Novel H-Bonded Synthons in Copper Supramolecular Frameworks with Aminoethylpiperazine-Based Ligands. Synthesis, Structure and Catalytic Activity
Abstract
:1. Introduction
2. Materials and Methods
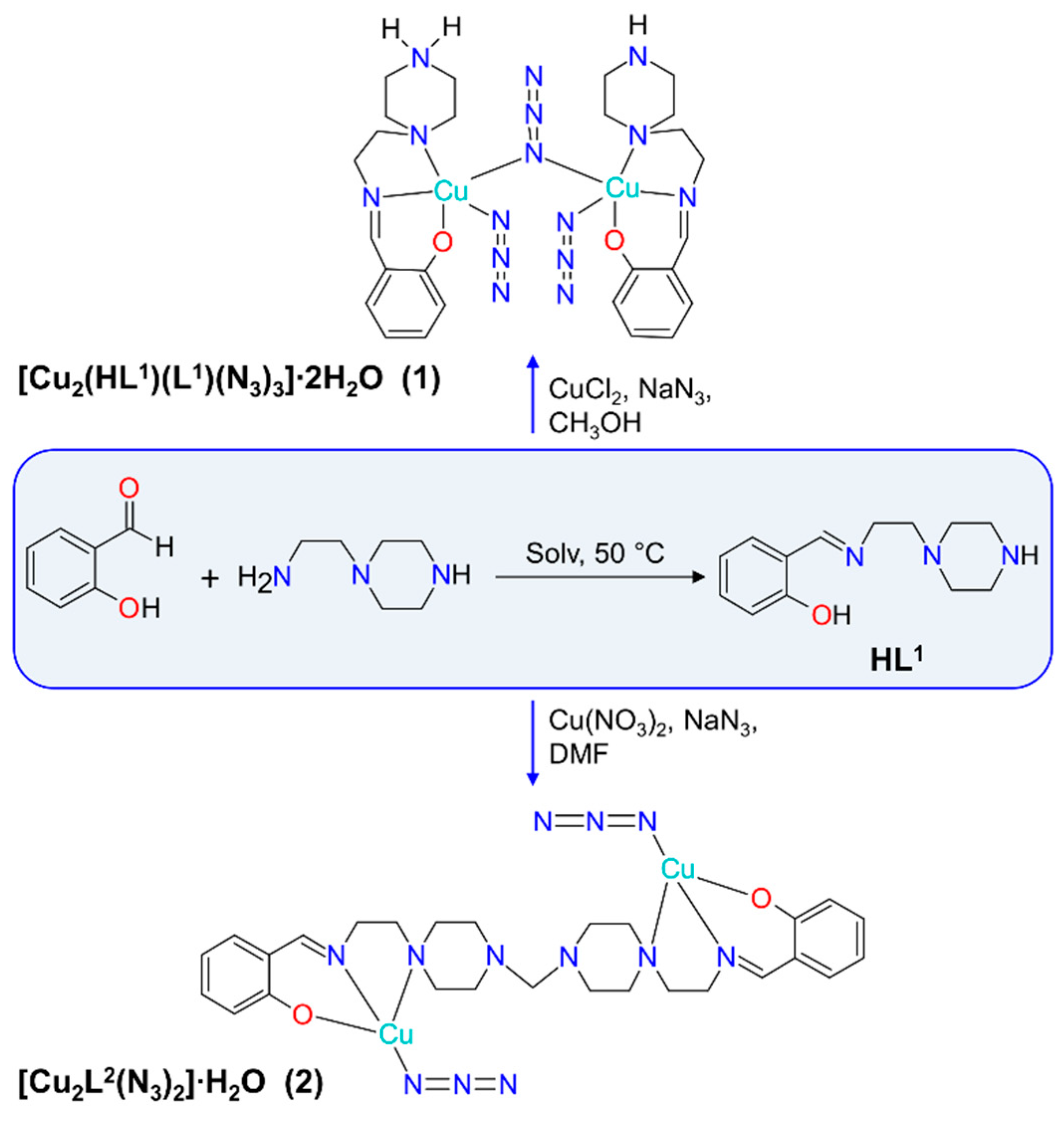
2.1. Synthesis of [Cu2(HL1)(L1)(N3)3]∙2H2O
