Formation Mechanism of High-Purity Ti2AlN Powders under Microwave Sintering
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials and Methods
2.2. Powder Characterization
3. Results and Discussion
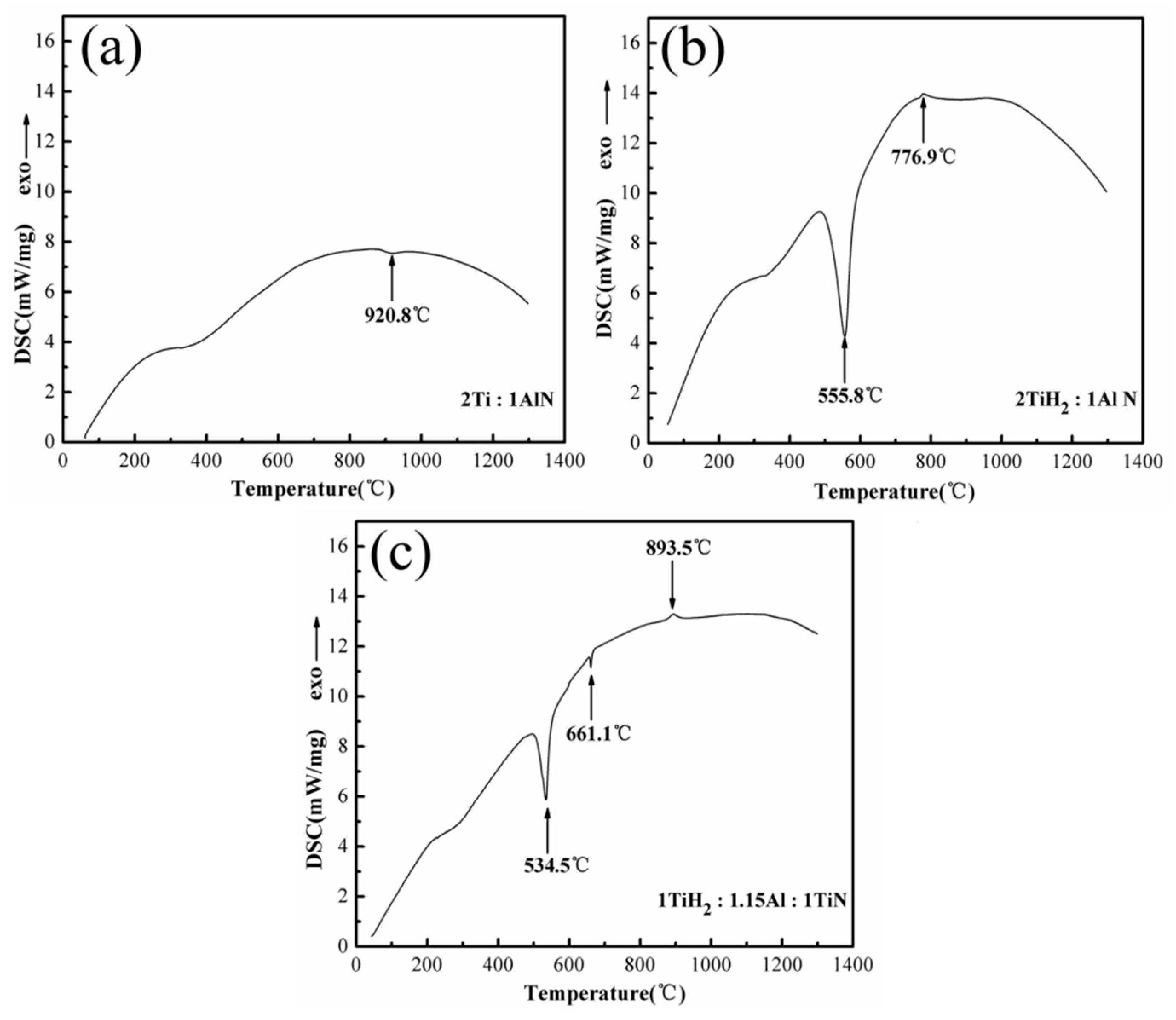
