Effects of Sintering Temperature Variation on Synthesis of Glass-Ceramic Phosphor Using Rice Husk Ash as Silica Source
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Synthesis of Silica (SiO2) from Rice Husk
2.2. Synthesis of Zinc-Boro-Silicate (ZBS) Glass-Ceramic
2.3. Characterization of Physical Properties
2.4. Characterization of Structural and Luminescence Properties
3. Results and Discussion
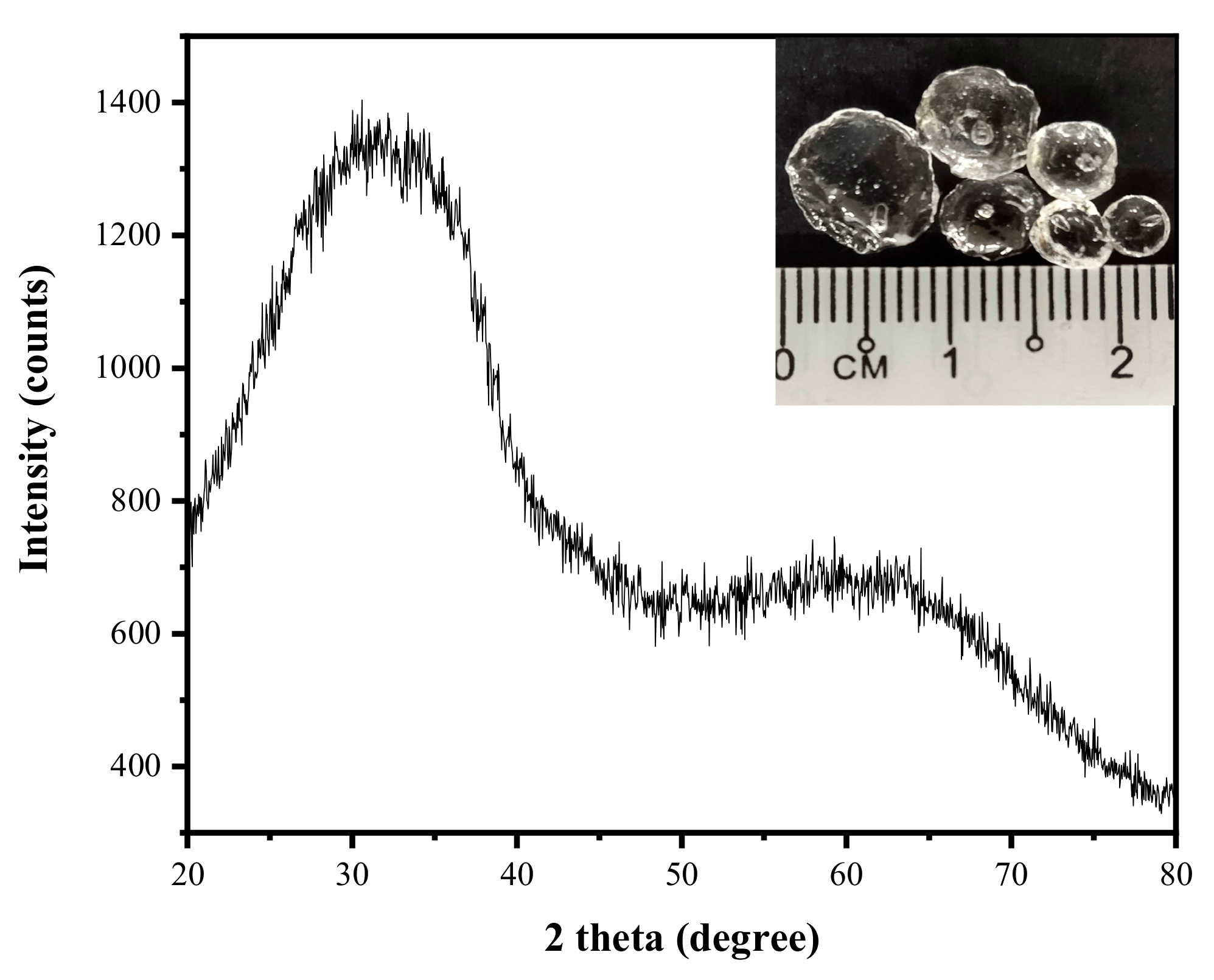
3.1. Chemical and Structural Characterization of Rice Husk Ash (RHA)
3.2. Characterization of the ZnO-B2O3-SiO2 (ZBS) Glass
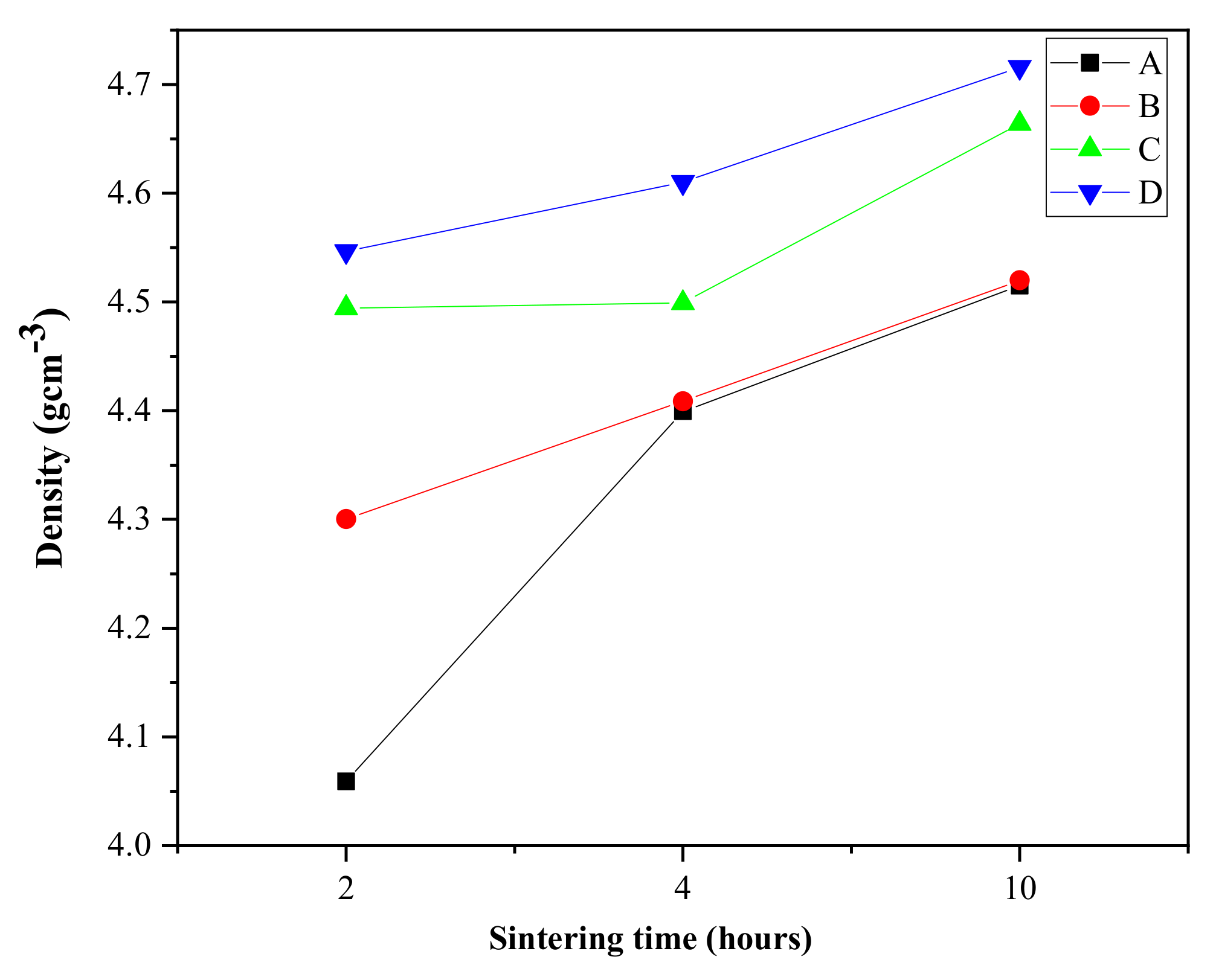
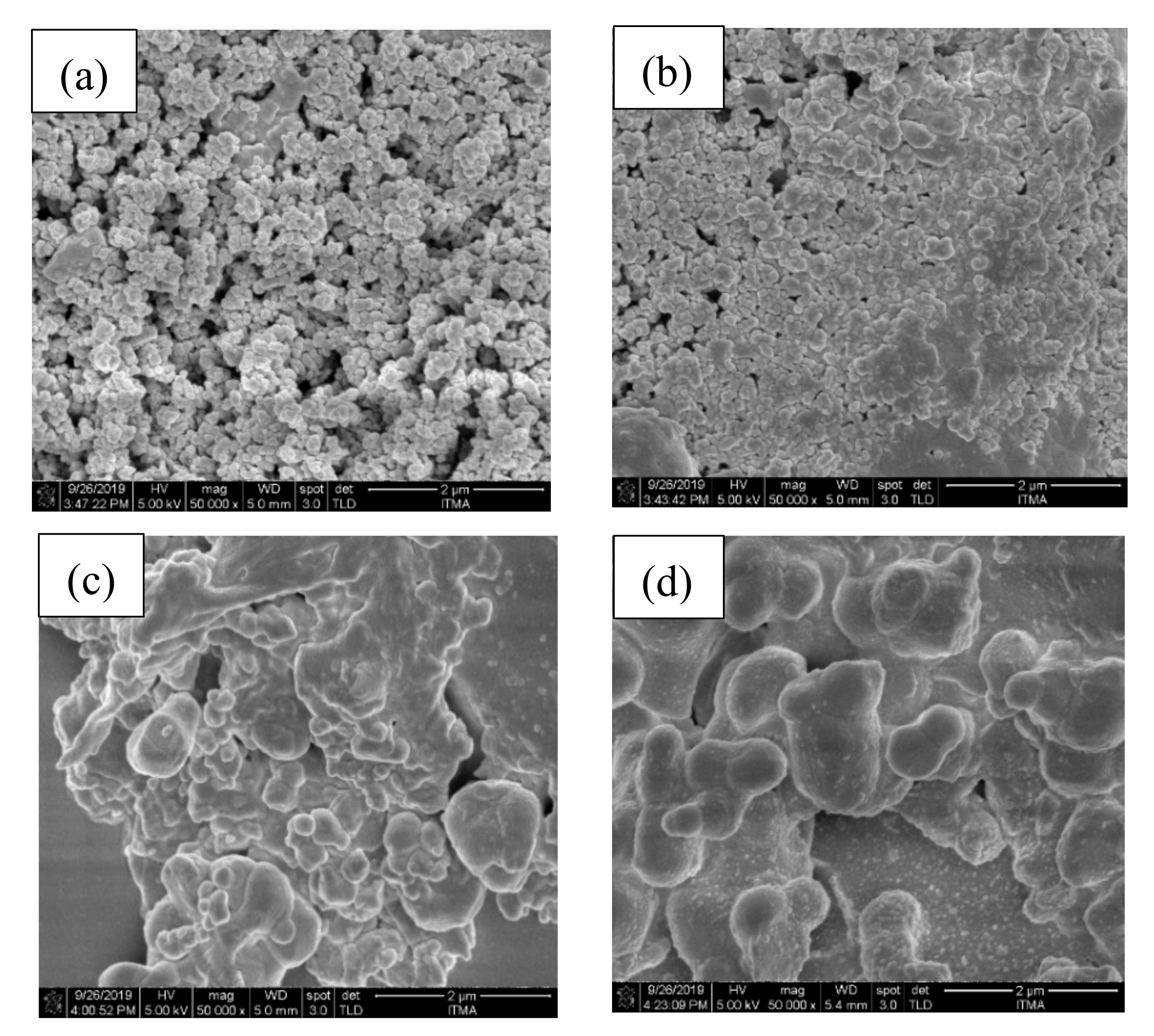
3.3. Physical Characterization of the ZnO-B2O3-SiO2 (ZBS) Glass-Ceramics
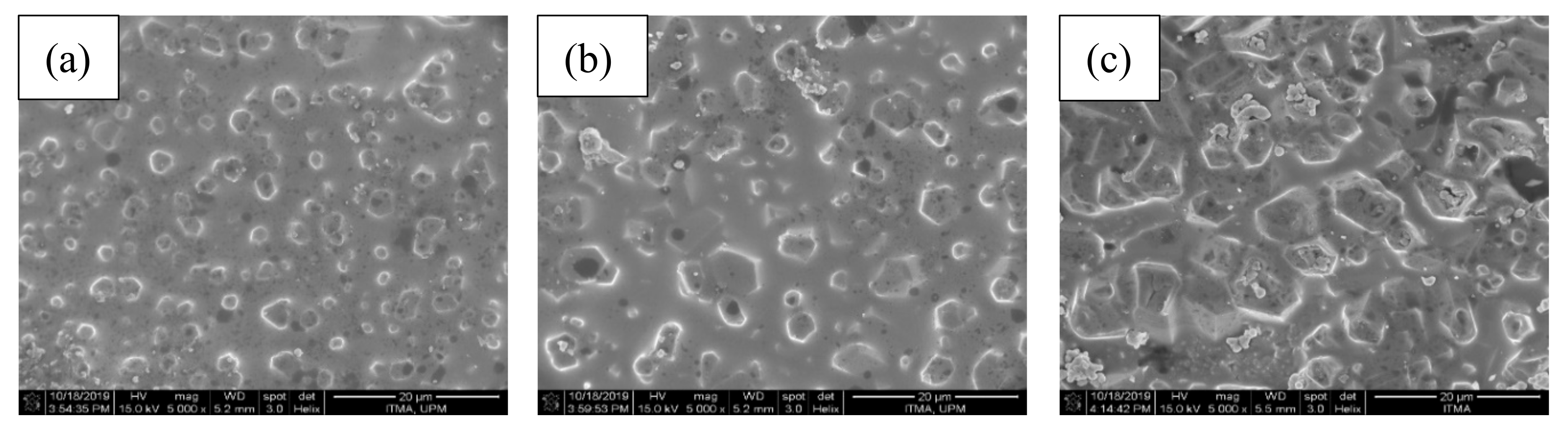
3.4. Structural Characterization of the ZnO-B2O3-SiO2 (ZBS) Glass-Ceramics
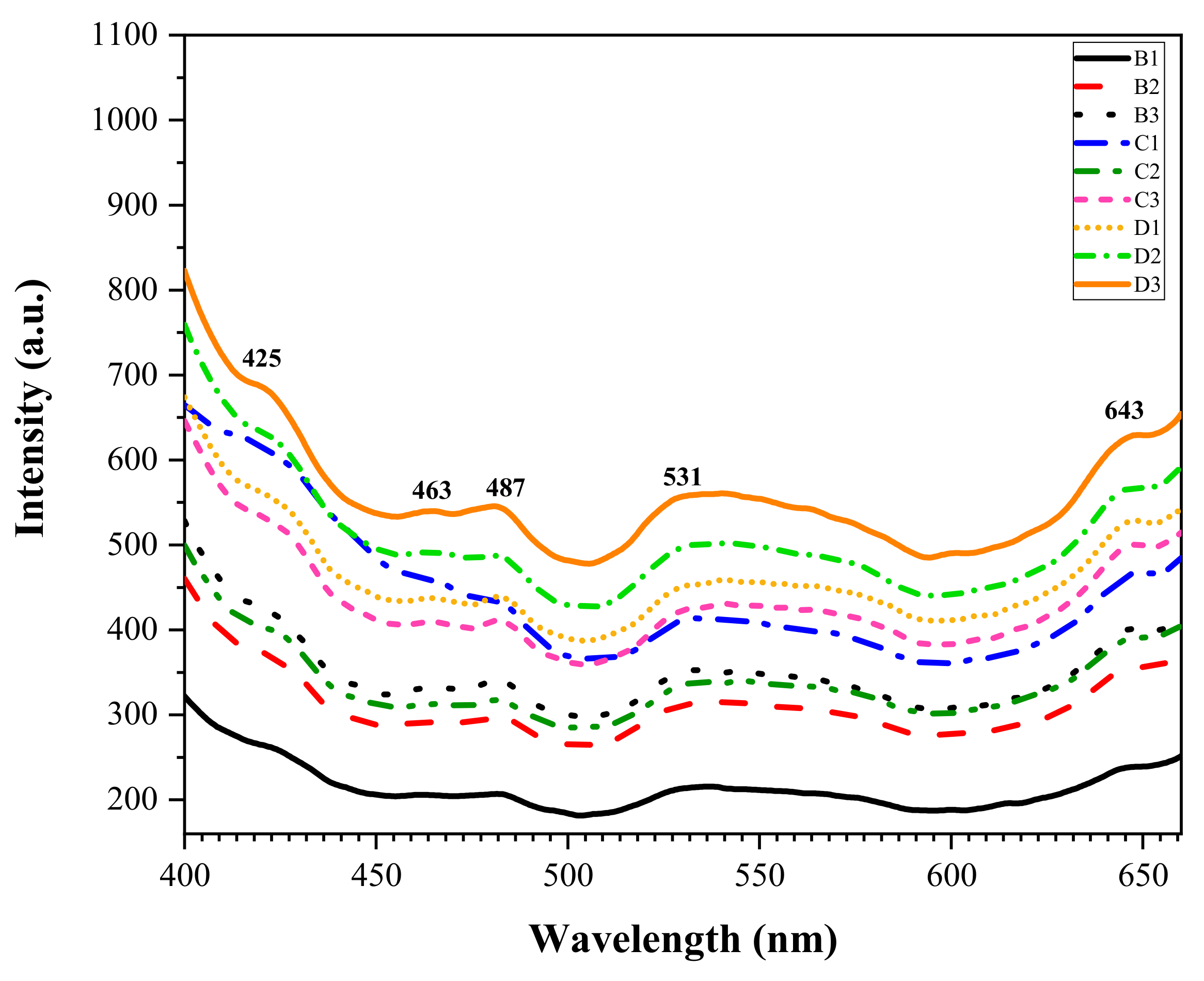
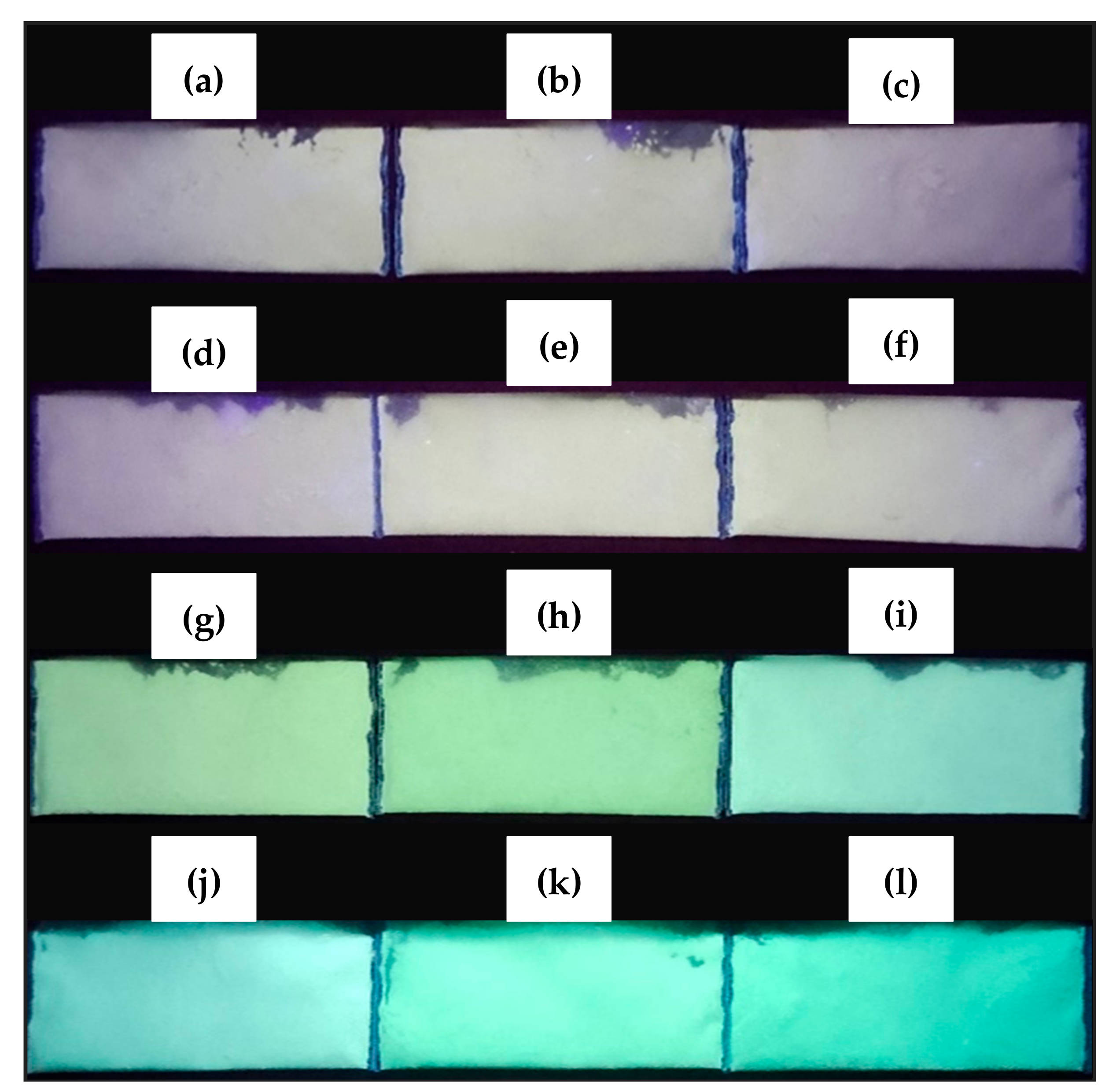
3.5. Optical Characterization of the ZnO-B2O3-SiO2 (ZBS) Glass-Ceramics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stein, V.; Schemmel, T. Sustainable rice husk ash-based high-temperature insulating materials. Int. Ceram. Rev. 2020, 69, 30–37. [Google Scholar] [CrossRef]
- Leenakul, W.; Tunkasiri, T.; Tongsiri, N.; Pengpat, K.; Ruangsuriya, J. Effect of sintering temperature variations on fabrication of 45S5 bioactive glass-ceramics using rice husk as a source for silica. Mater. Sci. Eng. C 2016, 61, 695–704. [Google Scholar] [CrossRef]
- Khaidir, R.E.M.; Fen, Y.W.; Zaid, M.H.M.; Matori, K.A.; Omar, N.A.S.; Anuar, M.F.; Wahab, S.A.A.; Azman, A.Z.K. Addition of ZnO nanoparticles on waste rice husk as potential host material for red-emitting phosphor. Mater. Sci. Semicond. Process 2020, 106, 104774. [Google Scholar] [CrossRef]
- Sobrosa, F.Z.; Stochero, N.P.; Marangon, E.; Tier, M.D. Development of refractory ceramics from residual silica derived from rice husk ash. Ceram. Int. 2017, 43, 13875–13880. [Google Scholar] [CrossRef]
