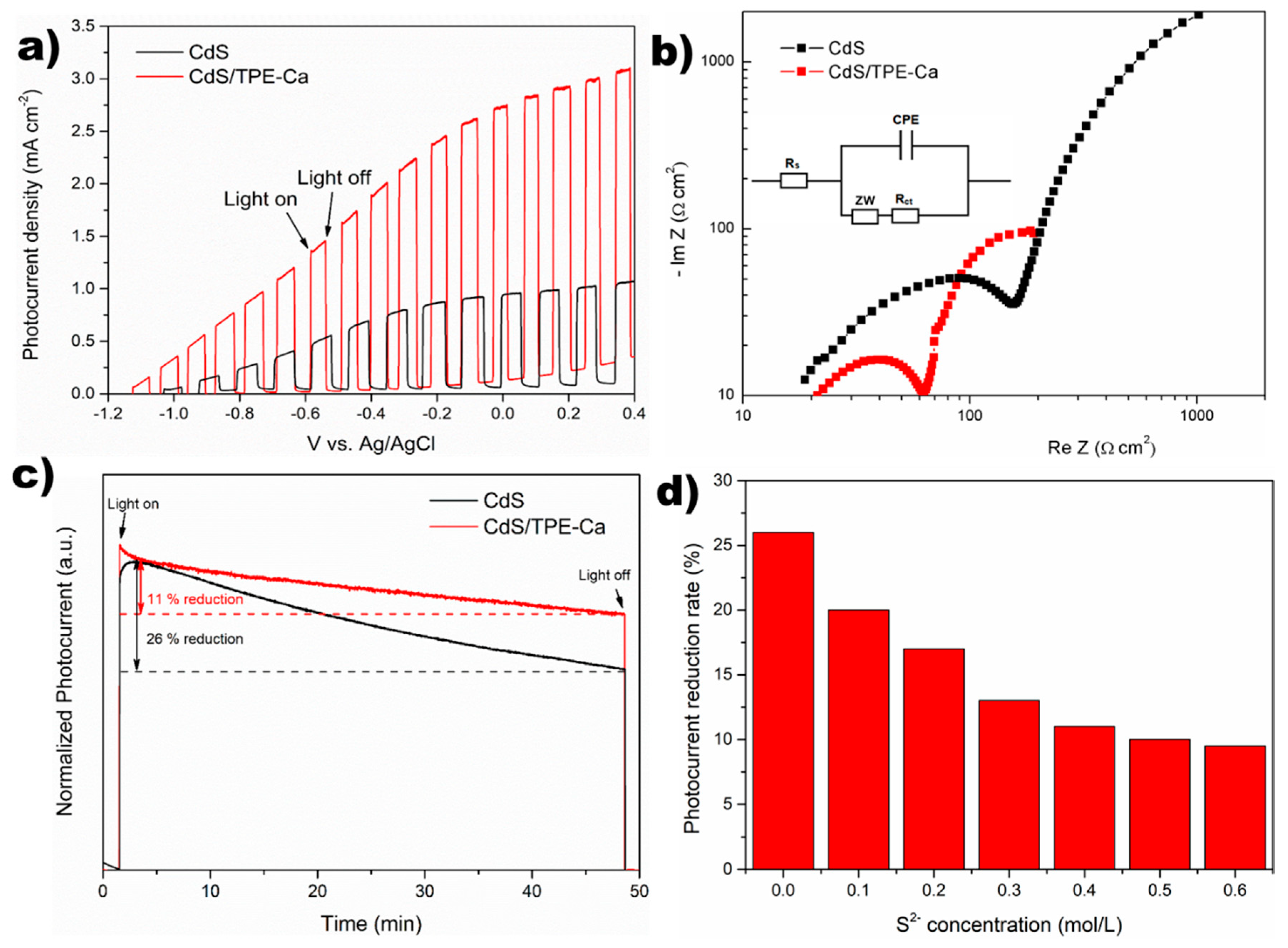
3.3. Photoelectrochemical Cell
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, Y.; Park, T.; Yi, J.W.; Henzie, J.; Kim, J.; Wang, Z.; Jiang, B.; Bando, Y.; Sugahara, Y.; Tang, J.; et al. Nanoarchitectonics for Transition-Metal-Sulfide-Based Electrocatalysts for Water Splitting. Adv. Mater. 2019, 31, e1807134. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, C.; Shen, J.; Zhong, Y.; Ning, J.; Hu, Y. One-step construction of a transition-metal surface decorated with metal sulfide nanoparticles: A high-efficiency electrocatalyst for hydrogen generation. J. Colloid Interface 2020, 558, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, X.; Zalnezhad, E.; Hui, K.S.; Ko, M.J. 3D hierarchical transition-metal sulfides deposited on MXene as binder-free electrode for high-performance supercapacitors. J. Ind. Eng. Chem. 2020, 82, 309–316. [Google Scholar] [CrossRef]
- Guo, D.; Song, X.; Tan, L.; Ma, H.; Pang, H.; Wang, X.; Zhang, L. Metal–Organic Framework Template-Directed Fabrication of Well-Aligned Pentagon-like Hollow Transition-Metal Sulfides as the Anode and Cathode for High-Performance Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 42621–42629. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Zhou, G.; Lv, W.; Ling, G.; Zhi, L.; Yang, Q.H. Catalytic Effects in Lithium-Sulfur Batteries: Promoted Sulfur Transformation and Reduced Shuttle Effect. Adv. Sci. 2017, 5, 1700270. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Singh, G.; Cha, W.; Kim, S.; Yi, J.; Hwang, S.-J.; Vinu, A. Recent Advances in Developing Hybrid Materials for Sodium-Ion Battery Anodes. ACS Energy Lett. 2020, 5, 1939–1966. [Google Scholar] [CrossRef]
- Li, C.; Cao, Q.; Wang, F.; Xiao, Y.; Li, Y.; Delaunay, J.-J.; Zhu, H. Engineering graphene and TMDs based van der Waals heterostructures for photovoltaic and photoelectrochemical solar energy conversion. Chem. Soc. Rev. 2018, 47, 4981–5037. [Google Scholar] [CrossRef]
- Hao, H.; Lang, X. Metal Sulfide Photocatalysis: Visible-Light-Induced Organic Transformations. ChemCatChem 2019, 11, 1378–1393. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Li, L.; Lv, C.; Zatovsky, I.V.; Han, W. Metal Sulfides@Carbon Microfiber Networks for Boosting Lithium Ion/Sodium Ion Storage via a General Metal–Aspergillus niger Bioleaching Strategy. ACS Appl. Mater. Interfaces 2019, 11, 8072–8080. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, D.; Zheng, D.; Wang, G.; Huang, X.; Harris, J.; Qu, D.; Qu, D. Dual carbon-protected metal sulfides and their application to sodium-ion battery anodes. J. Mater. Chem. A 2018, 6, 13294–13301. [Google Scholar] [CrossRef]
- Yadav, P.; Dwivedi, P.K.; Tonda, S.; Boukherroub, R.; Shelke, M.V. Metal and Non-metal Doped Metal Oxides and Sulfides. Pollut. Build. Water Living Org. 2019, 89–132. [Google Scholar] [CrossRef]
- Kisch, H.; Hetterich, W.; Twardzik, G. Heterogeneous Photocatalysis by Metal Sulfide Semiconductors. In Photochemistry and Photophysics of Coordination Compounds; Springer: Berlin, Germany, 1987; pp. 301–306. [Google Scholar]