3.1. DSC Analysis
3.2. Factors Influencing the Synthesis of Ti2AlN Powders
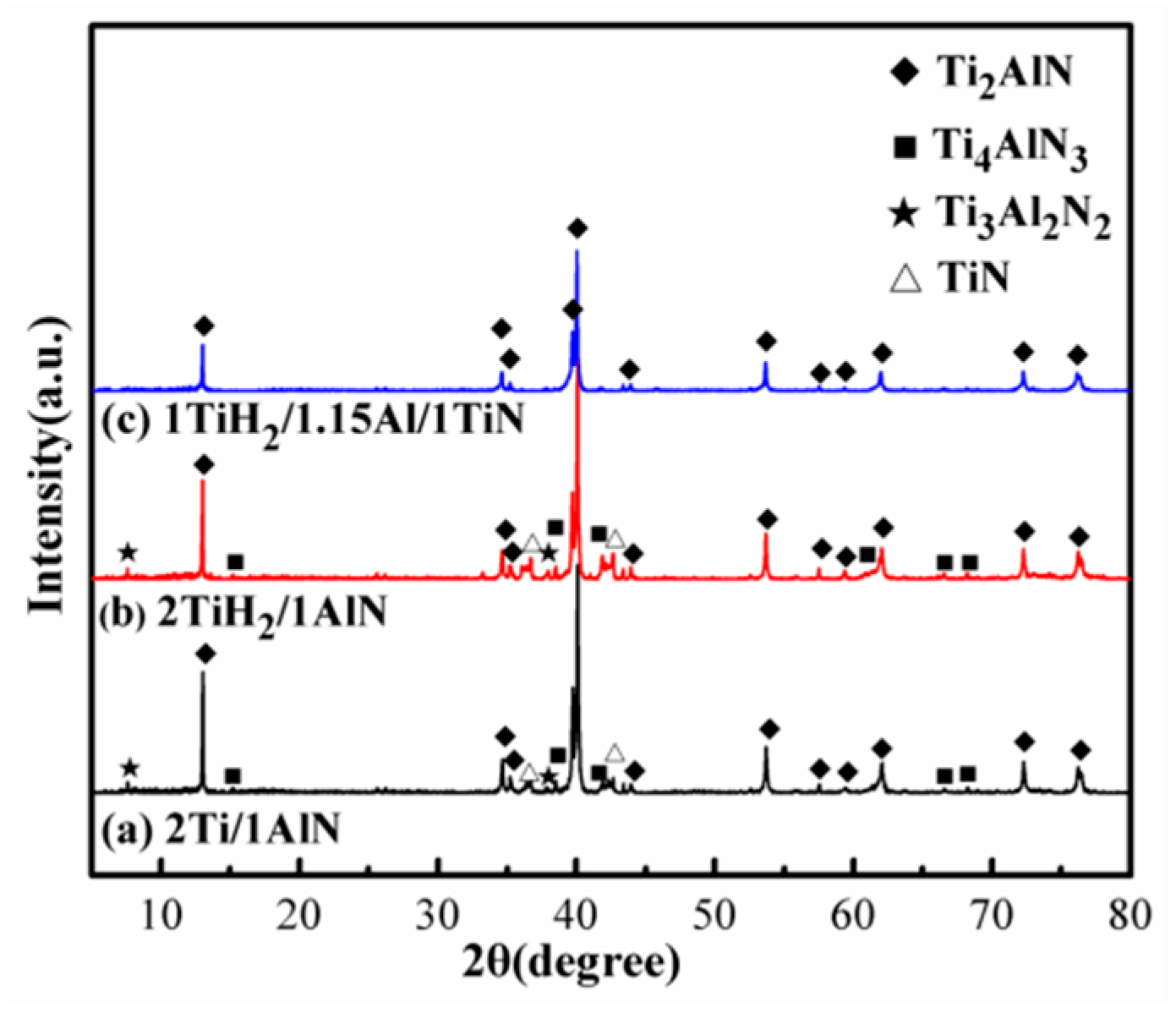
3.2.1. Raw Material Systems
3.2.2. Aluminum Content
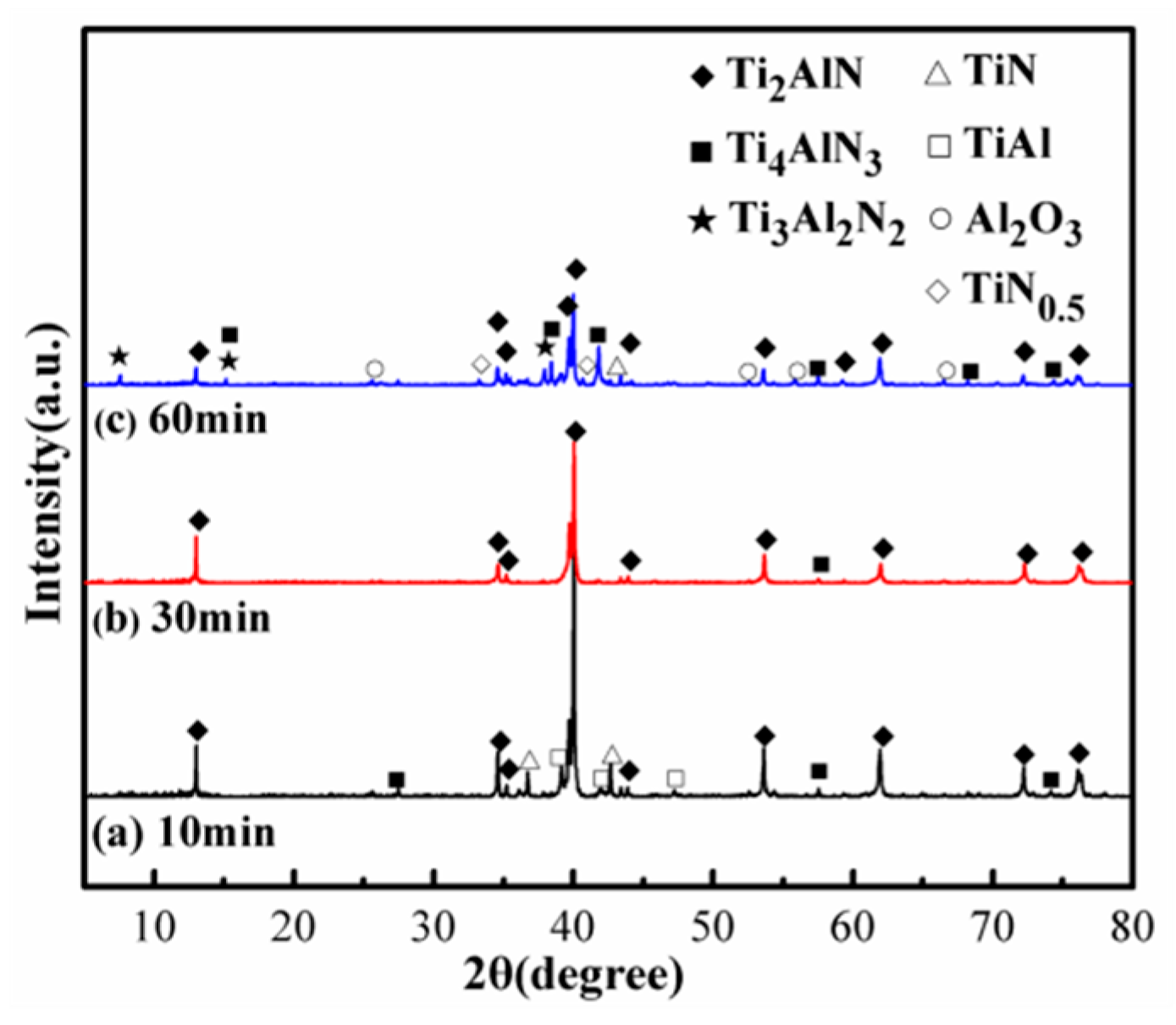
3.2.3. Holding Time