2.2. Synthesis of [Cu2L2(N3)2]∙H2O
2.3. Crystallography
2.4. DFT Calculations
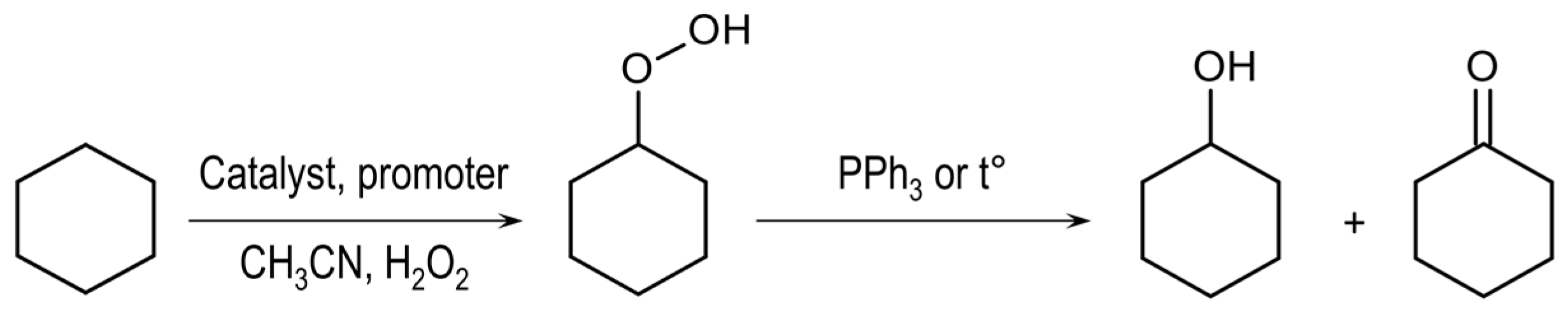
2.5. Catalytic Oxidation of Cyclohexane