- Qing, Z. The effects of B2O3 on the microstructure and properties of lithium amluminosilicate glass-ceramics for LTCC applications. Mater. Lett. 2018, 212, 126–129. [Google Scholar] [CrossRef]
- Rui, X.; Yuetan, M.; Xi, J.; Miaomiao, Z.; Yiyuan, Z.; Yanhai, Z.; Baoshan, H.; Qiang, H. Strength, microstructure, efflorescence behaviour and environmental impacts of waste glass geopolymers cured at ambient temperature. J. Clean. Prod. 2020, 252, 119610. [Google Scholar]
- Rui, X.; Xi, J.; Miaomiao, Z.; Pawel, P.; Baoshan, H. Analytical investigation of phase assemblages of alkali-activated materials in CaO-SiO2-Al2O3 system: The management of reactionproducts and designing of precursors. Mater. Des. 2020, 194, 108975. [Google Scholar]
- Rui, X.; Pawel, P.; Miaomiao, Z.; Xi, J.; Yiyuan, Z.; Baoshan, H.; Wei, H. Evaluation of glass powder-based geopolymer stabilized road bases containing recycled waste glass aggregate. Transp Res. Rec. 2020, 2674, 22–32. [Google Scholar]
- Tong, K.T.; Vinai, R.; Soutsos, M.N. Use of Vietnamese rice husk ash for the production of sodium silicate as the activator for alkali-activated binders. J. Clean. Prod. 2018, 201, 272–286. [Google Scholar] [CrossRef]
- Ruengsri, S.; Insiripong, S.; Sangwaranatee, N.; Kaewkhao, J. Development of barium borosilicate glasses for radiation shielding materials using rice husk ash as a silica source. Prog. Nucl. Energy 2015, 83, 99–104. [Google Scholar] [CrossRef]
- Nayak, J.P.; Bera, J. Effect of sintering temperature on mechanical behaviour and bioactivity of sol-gel synthesized bioglass-ceramics using rice husk ash as a silica source. Appl. Surf. Sci. 2010, 257, 458–462. [Google Scholar] [CrossRef]
- Yusoff, N.M.; Johari, Y.; Rahman, I.A.; Mohamad, D.; Khamis, M.F.; Ariffin, Z.; Husein, A. Physical and mechanical properties of flowable composite incorporated with nanohybrid silica synthesised from rice husk. J. Mater. Res. Technol. 2019, 8, 2777–2785. [Google Scholar] [CrossRef]
- Zaid, M.H.M.; Matori, K.A.; Wah, L.C.; Sidek, H.A.A.; Halimah, M.K.; Wahab, Z.A.; Azmi, B.Z. Elastic moduli prediction and correlation in soda lime silicate glasses containing ZnO. Int. J. Phys. Sci. 2011, 6, 1404–1410. [Google Scholar]