- Mohammed, M.K.A. Studying the Structural, Morphological, Optical, and Electrical Properties of CdS/PbS Thin Films for Photovoltaic Applications. Plasmonics 2020, 15, 1989–1996. [Google Scholar] [CrossRef]
- Zhang, P.; Guan, B.Y.; Yu, L.; Lou, X.W. (David) Facile Synthesis of Multi-shelled ZnS-CdS Cages with Enhanced Photoelectrochemical Performance for Solar Energy Conversion. Chem 2018, 4, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-Y.; Jang, Y.J.; Park, J.; Kim, J.; Kang, J.S.; Chung, D.Y.; Sung, Y.-E.; Lee, C.; Lee, J.; Ko, M.J. Highly loaded PbS/Mn-doped CdS quantum dots for dual application in solar-to-electrical and solar-to-chemical energy conversion. Appl. Catal. B Environ. 2018, 227, 409–417. [Google Scholar] [CrossRef]
- Li, J.-Y.; Li, Y.-H.; Qi, M.-Y.; Lin, Q.; Tang, Z.-R.; Xu, Y.-J. Selective Organic Transformations over Cadmium Sulfide-Based Photocatalysts. ACS Catal. 2020, 10, 6262–6280. [Google Scholar] [CrossRef]
- Zheng, X.; Quan, H.; Chen, Z.; Shao, Y.; Zheng, X. New insight into an efficient visible light-driven photocatalytic organic transformation over CdS/TiO2 photocatalysts. Photochem. Photobiol. Sci. 2018, 17, 51–59. [Google Scholar] [CrossRef]
- Wu, H.; Kang, Z.; Zhang, Z.; Zhang, Z.; Si, H.; Liao, Q.; Zhang, S.; Wu, J.; Zhang, X.; Zhang, Y. Interfacial Charge Behavior Modulation in Perovskite Quantum Dot-Monolayer MoS2 0D-2D Mixed-Dimensional van der Waals Heterostructures. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Khan, U.A.; Liu, J.; Pan, J.; Ma, H.; Zuo, S.; Yu, Y.; Ahmad, A.; Ullah, S.; Li, B. Fabrication of Highly Efficient and Hierarchical CdS QDs/CQDs/H-TiO2 Ternary Heterojunction: Surpassable Photocatalysis under Sun-like Illumination. Ind. Eng. Chem. Res. 2018, 58, 79–91. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, S.; Zhang, X.; Liu, B.; Tang, N. Highly efficient visible-light-induced photoactivity of the CdS–Mn/MoS2/CdTe/TiO2 quaternary photocatalyst for label-free immunoassay of tris-(2,3-dibromopropyl) isocyanurate and enhanced solar hydrogen generation. Anal. Methods 2018, 10, 3462–3469. [Google Scholar] [CrossRef]
- Sharma, S.; Dutta, V.; Raizada, P.; Hosseini-Bandegharaei, A.; Singh, P.; Nguyen, V.-H. Tailoring cadmium sulfide-based photocatalytic nanomaterials for water decontamination: A review. Environ. Chem. Lett. 2020, 1–36. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, S.; Zhang, J.; Zhou, T.; Zhang, N.; Zheng, X.-S.; Chu, W.; Hu, Z.; Wu, C.; Xie, Y. Surface Nitrogen-Injection Engineering for High Formation Rate of CO2 Reduction to Formate. Nano Lett. 2020, 20, 6097–6103. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.M.; Takayama, T.; Sato, S.; Iwase, A.; Kudo, A.; Morikawa, T. Enhancement of CO2 reduction activity under visible light irradiation over Zn-based metal sulfides by combination with Ru-complex catalysts. Appl. Catal. B Environ. 2018, 224, 572–578. [Google Scholar] [CrossRef]