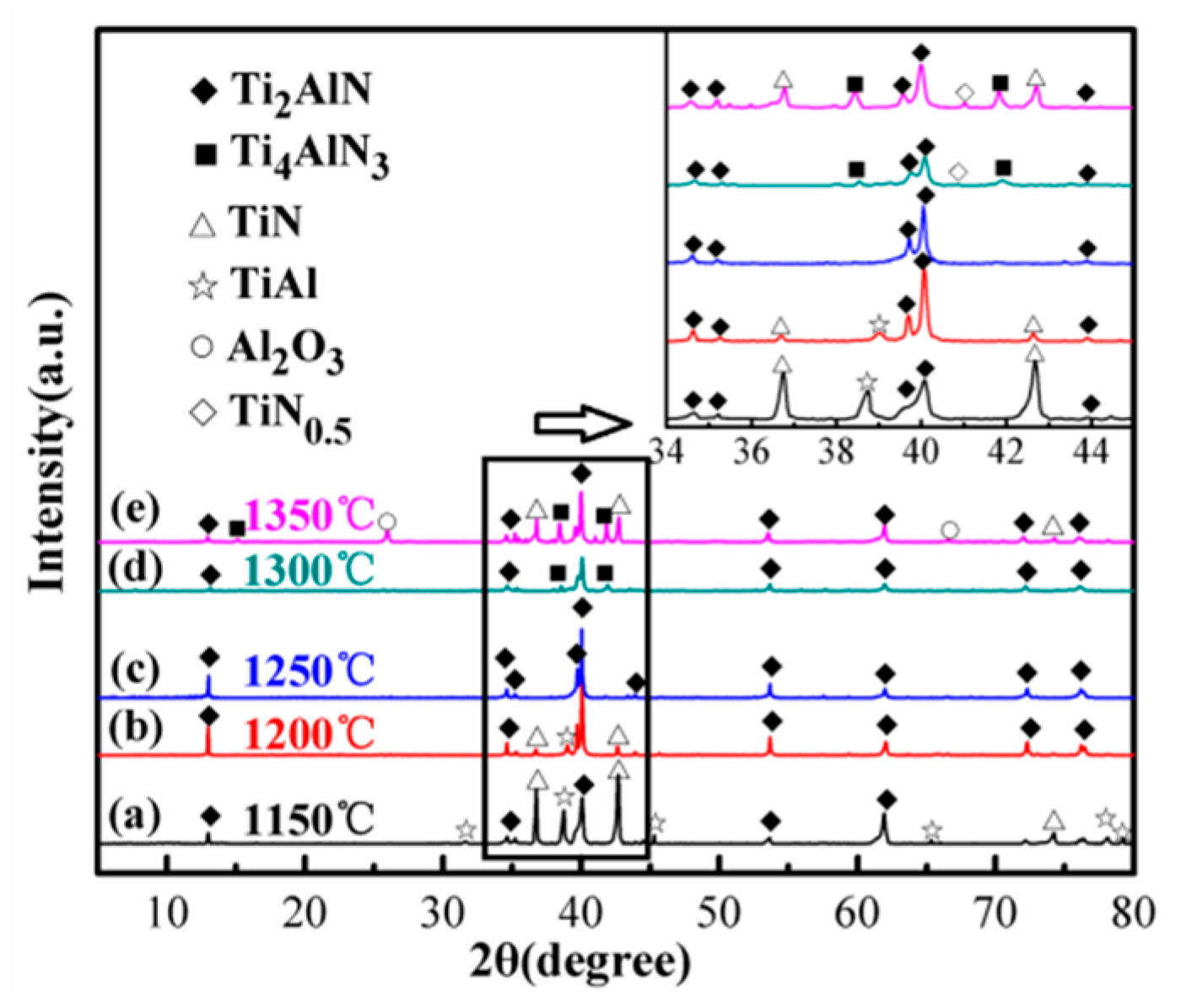
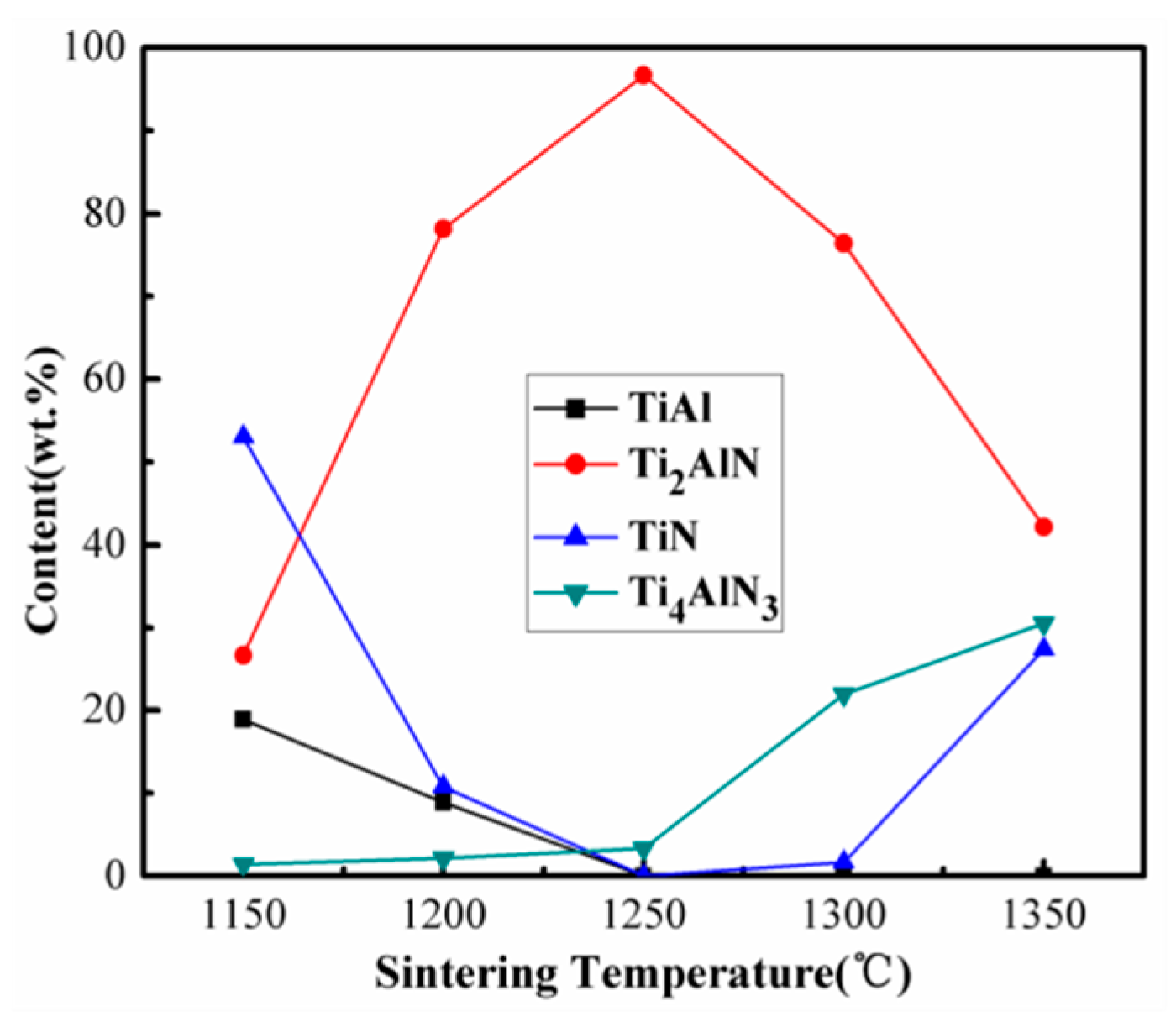
3.2.4. Sintering Temperature
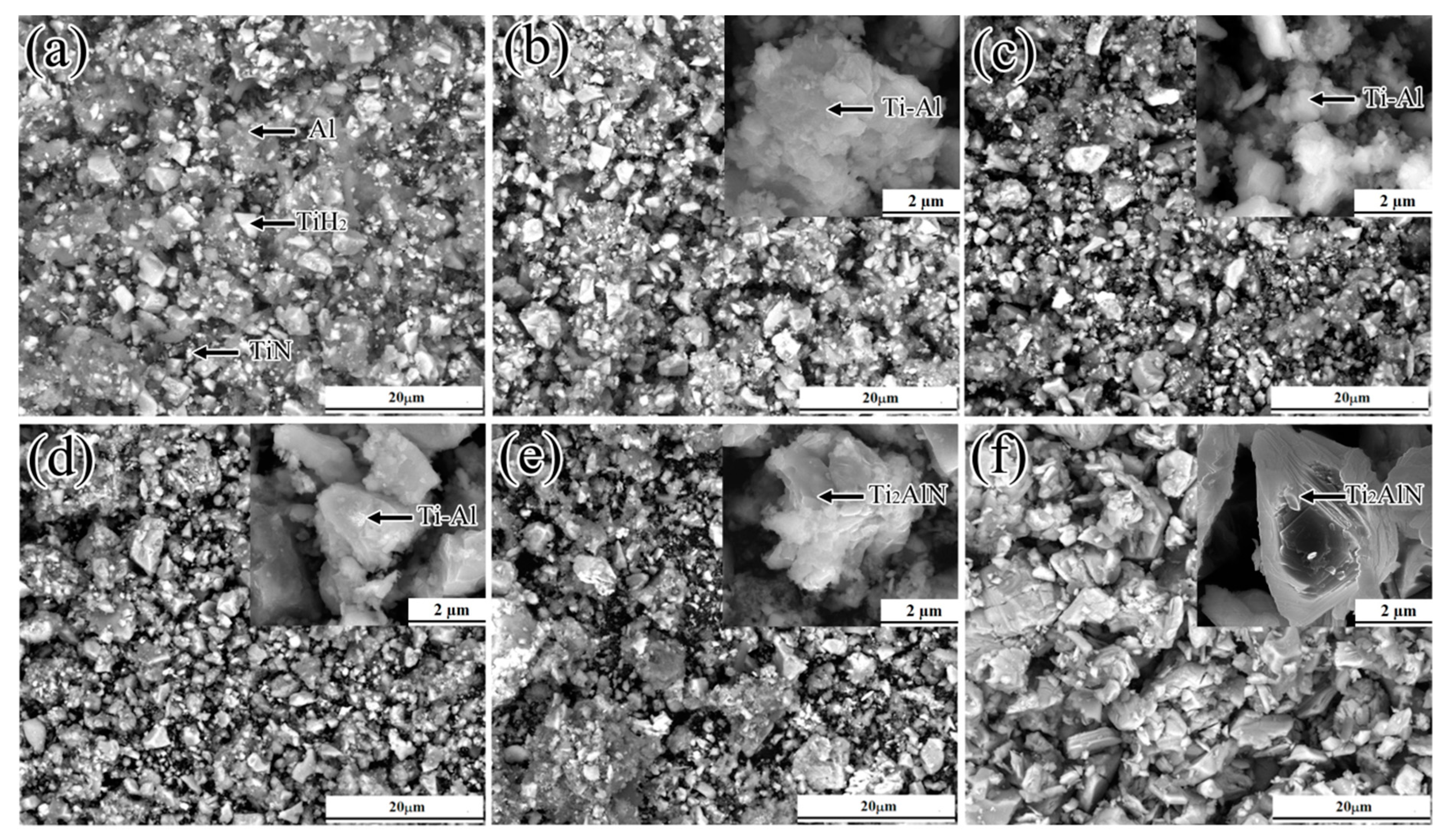
3.3. Morphology and Structure of Ti2AlN Powders
3.4. Reaction Mechanism for Ti2AlN Formation