- Zaid, M.H.M.; Matori, K.A.; Sidek, H.A.A.; Kamari, H.M.; Wahab, Z.A.; Effendy, N.; Alibe, I.M. Comprehensive study on compositional dependence of optical band gap in zinc soda lime silica glass system for optoelectronic applications. J. Non-Cryst. Solids 2016, 449, 107–112. [Google Scholar] [CrossRef]
- Wahab, S.A.A.; Matori, K.A.; Sidek, H.A.A.; Zaid, M.H.M.; Kechik, M.M.A.; Azman, A.Z.K.; Khaidir, R.E.M.; Khiri, M.Z.A.; Effendy, N. Effect of ZnO on the phase transformation and optical properties of silicate glass frits using rice husk ash as SiO2 source. J. Mater. Res. Technol. 2020, 9, 11013–11021. [Google Scholar] [CrossRef]
- Alibe, I.M.; Matori, K.A.; Sidek, H.A.A.; Yaakob, Y.; Rashid, U.; Alibe, A.M.; Zaid, M.H.M.; Khiri, M.Z.A. Effects of calcination holding time on properties of wide bandgap willemite semiconductor nanoparticles by the polymer thermal treatment method. Molecules 2018, 23, 873. [Google Scholar] [CrossRef]
- Lee, T.; Othman, R.; Yeoh, F.Y. Development of photoluminescent glass derived from rice husk. Biomass Bioenergy 2013, 59, 380–392. [Google Scholar] [CrossRef]
- Hameed, S.A.M.A.; Margha, F.H. Preparation, crystallization and photoluminescence properties of un-doped nano willemite glass ceramics with high ZnO additions. Optik 2020, 206, 164374. [Google Scholar] [CrossRef]
- Wahab, S.A.A.; Matori, K.A.; Sidek, H.A.A.; Zaid, M.H.M.; Kechik, M.M.A.; Azman, A.Z.K.; Khaidir, R.E.M.; Khiri, M.Z.A.; Effendy, N. Synthesis of cobalt oxide Co3O4 doped zinc silicate based glass-ceramic derived for LED applications. Optik 2019, 179, 919–926. [Google Scholar] [CrossRef]
- Tarafder, A.; Molla, A.R.; Dey, C.; Karmakar, B. Thermal, structural, and enhanced photoluminescence properties of Eu3+-doped transparent willemite glass–ceramic nanocomposites. J. Am. Ceram. Soc. 2013, 96, 2424–2431. [Google Scholar] [CrossRef]
- Khalkhali, Z.; Hamnabard, Z.; Qazvini, S.S.A.; Baghshahi, S.; Maghsoudipour, A. Preparation, phase formation and photoluminescence properties of ZnO-SiO2-B2O3 glasses with different ZnO/B2O3 ratios. Opt. Mater. 2012, 34, 850–855. [Google Scholar] [CrossRef]