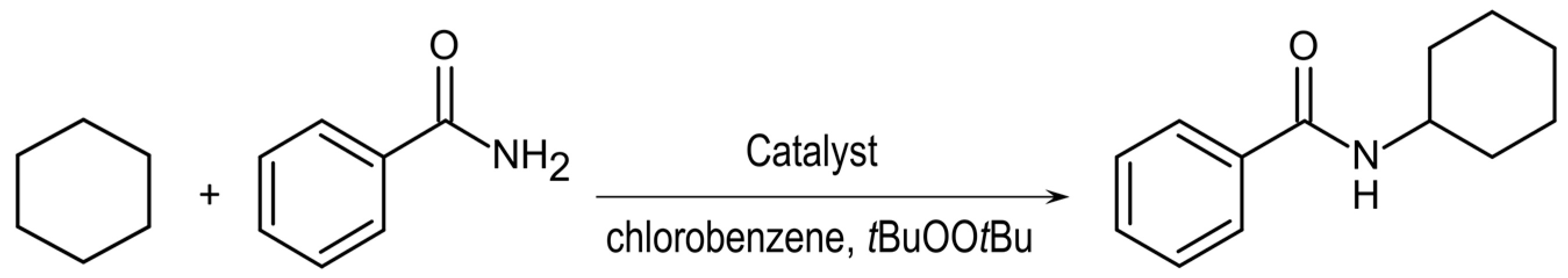
2.6. Catalytic Amidation
2.7. Gas Chromatography
3. Results
3.1. Synthesis and Spectroscopic Analysis