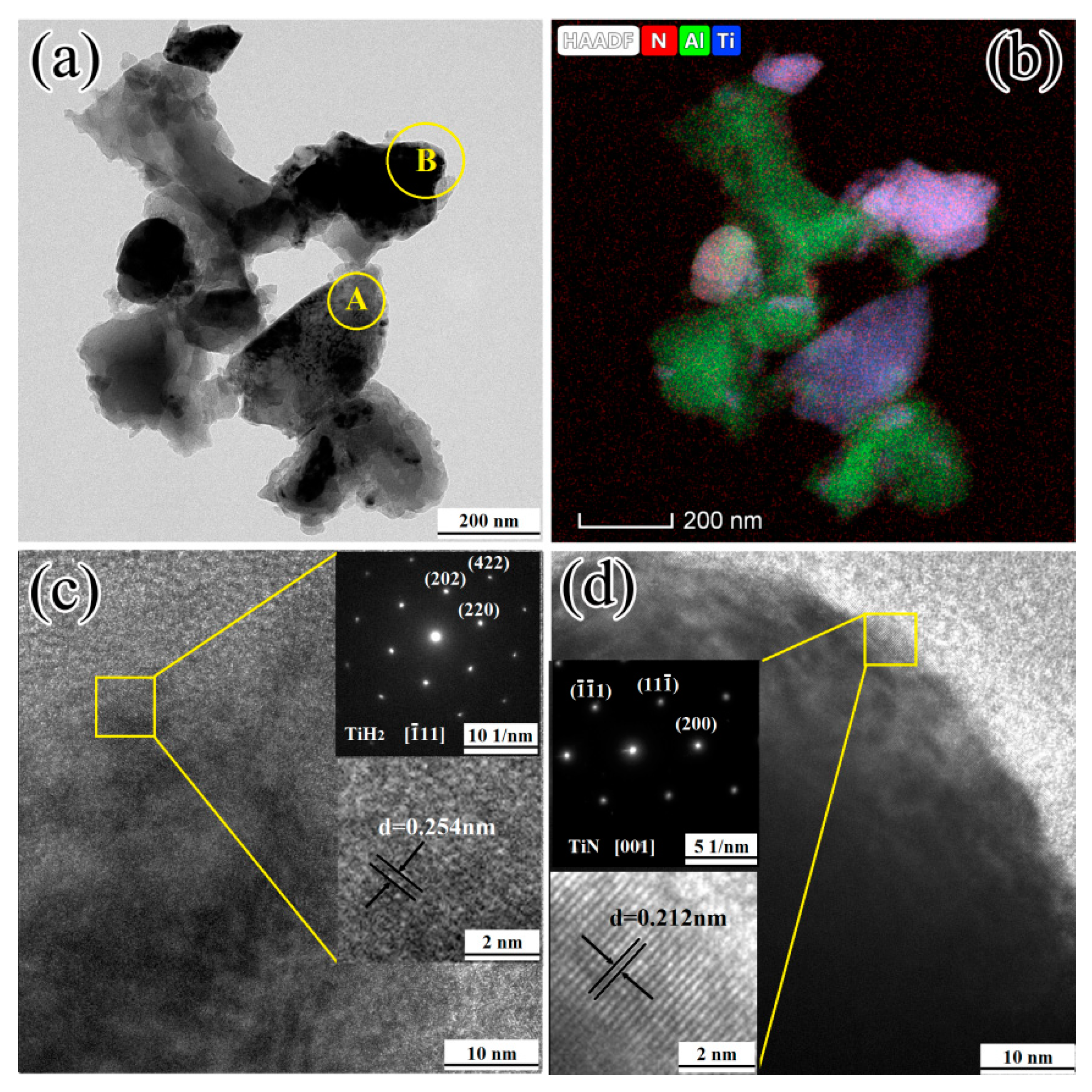
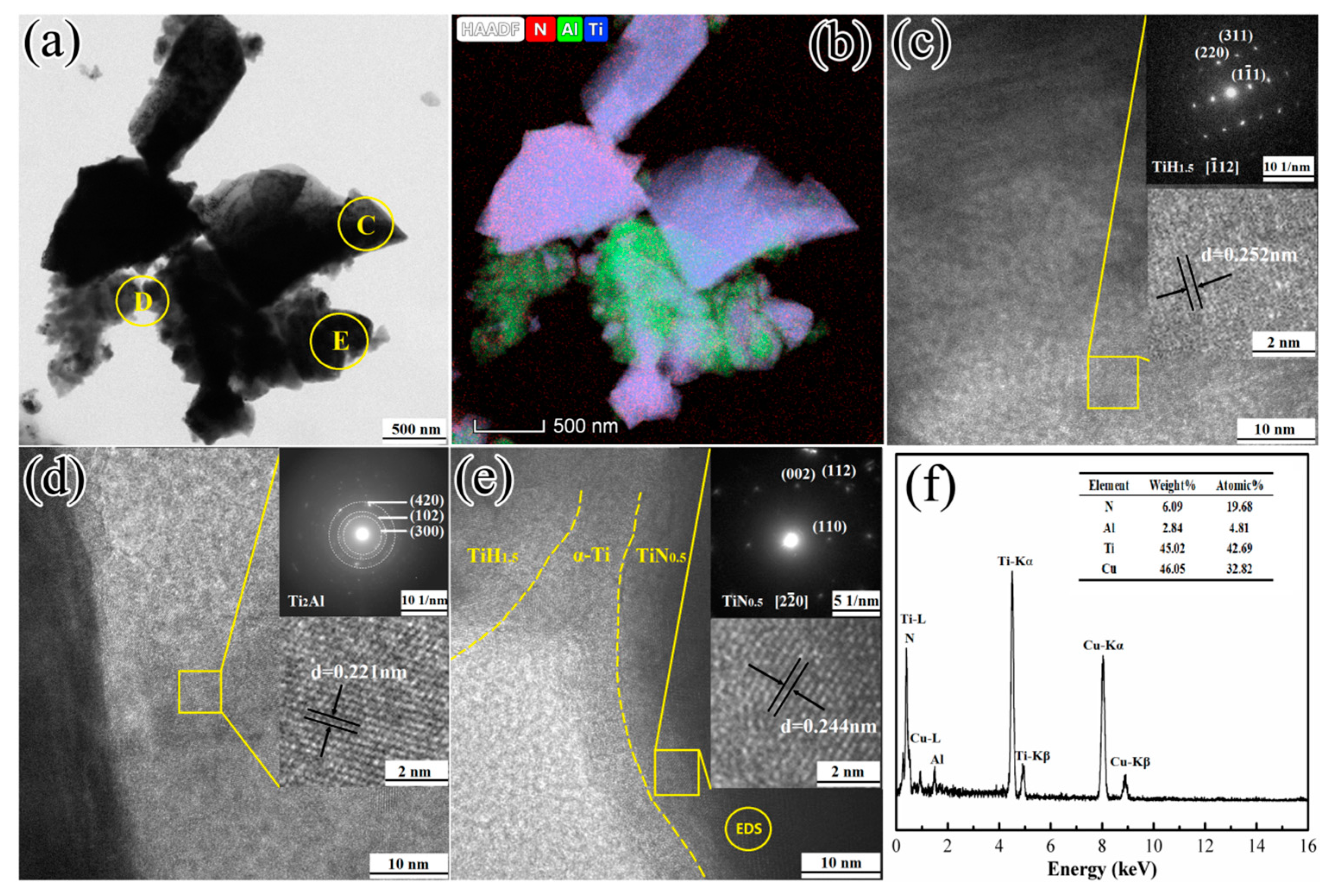
3.4.1. Reactions at Temperatures Lower than 660 °C
3.4.2. Reactions at 660–720 °C
3.4.3. Reactions at 720–1150 °C
3.4.4. Reactions at 1150–1350 °C
4. Conclusions
- (1)
- During microwave sintering, TiH2 was decomposed and formed active Ti atoms at temperatures lower than the melting point of Al. Subsequently, the Ti2Al and TiN0.5 phases were formed through the reactions of active Ti atoms with Al and TiN, respectively.