- Fang, M.; Lv, J. Zn2SiO4 as an ultralow solar absorptive pigment for thermal control coating. Mater. Lett. 2019, 255, 126538. [Google Scholar] [CrossRef]
- Effendy, N.; Wahab, Z.A.; Sidek, H.A.A.; Matori, K.A.; Zaid, M.H.M.; Rashid, S.S.A. Characterization and optical properties of erbium oxide doped ZnO-SLS glass for potential optical and optoelectronic materials. Mater. Express 2017, 7, 59–65. [Google Scholar] [CrossRef]
- Babu, B.C.; Rao, B.V.; Ravi, M.; Babu, S. Structural, microstructural, optical, and dielectric properties of Mn2+: Willemite Zn2SiO4 nanocomposites obtained by a sol-gel method. J. Mol. Struct. 2017, 1127, 6–14. [Google Scholar] [CrossRef]
- Alibe, I.M.; Matori, K.A.; Sidek, H.A.A.; Yakob, Y.; Rashid, U.; Alibe, A.M.; Zaid, M.H.M.; Nasir, S.; Nasir, M.M. Effects of polyvinylpyrrolidone on structural and optical properties of willemite semiconductor nanoparticles by polymer thermal treatment method. J. Therm. Anal. 2019, 136, 2249–2268. [Google Scholar] [CrossRef]
- Pozas, R.; Orera, V.M.; Ocana, M. Hydrothermal synthesis of Co-doped willemite powders with controlled particle size and shape. J. Eur. Ceram. Soc. 2005, 25, 3165–3172. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, J.; Xie, Z.; Zheng, L. Syntheses and optical properties of α-and β-Zn2SiO4: Mn nanoparticles by solvothermal method in ethylene glycol-water system. Mater. Chem. Phys. 2010, 120, 313–318. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, Z.; Tait, W.R.T.; Pokhrel, M.; Mao, Y.; Liu, J.; Zhang, L.; Wang, W.; Sun, L. Synthesis of green phosphors from highly active amorphous silica derived from rice husks. J. Mater. Sci. 2018, 53, 1824–1832. [Google Scholar] [CrossRef]