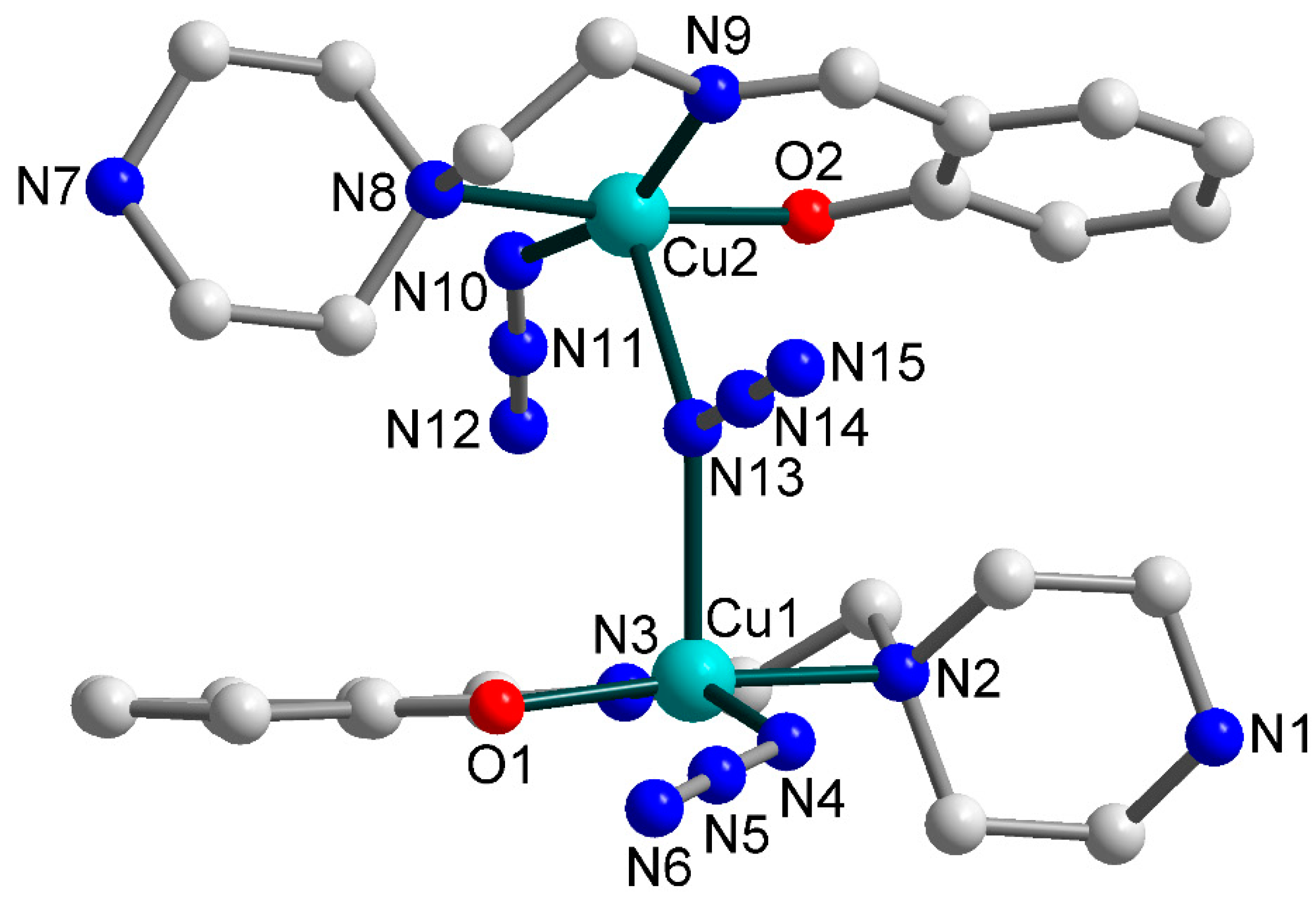
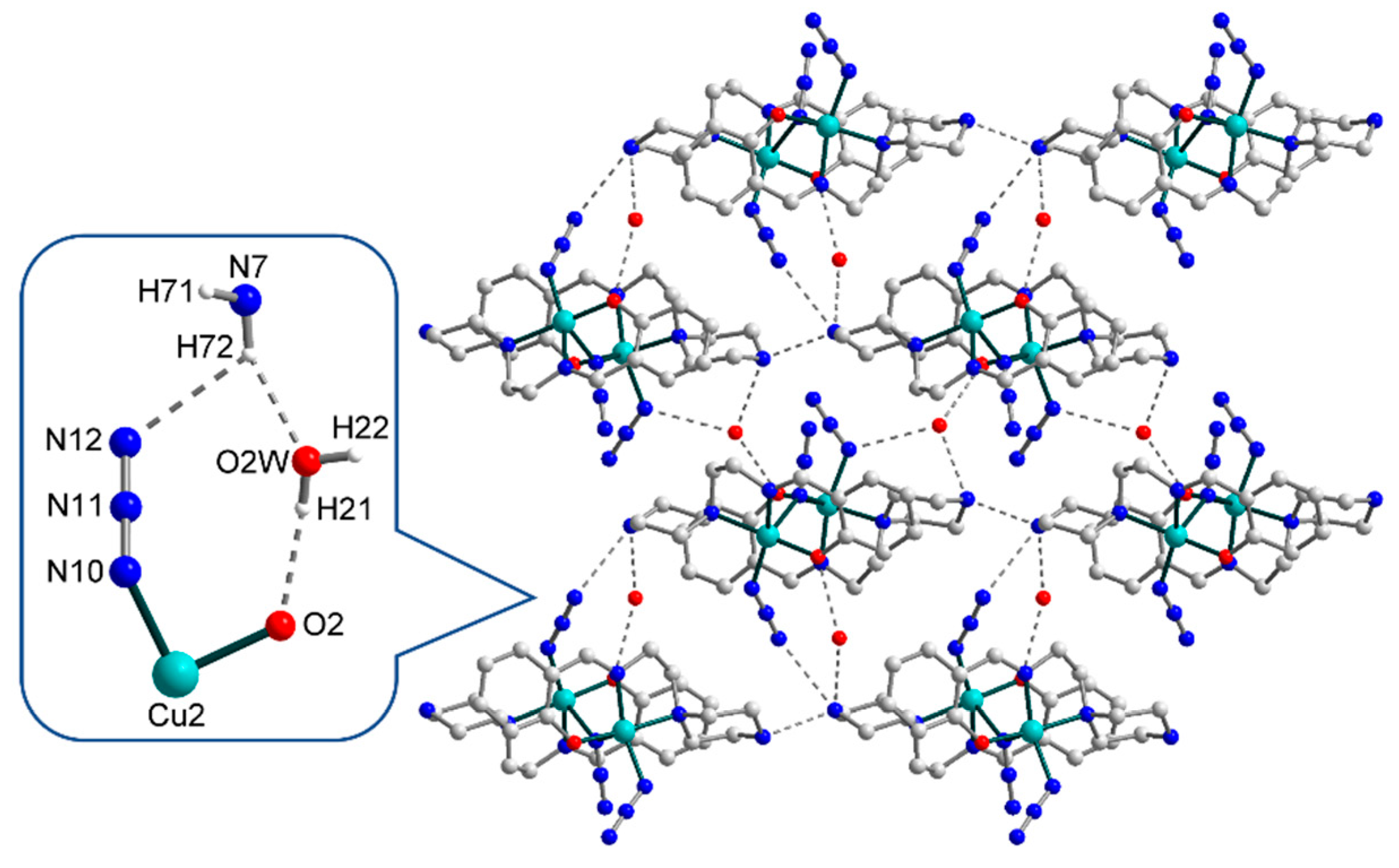
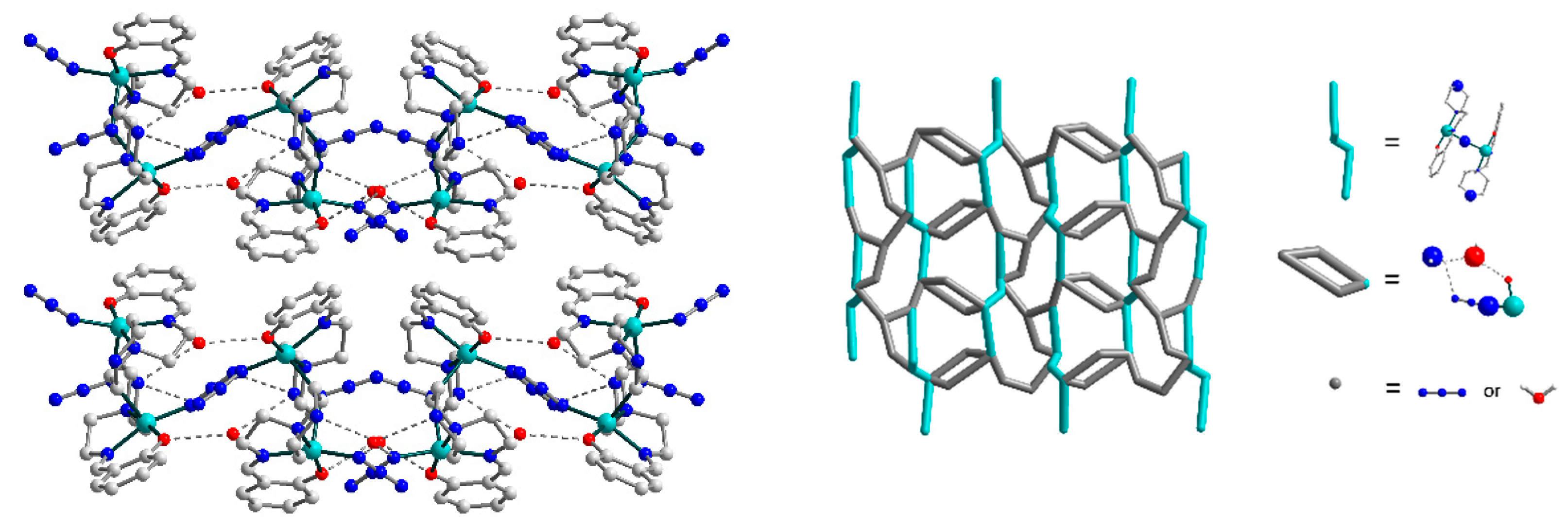