- (2)
- At temperatures higher than the melting point of Al, the liquid Al easily reacted with Ti, Ti2Al, and TiN0.5 to form TiAl2, TiAl, and Ti2AlN, respectively. Subsequently, TiAl2 and Ti2Al reacted with each other to form TiAl.
- (3)
- TiAl further reacted with TiN to form Ti2AlN. Although Ti2AlN was identified as a dominant synthesized product, Ti4AlN3 did not react completely in the sintering process.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Barsoum, M.W.; Heintz, M.R. Elastic and mechanical Properties of the MAX Phases. Annu. Rev. Mater. Res. 2011, 41, 195–227. [Google Scholar] [CrossRef]
- Cover, M.F.; Warschkow, O.; Bilek, M.M.M.; Mckenzie, D.R. Elastic properties of Tin+1AlCn and Tin+1AlNn MAX phases. Adv. Energy Mater. 2008, 10, 935–938. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhou, Y.C. Layered machinable and electrically conductive Ti2AlC and Ti3AlC2 ceramics: A review. J. Mater. Sci. Technol. 2010, 26, 385–416. [Google Scholar] [CrossRef]
- Sun, Z.M. Progress in research and development on MAX phases: A family of layered ternary compounds. Int. Mater. Rev. 2011, 56, 143–166. [Google Scholar] [CrossRef]
- Keast, V.J.; Harris, S.; Smith, D.K. Prediction of the stability of the Mn+1AXn phases from first principles. Phys. Rev. B 2009, 80, 1–7. [Google Scholar] [CrossRef]
- Filippatos, P.P.; Hadi, M.A.; Christopoulos, S.R.G.; Kordatos, A.; Kelaidis, N.; Fitzpatrick, M.E.; Vasilopoulou, M.; Chroneos, A. 312 MAX Phases: Elastic properties and lithiation. Materials 2019, 12, 4098. [Google Scholar] [CrossRef]
- Eklund, P.; Beckers, M.; Jansson, U.; Högberg, H.; Hultman, L. The Mn+1AXn phases: Materials science and thin-film processing. Thin Solid Film. 2010, 518, 1851–1878. [Google Scholar] [CrossRef]
- Benitez, R.; Kan, W.H.; Gao, H.; O’Neal, M.; Proust, G.; Srivastava, A.; Radovic, M. Mechanical properties and microstructure evolution of Ti2AlC under compression in 25–1100 °C temperature range. Acta Mater. 2020, 189, 154–165. [Google Scholar] [CrossRef]
- Goc, K.; Prendota, W.; Chlubuny, L.; Strączek, T.; Tokarz, W.; Borowiak, P.; Witulska, K.; Bućko, K.; Przewoźnik, J.; Lis, J. Structure, morphology and electrical transport properties of the Ti3AlC2 materials. Ceram. Int. 2018, 44, 18322–18328. [Google Scholar] [CrossRef]
- Lslak, B.T.; Ayas, E. Evaluation of properties of spark plasma sintered Ti3SiC2 and Ti3SiC2/SiC composites. Ceram. Int. 2019, 45, 12297–12306. [Google Scholar] [CrossRef]
- Hoffman, E.N.; Vinson, D.W.; Sindelar, R.L.; Tallman, D.J.; Kohse, G.; Barsoum, M.W. MAX phase carbides and nitrides: Properties for future nuclear power plant in-core applications and neutron transmutation analysis. Nucl. Eng. Des. 2012, 244, 17–24. [Google Scholar] [CrossRef]
- Qu, L.; Bei, G.; Nijemeisland, M.; Cao, D.; Zwaag, S.V.D.; Sloof, W.G. Point contact abrasive wear behavior of MAX phase materials. Ceram. Int. 2020, 46, 1722–1729. [Google Scholar] [CrossRef]
- Zhu, J.; Ha, E.; Zhao, G.; Zhou, Y.; Huang, D.; Yue, G.; Hu, L.; Sun, N.; Wang, Y.; Lee, L.Y.S.; et al. Recent advance in MXenes: A promising 2D material for catalysis, sensor and chemical adsorption. Coordin. Chem. Rev. 2017, 352, 306–327. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, M.Q.; Wang, Y.; Yao, W.; Chen, C.; Anasori, B.; Sarycheva, A.; Ren, C.E.; Mathis, T.; Gomes, L.; et al. Demonstration of Li-Ion capacity of MAX phases. ACS Energy Lett. 2016, 1, 1094–1099. [Google Scholar] [CrossRef]
- Haddad, N.; Garcia-Caurel, E.; Hultman, L.; Barsoum, M.W.; Hug, G. Dielectric properties of Ti2AlC and Ti2AlN MAX phases: The conductivity anisotropy. J. Appl. Phys. 2008, 104, 023531. [Google Scholar] [CrossRef]
- Zong, H.; Hu, L.; Wang, Z.G.; Yu, K.; Gong, S.J.; Zhu, Z.Q. Interfacial superassembly of MoSe2@Ti2N MXene hybrids enabling promising lithium-ion storage. CrystEngComm 2020, 22, 5995–6002. [Google Scholar] [CrossRef]