- Lee, C.S.; Matori, K.A.; Sidek, H.A.A.; Kamari, H.M.; Ismail, I.; Zaid, M.H.M. Fabrication and characterization of glass and glass-ceramic from rice husk ash as a potent material for opto-electronic applications. J. Mater. Sci. Mater. Electron. 2017, 28, 17611–17621. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, R.; Zhu, W.; Chen, G. Impact of heat treatent on the Mn2+ doped transparent glass ceramics containing NaZnPO4 nanocrystals. Mater. Lett. 2017, 189, 172–175. [Google Scholar] [CrossRef]
- Khaidir, R.E.M.; Fen, Y.W.; Zaid, M.H.M.; Matori, K.A.; Omar, N.A.S.; Anuar, M.F.; Wahab, S.A.A.; Azman, A.Z.K. Optical band gap and photoluminescenece studies of Eu3+-doped zinc silicate derived from waste rice husks. Optik 2019, 182, 486–495. [Google Scholar] [CrossRef]
- Lee, C.S.; Matori, K.A.; Sidek, H.A.A.; Kamari, H.M.; Ismail, I.; Zaid, M.H.M. Influence of zinc oxide on the physical, structural and optical band gap of zinc silicate glass system from waste rice husk ash. Optik 2017, 136, 129–135. [Google Scholar] [CrossRef]
- Zaid, M.H.M.; Matori, K.A.; Sidek, H.A.A.; Kamari, H.M.; Yunus, W.M.M.; Wahab, Z.A.; Samsudin, N.F. Fabrication and crystallization of ZnO-SLS glass derived willemite glass-ceramics as a potential material for optics applications. J. Spectrosc. 2016, 2016, 8084301. [Google Scholar]
- Ehrt, D.; Flugel, S. Properties of zinc silicate glasses and melts. J. Mater. Sci. Eng. A 2011, 1, 312–320. [Google Scholar]
- Kullberg, A.T.G.; Lopes, A.A.S.; Veiga, J.P.B.; Lima, M.M.R.A.; Monteiro, R.C.C. Formation and crystallization of zinc borosilicate glasses: Influence of the ZnO/B2O3 ratio. J. Non-Cryst. Solids 2016, 441, 79–85. [Google Scholar] [CrossRef]
- Kullberg, A.T.G.; Lopes, A.A.S.; Veiga, J.P.B.; Monteiro, R.C.C. Crystal growth in zinc borosilicate glasses. J. Cryst. Growth 2017, 457, 239–243. [Google Scholar] [CrossRef]
- Tarafder, A.; Molla, A.R.; Mukhopadhyay, S.; Karmakar, B. Fabrication and enhanced photoluminescence properties of Sm3+-doped ZnO-Al2O3-B2O3-SiO2 glass derived willemite glass-ceramic nanocomposites. Opt. Mater. 2014, 36, 1463–1470. [Google Scholar] [CrossRef]