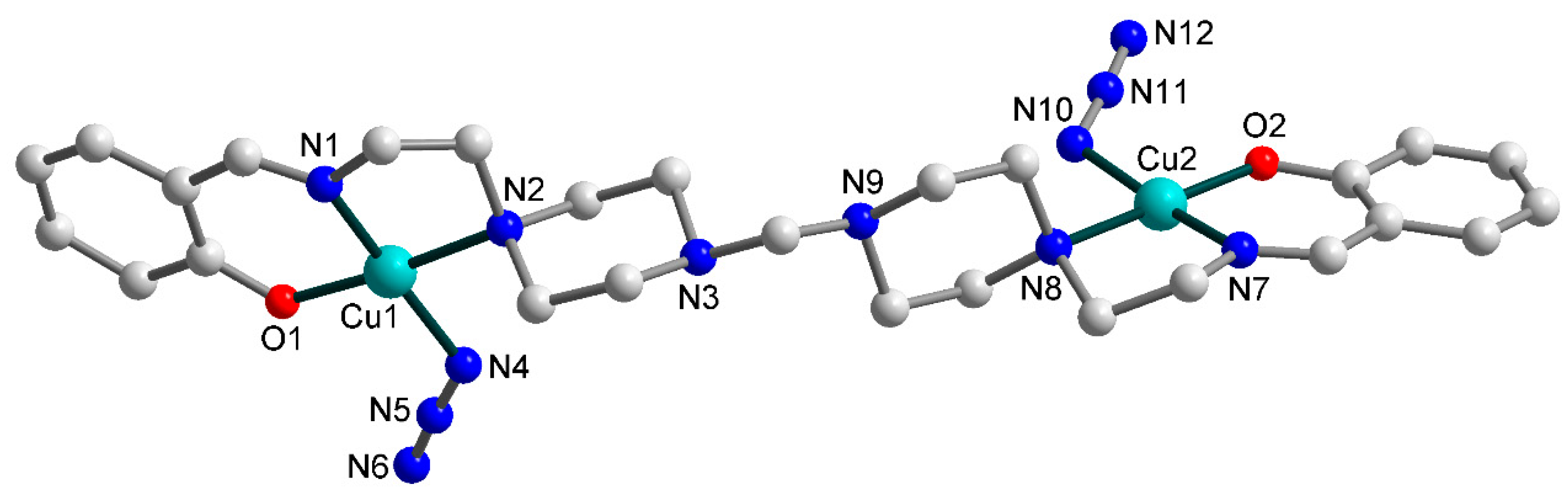
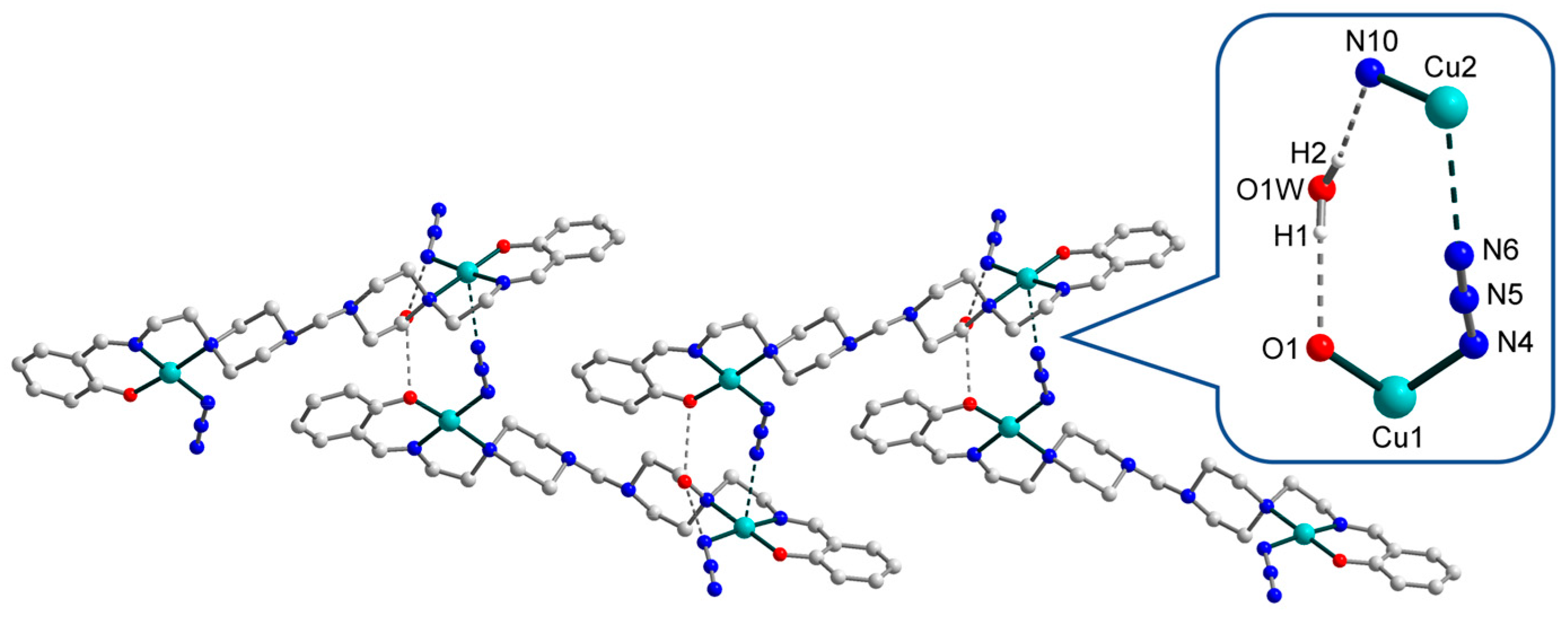
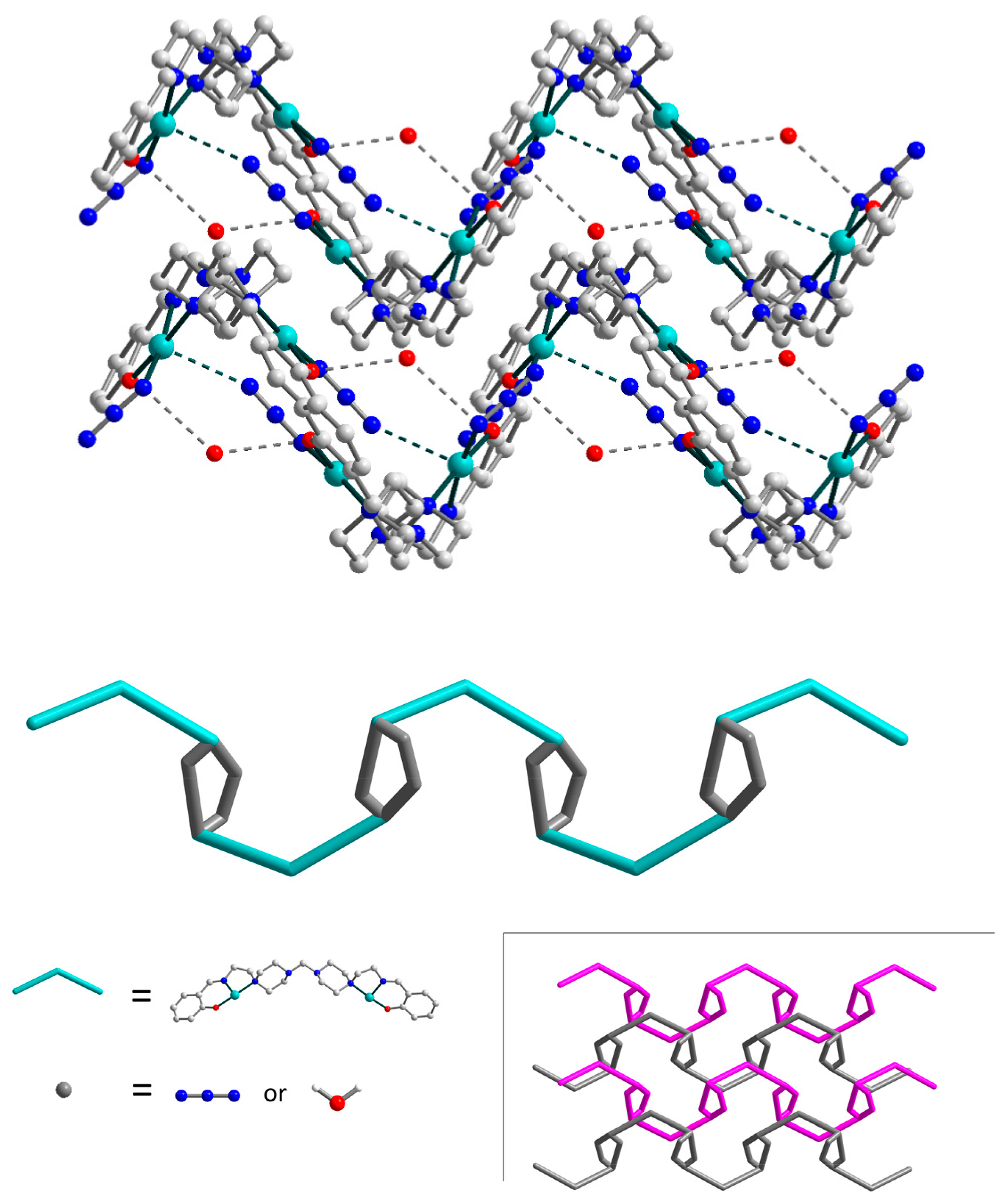
3.2. Crystal Structures