- Salvo, C.; Chicardi, E.; García-Garrido, C.; Jiménez, J.A.; Aguilar, C.; Usuba, J.; Mangalaraja, R.V. The influence of mechanical activation process on the microstructure and mechanical properties of bulk Ti2AlN MAX phase obtained by reactive hot pressing. Ceram. Int. 2019, 45, 17793–17799. [Google Scholar] [CrossRef]
- Barsoum, M.W.; Ei-raghy, T.; Ali, M. Processing and characterization of Ti2AlC, Ti2AlN, and Ti2AlC0.5N0.5. Metall. Mater. Trans. A 2000, 31, 1857–1865. [Google Scholar] [CrossRef]
- Yan, M.; Mei, B.C.; Zhu, J.Q.; Tian, C.G.; Wang, P. Synthesis of high-purity bulk Ti2AlN by spark plasma sintering (SPS). Ceram. Int. 2008, 34, 1439–1442. [Google Scholar] [CrossRef]
- Yeh, C.L.; Kuo, C.W.; Wu, F.S. Formation of Ti2AlN by solid–gas combustion synthesis with AlN-and TiN-diluted samples in nitrogen. Int. J. Appl. Ceram. Technol. 2010, 7, 730–737. [Google Scholar] [CrossRef]
- Roy, C.; Banerjee, P.; Bhattacharyya, S. Molten salt shielded synthesis (MS3) of Ti2AlN and V2AlC MAX phase powders in open air. J. Eur. Ceram. Soc. 2020, 40, 923–929. [Google Scholar] [CrossRef]
- Hashimoto, H.; Abe, T.; Sun, Z.M. Nitrogen-induced powder formation of titanium aluminides during mechanical alloying. Intermetallics 2000, 8, 721–728. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.L.; Xiao, W.W.; Zhang, L.F.; Pu, Y.P.; Guo, S.W. Rapid synthesis of Ti2AlN ceramic via thermal explosion. Mater. Lett. 2015, 149, 5–7. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Li, F.; Cui, H.; Zhang, L.; Guo, S. Synthesis and microstructure of Ti2AlN ceramic by thermal explosion. Ceram. Int. 2017, 43, 13618–13624. [Google Scholar] [CrossRef]
- Chen, W.H.; Tang, J.C.; Shi, X.W.; Ye, N.; Yue, Z.H.; Lin, X.H. Synthesis and formation mechanism of high-purity Ti3AlC2 powders by microwave sintering. Int. J. Appl. Ceram. Technol. 2020, 17, 778–789. [Google Scholar] [CrossRef]
- Liu, W.L.; Qiu, C.J.; Zhou, J.; Ding, Z.H.; Zhou, X.B.; Du, S.Y.; Han, Y.H.; Huang, Q. Fabrication of Ti2AlN ceramics with orientation growth behavior by the microwave sintering method. J. Eur. Ceram. Soc. 2015, 35, 1385–1391. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, C.; Cai, S.; Sakka, Y.; Grasso, S.; Huang, Q. Synthesis of High-Purity Ti3SiC2 by Microwave Sintering. Int. J. Appl. Ceram. Technol. 2014, 11, 911–918. [Google Scholar] [CrossRef]
- Yang, J.S.; Liao, C.J.; Wang, J.F.; Jiang, Y.; He, Y.H. Effects of the Al content on pore structures of porous Ti3AlC2 ceramics by reactive synthesis. Ceram. Int. 2014, 40, 4643–4648. [Google Scholar] [CrossRef]
- Li, S.B.; Xiang, W.H.; Zhai, H.X.; Zhou, Y.; Li, C.W.; Zhang, Z.L. Formation of a single-phase Ti3AlC2 from a mixture of Ti, Al and TiC powders with Sn as an additive. Mater. Res. Bull. 2008, 43, 2092–2099. [Google Scholar] [CrossRef]
- Ai, M.X.; Zhai, H.X.; Zhou, Y.; Tang, Z.Y.; Huang, Z.Y.; Zhang, Z.L.; Li, S.B. Synthesis of Ti3AlC2 powders using Sn as an additive. J. Am. Ceram. Soc. 2006, 89, 1114–1117. [Google Scholar] [CrossRef]
- Colomban, P.; Badot, J.C. Elaboration de ceramiques superconductrices anisotropes (Na+ β Al2O3) par chauffage microondes. Mater. Res. Bull. 1978, 13, 135–139. [Google Scholar] [CrossRef]
- Salehi, M.; Maleksaeed, S.; Nai, M.L.S.; Gupta, M. Towards additive manufacturing of magnesium alloys through integration of binderless 3D printing and rapid microwave sintering. Addit. Manuf. 2019, 29, 100790. [Google Scholar] [CrossRef]
- Breen, A.; Milosavljević, V.; Dowling, D.P. Influence of gastype on the thermal efficiency of microwave plasmas for the sintering of metal powders. Plasma Chem. Plasmap. 2011, 31, 771–785. [Google Scholar] [CrossRef]
- Haemers, J.; Gusmão, R.; Sofer, Z. Synthesis Protocols of the most common layered carbide and nitride MAX phases. Small Methods 2020, 4, 1900780. [Google Scholar] [CrossRef]
- Kovalev, D.Y.; Luginina, M.A.; Sytschev, A.E. Reaction synthesis of the Ti2AlN MAX-phase. Russ. J. Non Ferr. Met. 2017, 58, 303–307. [Google Scholar] [CrossRef]