- John, J.; Lee, D.-K.; Sim, U. Photocatalytic and electrocatalytic approaches towards atmospheric nitrogen reduction to ammonia under ambient conditions. Nano Converg. 2019, 6, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.-Z.; Shang, X.; Dong, B.; Chai, Y.-M. Triple Ni-Co-Mo metal sulfides with one-dimensional and hierarchical nanostructures towards highly efficient hydrogen evolution reaction. J. Catal. 2018, 361, 204–213. [Google Scholar] [CrossRef]
- Landers, A.T.; Fields, M.; Torelli, D.A.; Xiao, J.; Hellstern, T.R.; Francis, S.A.; Tsai, C.; Kibsgaard, J.; Lewis, N.S.; Chan, K.; et al. The Predominance of Hydrogen Evolution on Transition Metal Sulfides and Phosphides under CO2 Reduction Conditions: An Experimental and Theoretical Study. ACS Energy Lett. 2018, 3, 1450–1457. [Google Scholar] [CrossRef] [Green Version]
- Aslan, E.; Sarilmaz, A.; Ozel, F.; Patir, I.H.; Girault, H.H. 1D Amorphous Tungsten-Based Ternary Refractory Metal Sulfides for Catalytic Hydrogen Evolution at Soft Interfaces. ChemNanoMat 2019, 5, 1461–1466. [Google Scholar] [CrossRef]
- Li, Y.; Niu, S.; Rakov, D.; Wang, Y.; Cabán-Acevedo, M.; Zheng, S.; Song, B.; Xu, P. Metal organic framework-derived CoPS/N-doped carbon for efficient electrocatalytic hydrogen evolution. Nanoscale 2018, 10, 7291–7297. [Google Scholar] [CrossRef]
- Yua, J.; Gao, X.; Cui, Z.; Jiao, Y.; Zhang, Q.; Dong, H.; Yu, L.; Dong, L. Facile Synthesis of Binary Transition Metal Sulfide Tubes Derived from NiCo-MOF-74 for High-Performance Supercapacitors. Energy Technol. 2019, 7. [Google Scholar] [CrossRef]
- Yi, M.; Zhang, C.; Cao, C.; Xu, C.; Sa, B.; Cai, D.; Zhan, H. MOF-Derived Hybrid Hollow Submicrospheres of Nitrogen-Doped Carbon-Encapsulated Bimetallic Ni–Co–S Nanoparticles for Supercapacitors and Lithium Ion Batteries. Inorg. Chem. 2019, 58, 3916–3924. [Google Scholar] [CrossRef]
- Nijamudheen, A.; Akimov, A.V. Criticality of Symmetry in Rational Design of Chalcogenide Perovskites. J. Phys. Chem. Lett. 2017, 9, 248–257. [Google Scholar] [CrossRef]
- Niu, S.; Milam-Guerrero, J.; Zhou, Y.; Ye, K.; Zhao, B.; Melot, B.C.; Ravichandran, J. Thermal stability study of transition metal perovskite sulfides. J. Mater. Res. 2018, 33, 4135–4143. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Liu, Y.; Zhang, L.; Li, H.; Liu, H.; Li, W. Transition metal-doped amorphous molybdenum sulfide/graphene ternary cocatalysts for excellent photocatalytic hydrogen evolution: Synergistic effect of transition metal and graphene. J. Colloid Interface Sci. 2019, 533, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Kim, K.M.; Choi, H.; Ali, G.; Chung, K.-Y.; Hong, Y.-R.; Choi, J.; Kwon, J.; Lee, S.W.; Lee, J.W.; et al. Parallelized Reaction Pathway and Stronger Internal Band Bending by Partial Oxidation of Metal Sulfide–Graphene Composites: Important Factors of Synergistic Oxygen Evolution Reaction Enhancement. ACS Catal. 2018, 8, 4091–4102. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, Y.; Zhu, G.; Li, B.; Gou, J.; Cheng, X. Synthesis of PbS/TiO2 nano-tubes photoelectrode and its enhanced visible light driven photocatalytic performance and mechanism for purification of 4-chlorobenzoic acid. Sep. Purif. Technol. 2019, 227, 115697. [Google Scholar] [CrossRef]