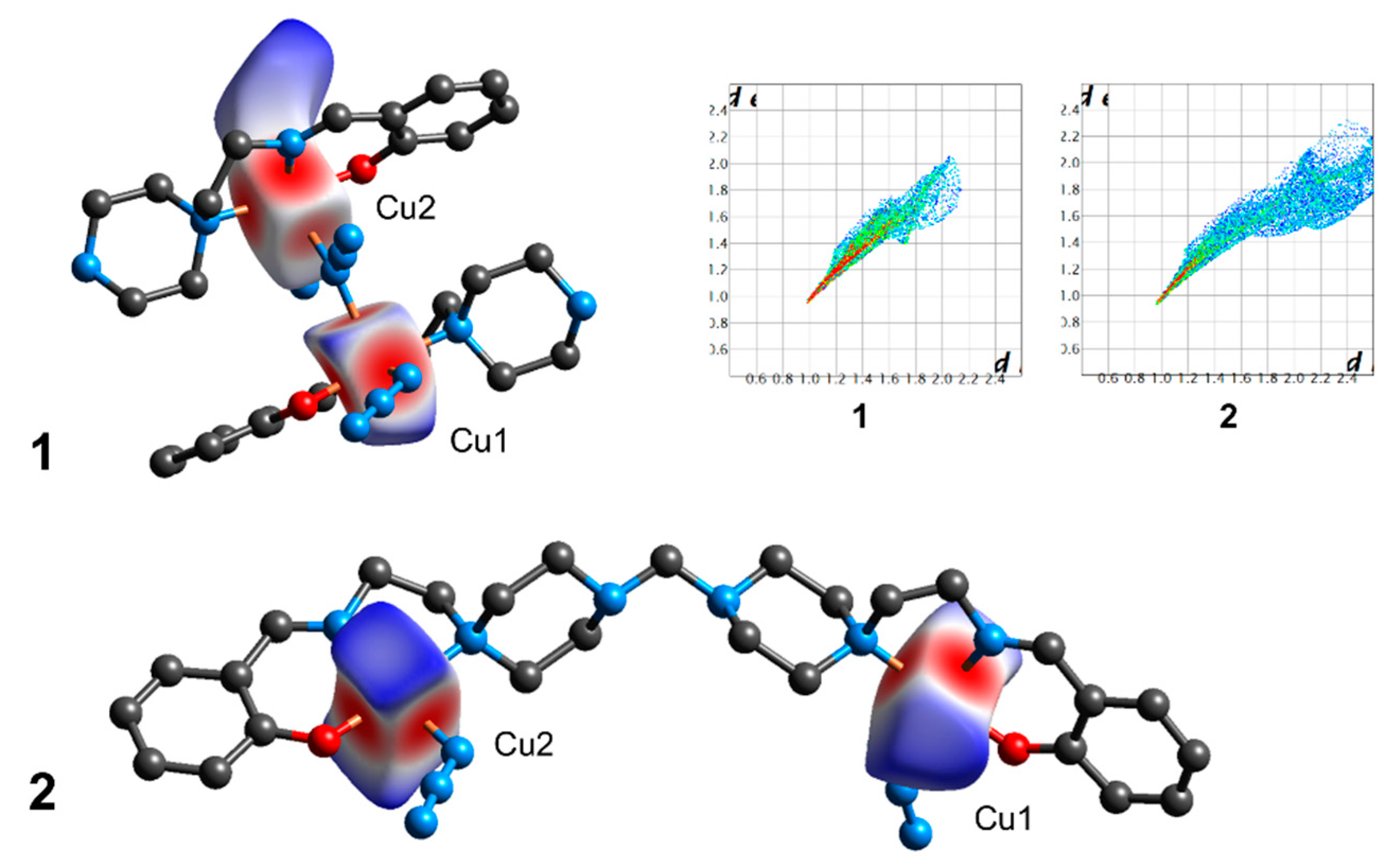
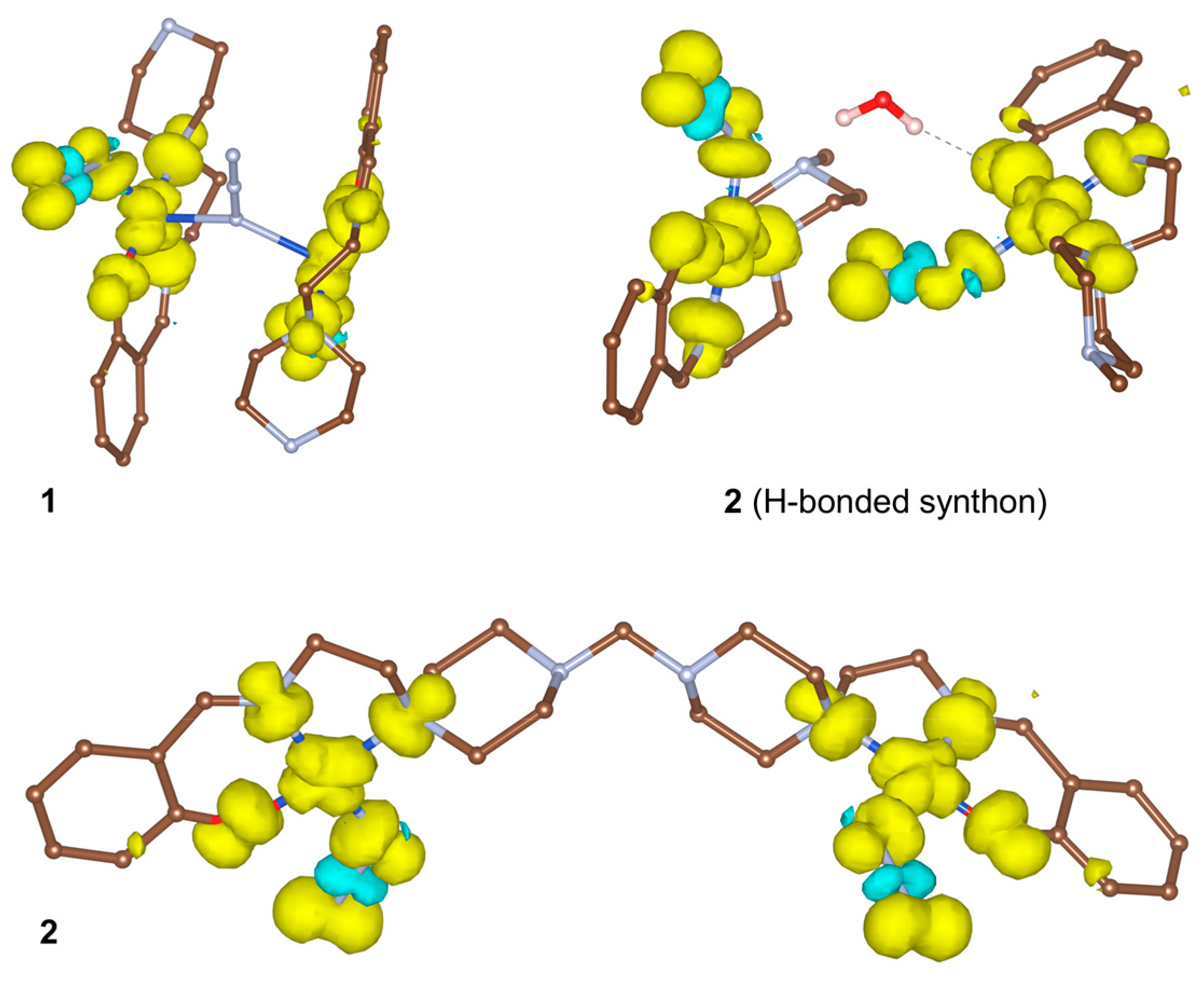
3.3. Hirshfeld Surface Analysis
3.4. DFT Calculations