- Lin, Z.J.; Zhuo, M.J.; Li, M.S.; Wang, J.Y.; Zhou, Y.C. Synthesis and microstructure of layered-ternary Ti2AlN ceramic. Scripta Mater. 2007, 56, 1115–1118. [Google Scholar] [CrossRef]
- Pang, W.K.; Low, I.M.; Kennedy, S.J.; Smith, R.I. In situ diffraction study on decomposition of Ti2AlN at 1500–1800 °C in vacuum. Mater. Sci. Eng. A 2010, 11, 137–142. [Google Scholar] [CrossRef]
- Low, I.M.; Pang, W.K.; Kennedy, S.J.; Smith, R.I. High-temperature thermal stability of Ti2AlN and Ti4AlN3: A comparative diffraction study. J. Eur. Ceram. Soc. 2011, 31, 159–166. [Google Scholar] [CrossRef]
- Liu, H.; He, P.; Feng, J.C.; Cao, J. Kinetic study on nonisothermal dehydrogenation of TiH2 powders. Int. J. Hydrogen Energy 2009, 34, 3018–3025. [Google Scholar] [CrossRef]
- Yang, Y.F.; Mu, D.K. Rapid dehydrogenation of TiH2 and its effect on formation mechanism of TiC during self-propagation high-temperature synthesis from TiH2–C system. Powder Technol. 2013, 249, 208–211. [Google Scholar] [CrossRef]
- Oghbaei, M.; Mirzaee, O. Microwave versus conventional sintering: A review of fundamentals, advantages and applications. J. Alloy. Compd. 2010, 494, 175–189. [Google Scholar] [CrossRef]
- Han, Y.S.; Kalmykov, K.B.; Dunaev, S.F.; Zaitsev, A.I. Solid-state phase equilibria in the Titanium-Aluminum-Nitrogen system. J. Phase Equilibria Diff. 2004, 25, 427–436. [Google Scholar] [CrossRef]
- Barsoum, M.W.; Farber, L.; Levin, I.; Procopio, A.; Ei-raghy, T.; Berner, A. High-Resolution Transmission Electron Microscopy of Ti4AlN3, or Ti3Al2N2 Revisited. J. Am. Ceram. Soc. 1999, 82, 2545–2547. [Google Scholar] [CrossRef]
- Joulain, A.; Thilly, L.; Rabier, J. Revisiting the defect structure of MAX phases: The case of Ti4AlN3. Philos. Mag. 2008, 88, 1307–1320. [Google Scholar] [CrossRef]



| Raw Materials | Manufacturer | Purity (wt %) | Particle Size |
|---|---|---|---|
| Titanium hydride (TiH2) | Aladdin reagent | 99.0 | <45 µm |
| Titanium (Ti) | Aladdin reagent | 99.9 | <45 µm |
| Titanium nitride (TiN) | Aladdin reagent | 99.0 | <4 µm |
| Aluminum (Al) | Aladdin reagent | 99.9 | <25 µm |
| Aluminum nitride (AlN) | Aladdin reagent | 99.5 | <75 µm |
| Tin (Sn) | Aladdin reagent | 99.9 | <25 µm |
| Sintering Temperature (°C) | Holding Time (min) | Phase Components Determined by XRD | Major Phase | The Content of the Ti2AlN (wt %) |
|---|---|---|---|---|
| Mixed powders | / | TiN, TiH2, Al, Sn | TiN, TiH2, Al | / |
| 480 °C | 1 min | TiN, TiH2, TiH1.5, Al, Sn | TiN, TiH2, Al | / |
| 620 °C | 1 min | TiN, TiH1.5, α-Ti, TiN0.5, Ti2Al, Al | TiN, TiH1.5, Al | / |
| 720 °C | 1 min | TiN, Ti2Al, TiAl2, TiAl, Ti2AlN | TiN, Ti2Al, TiAl2 | / |
| 820 °C | 1 min | TiN, TiAl, Ti2Al, TiAl2, Ti2AlN | TiN, Ti2Al, TiAl2 | / |
| 920 °C | 1 min | TiN, TiAl, Ti2Al, TiAl2, Ti2AlN | TiN, TiAl | / |
| 1150 °C | 30 min | Ti2AlN, TiN, TiAl | TiN, Ti2AlN | 26.68 |
| 1200 °C | 30 min | Ti2AlN, TiN, TiAl | Ti2AlN | 78.16 |
| 1250 °C | 30 min | Ti2AlN, Ti4AlN3 | Ti2AlN | 96.68 |
| 1300 °C | 30 min | Ti2AlN, Ti4AlN3, TiN, TiN0.5 | Ti2AlN | 76.39 |
| 1350 °C | 30 min | Ti2AlN, Ti4AlN3, TiN, TiN0.5, Al2O3 | Ti2AlN, Ti4AlN3, TiN | 42.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Tang, J.; Lin, X.; Ai, Y.; Ye, N. Formation Mechanism of High-Purity Ti2AlN Powders under Microwave Sintering. Materials 2020, 13, 5356. https://doi.org/10.3390/ma13235356
Chen W, Tang J, Lin X, Ai Y, Ye N. Formation Mechanism of High-Purity Ti2AlN Powders under Microwave Sintering. Materials. 2020; 13(23):5356. https://doi.org/10.3390/ma13235356
Chicago/Turabian StyleChen, Weihua, Jiancheng Tang, Xinghao Lin, Yunlong Ai, and Nan Ye. 2020. "Formation Mechanism of High-Purity Ti2AlN Powders under Microwave Sintering" Materials 13, no. 23: 5356. https://doi.org/10.3390/ma13235356
APA StyleChen, W., Tang, J., Lin, X., Ai, Y., & Ye, N. (2020). Formation Mechanism of High-Purity Ti2AlN Powders under Microwave Sintering. Materials, 13(23), 5356. https://doi.org/10.3390/ma13235356