- Yang, T.; Huang, Y.; Yang, L.; Li, X.; Wang, X.; Zhang, G.; Luo, Y.; Jiang, J. Protecting Single Atom Catalysts with Graphene/Carbon-Nitride “Chainmail”. J. Phys. Chem. Lett. 2019, 10, 3129–3133. [Google Scholar] [CrossRef]
- Hu, Z.; Li, Y.; Kang, M.; Islam, M.; Chen, M.; Zhang, J.; Xiao, Y.; Feng, X.; Redshaw, C.; Zhang, M.; et al. Aggregation-induced emission luminogen: A new perspective in the photo-degradation of organic pollutants. EcoMat 2020, 2. [Google Scholar] [CrossRef]
- Tao, L.; Zhang, M. 3 Dimensional ordered macroporous FTO electrode decorated with CdxZn1−xS photocatalyst for improved photocurrent generation. Mater. Lett. 2018, 212, 307–310. [Google Scholar] [CrossRef]
- Yang, Z.; Qin, W.; Leung, N.L.C.; Arseneault, M.; Lam, J.W.Y.; Liang, G.; Sung, H.H.-Y.; Williams, I.D.; Tang, B.Z. A mechanistic study of AIE processes of TPE luminogens: Intramolecular rotation vs. configurational isomerization. J. Mater. Chem. C 2016, 4, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Ho, T.A.; Bae, C.; Joe, J.; Yang, H.; Kim, S.; Park, J.H.; Shin, H. Heterojunction Photoanode of Atomic-Layer-Deposited MoS2 on Single-Crystalline CdS Nanorod Arrays. ACS Appl. Mater. Interfaces 2019, 11, 37586–37594. [Google Scholar] [CrossRef]
- Jones, D.R.; Phillips, R.; Gannon, W.J.F.; Rome, B.; Warwick, M.E.A.; Dunnill, C.W. Photocapacitive CdS/WOx nanostructures for solar energy storage. Sci. Rep. 2019, 9, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, J.S.; Joshi, A.U.A.; Lee, J.S. Solvothermal Synthesis of CdS Nanowires for Photocatalytic Hydrogen and Electricity Production. J. Phys. Chem. C 2007, 111, 13280–13287. [Google Scholar] [CrossRef]
- Macdonald, J. Impedance Spectroscopy. Encycl. Phys. Sci. Technol. 2003, 20, 703–715. [Google Scholar] [CrossRef]
- Xu, Y.; Cai, H.; He, P.-G.; Fang, Y.-Z. Probing DNA Hybridization by Impedance Measurement Based on CdS-Oligonucleotide Nanoconjugates. Electroanalysis 2004, 16, 150–155. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, X.; Wang, K.; Tu, C.; Huang, R.; Luo, D.; Zhang, M. Novel Aggregation-Induced Emission Materials/Cadmium Sulfide Composite Photocatalyst for Efficient Hydrogen Evolution in Absence of Sacrificial Reagent. Materials 2020, 13, 5287. https://doi.org/10.3390/ma13225287
Ke X, Wang K, Tu C, Huang R, Luo D, Zhang M. Novel Aggregation-Induced Emission Materials/Cadmium Sulfide Composite Photocatalyst for Efficient Hydrogen Evolution in Absence of Sacrificial Reagent. Materials. 2020; 13(22):5287. https://doi.org/10.3390/ma13225287
Chicago/Turabian StyleKe, Xi, Kunqiang Wang, Chen Tu, Runda Huang, Dongxiang Luo, and Menglong Zhang. 2020. "Novel Aggregation-Induced Emission Materials/Cadmium Sulfide Composite Photocatalyst for Efficient Hydrogen Evolution in Absence of Sacrificial Reagent" Materials 13, no. 22: 5287. https://doi.org/10.3390/ma13225287