- Sivakumar, V.; Lakshmanan, A.; Kalpana, S.; Rani, S.; Kumar, R.S.; Jose, M.T. Low-temperature synthesis of Zn2SiO4: Mn green photoluminescence phosphor. J. Lumin. 2012, 132, 1917–1920. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Matori, K.A.; Zaid, M.H.M.; Norhafizah, M.R.; Nurzilla, M.; Zamratul, M.I.M. Synthesis and optical properties of europium doped zinc silicate prepared using low cost solid state reaction method. J. Mater. Sci. Mater. Electron. 2016, 27, 1092–1099. [Google Scholar] [CrossRef]
- Wahab, S.A.A.; Matori, K.A.; Zaid, M.H.M.; Kechik, M.M.A.; Sidek, H.A.A.; Talib, R.A.; Azman, A.Z.K.; Khaidir, R.E.M.; Khiri, M.Z.A.; Effendy, N. A study on optical properties of zinc silicate glass-ceramics as a host for green phosphor. Appl. Sci. 2020, 10, 4938. [Google Scholar] [CrossRef]
- Montoya-Quesada, E.; Villaquirán-Caicedo, M.A.; de Gutiérrez, R.M.; Muñoz-Saldaña, J. Effect of ZnO content on the physical, mechanical and chemical properties of glass-ceramics in the CaO-SiO2-Al2O3 system. Ceram. Int. 2020, 46, 4322–4328. [Google Scholar] [CrossRef]
- Effendy, N.; Wahab, Z.A.; Kamari, H.M.; Matori, K.A.; Sidek, H.A.A.; Zaid, M.H.M. Structural and optical properties of Er3+-doped willemite glass-ceramics from waste materials. Optik 2016, 127, 11698–11705. [Google Scholar] [CrossRef]
- Loiko, P.; Dymshits, O.; Volokitina, A.; Alekseeva, I.; Shemchuk, D.; Tsenter, M.; Bachina, A.; Khubetsov, A.; Vilejshikova, E.; Petrov, P.; et al. Structural transformations and optical properties of glass-ceramics based on ZnO, β-and α-Zn2SiO4 nanocrystals and doped with Er2O3 and Yb2O3: Part I. The role of heat-treatment. J. Lumin. 2018, 202, 47–56. [Google Scholar] [CrossRef]
- Anuar, M.F.; Fen, Y.W.; Zaid, M.H.M.; Omar, N.A.S.; Khaidir, R.E.M. Sintering temperature effect on structural and optical properties of heat treated coconut husk ash derived SiO2 mixed with ZnO nanoparticles. Materials 2020, 13, 2555. [Google Scholar] [CrossRef]
- Anuar, M.F.; Fen, Y.W.; Zaid, M.H.M.; Matori, K.A.; Khaidir, R.E.M. Synthesis and structural properties of coconut husk as potential silica source. Res. Phys. 2018, 11, 1–4. [Google Scholar] [CrossRef]
- Sowri, B.K.; Ramachandra, R.A.; Sujatha, C.; Venugopal, R.K. Optimisation of UV emission intensity of ZnO nanoparticles by changing the excitation wavelength. Mater. Lett. 2013, 99, 97–100. [Google Scholar] [CrossRef]
- Spallino, L.; Vaccaro, L.; Sciortino, L.; Agnello, S.; Buscarino, G.; Cannas, M.; Gelardi, F.M. Visible-ultraviolet vibronic emission of silica nanoparticles. Phys. Chem. Chem. Phys. 2014, 16, 22028–22034. [Google Scholar] [CrossRef]
- Elhadi, S.E.; Liu, C.; Guo, Y.; Li, K. Structure and optical properties of Mn2+ ions doped ZnO/Zn2SiO4 composite thin films. J. Alloys Compd. 2019, 785, 798–807. [Google Scholar] [CrossRef]
- Jacob, S. Hanumantharayappa, C.; Nagabhushana, B.M. Precursors effect: Photo conversion from UV to green/yellow in willemite nano phosphors. Optik 2018, 173, 88–96. [Google Scholar] [CrossRef]
- Krasnenko, T.I.; Zaitseva, N.A.; Ivanova, I.V.; Baklanova, I.V.; Samigullina, R.F.; Rotermel, M.V. The effect of Mg introduction on structural and luminescence properties of Zn2SiO4: Mn phosphor. J. Alloys Compd. 2020, 845, 156296. [Google Scholar] [CrossRef]
- Shofri, M.F.S.M.; Zaid, M.H.M.; Matori, K.M.; Fen, Y.W.; Yaakob, Y.; Jaafar, S.H.; Wahab, S.A.A.; Iwamoto, Y. Phase transformation, optical and emission performance of zinc silicate glass-ceramics phosphor derived from the ZnO-B2O3-SLS glass system. Appl. Sci. 2020, 10, 4940. [Google Scholar] [CrossRef]
- Kumar, V.; Swart, H.C.; Ntwaeaborwa, O.M.; Kroon, R.E.; Terblans, J.J.; Shaat, S.K.K.; Yousif, A.; Duvenhage, M.M. Origin of the red emission in zinc oxide nanophosphors. Mater. Lett. 2013, 101, 57–60. [Google Scholar] [CrossRef]
- Tsai, M.T.; Lin, Y.H.; Yang, J.R. Characterization of manganese-doped willemite green phosphor gel powders. IOP Conf. Ser. Mater. Sci. Eng. 2011, 18, 032026. [Google Scholar] [CrossRef]
- Zhou, J.; Nomenyo, K.; Cesar, C.C.; Lusson, A.; Schwartzberg, A.; Yen, C.C.; Woon, W.Y.; Lerondel, G. Giant defect emission enhancement from ZnO nanowires through desulfurization process. Sci. Rep. 2020, 10, 4237. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Baccaro, S.; Falconieri, M.; Bei, J.; Cecilica, A.; Chen, G. Photoluminescent properties and raman spectra of ZnO-based scintillating glasses. J. Non-Cryst. Solids 2008, 354, 4626–4629. [Google Scholar] [CrossRef]



| Sample(s) | Sintering Temperature (°C) | Holding Time (Hours) |
|---|---|---|
| A1 | 600 | 2 |
| A2 | 600 | 4 |
| A3 | 600 | 10 |
| B1 | 700 | 2 |
| B2 | 700 | 4 |
| B3 | 700 | 10 |
| C1 | 800 | 2 |
| C2 | 800 | 4 |
| C3 | 800 | 10 |
| D1 | 900 | 2 |
| D2 | 900 | 4 |
| D3 | 900 | 10 |
| Raw Materials | Constituents Oxides (wt%) | |||||
|---|---|---|---|---|---|---|
| SiO2 | K2O | CaO | Al2O3 | Others | LOI | |
| RHA | 95.6 ± 0.1 | 1.4 ± 0.1 | 0.95 ± 0.1 | 0.71 ± 0.1 | 0.64 ± 0.1 | 0.7 ± 0.1 |
| Sample (s) | A | B | C | D |
|---|---|---|---|---|
| true density (g·cm−3) | 4.515 | 4.520 | 4.664 | 4.716 |
| bulk density (g·cm−3) | 3.119 | 3.440 | 3.559 | 3.674 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Wahab, R.A.; Mohd Zaid, M.H.; Aziz, S.H.A.; Amin Matori, K.; Fen, Y.W.; Yaakob, Y. Effects of Sintering Temperature Variation on Synthesis of Glass-Ceramic Phosphor Using Rice Husk Ash as Silica Source. Materials 2020, 13, 5413. https://doi.org/10.3390/ma13235413
Abdul Wahab RA, Mohd Zaid MH, Aziz SHA, Amin Matori K, Fen YW, Yaakob Y. Effects of Sintering Temperature Variation on Synthesis of Glass-Ceramic Phosphor Using Rice Husk Ash as Silica Source. Materials. 2020; 13(23):5413. https://doi.org/10.3390/ma13235413
Chicago/Turabian StyleAbdul Wahab, Rabiatul Adawiyah, Mohd Hafiz Mohd Zaid, Sidek Hj. Ab Aziz, Khamirul Amin Matori, Yap Wing Fen, and Yazid Yaakob. 2020. "Effects of Sintering Temperature Variation on Synthesis of Glass-Ceramic Phosphor Using Rice Husk Ash as Silica Source" Materials 13, no. 23: 5413. https://doi.org/10.3390/ma13235413
APA StyleAbdul Wahab, R. A., Mohd Zaid, M. H., Aziz, S. H. A., Amin Matori, K., Fen, Y. W., & Yaakob, Y. (2020). Effects of Sintering Temperature Variation on Synthesis of Glass-Ceramic Phosphor Using Rice Husk Ash as Silica Source. Materials, 13(23), 5413. https://doi.org/10.3390/ma13235413