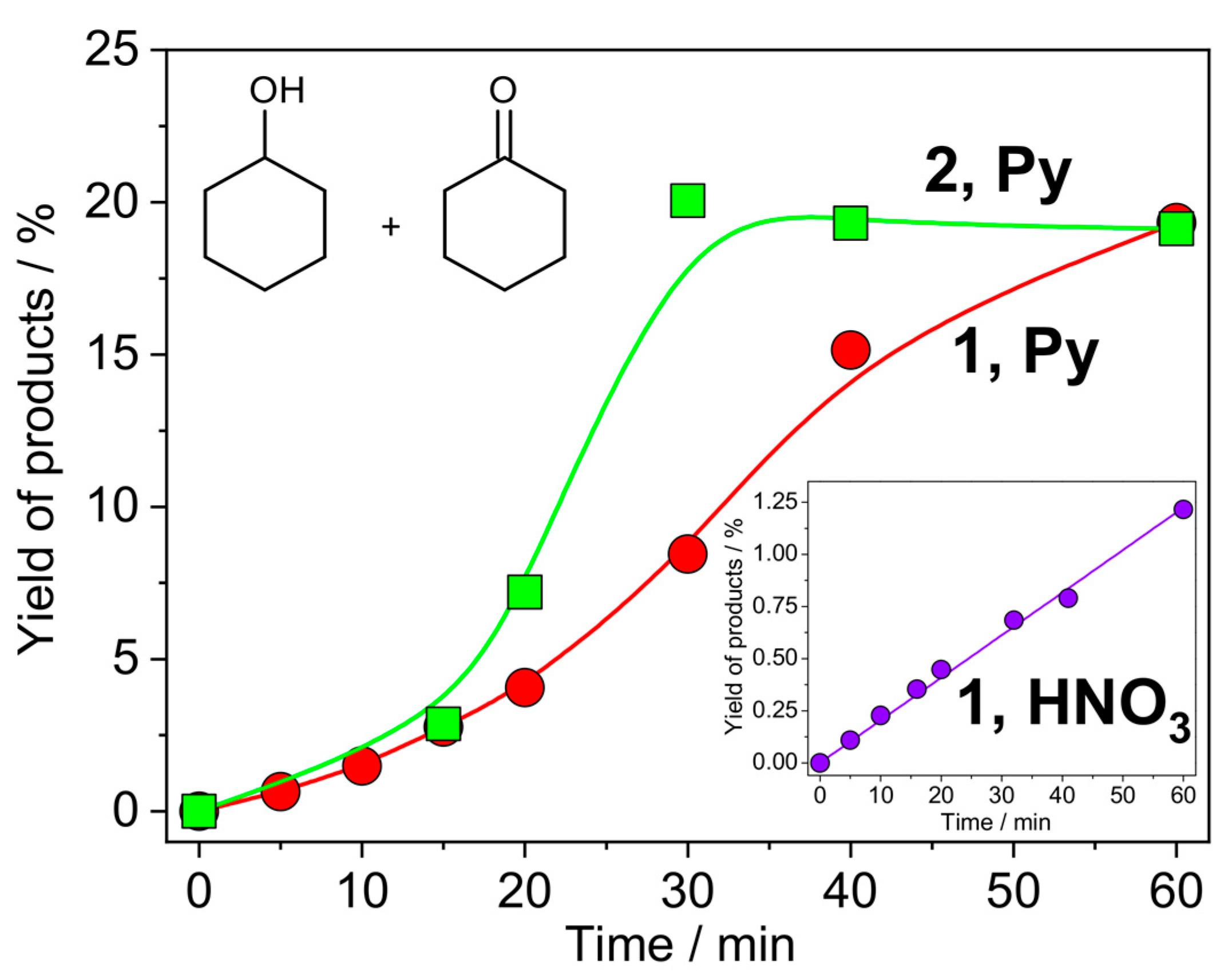
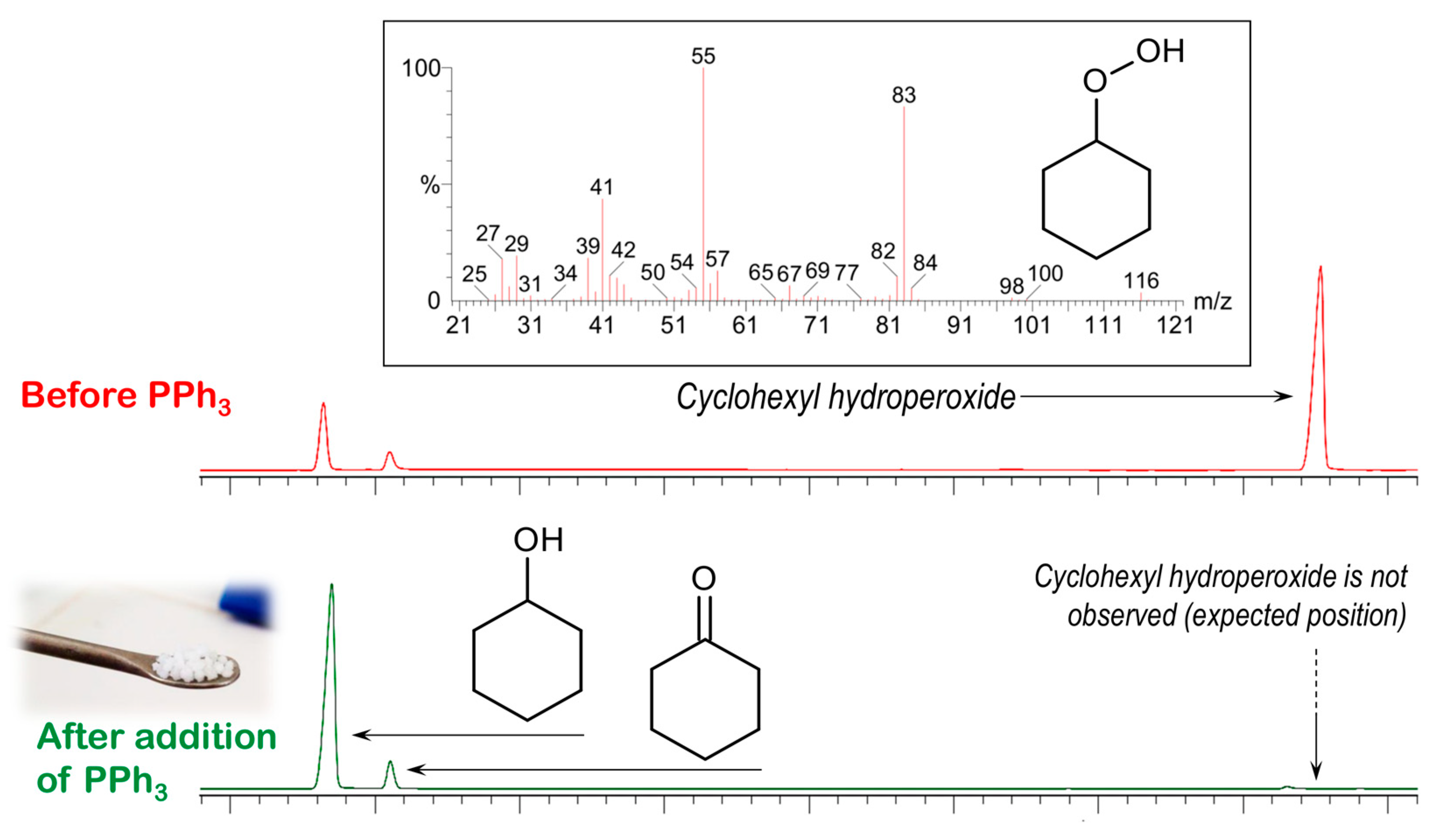
3.5. Catalytic Oxidation of Cyclohexane
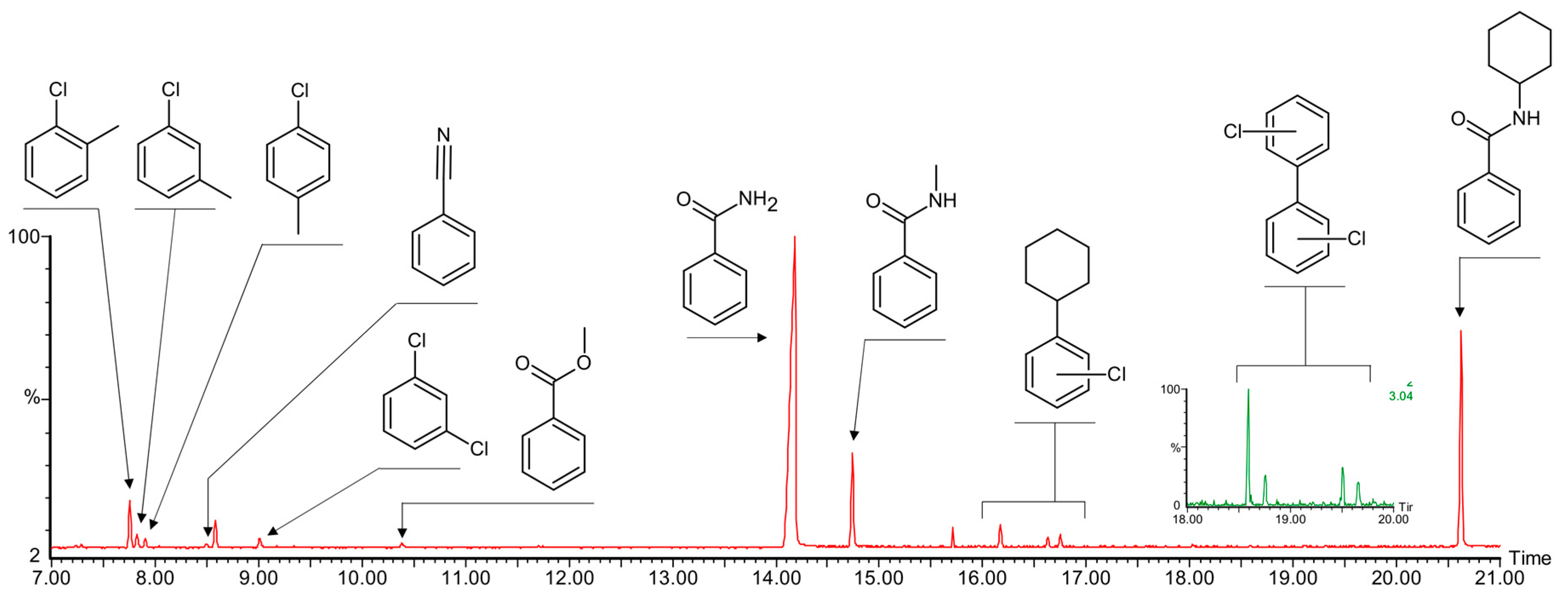
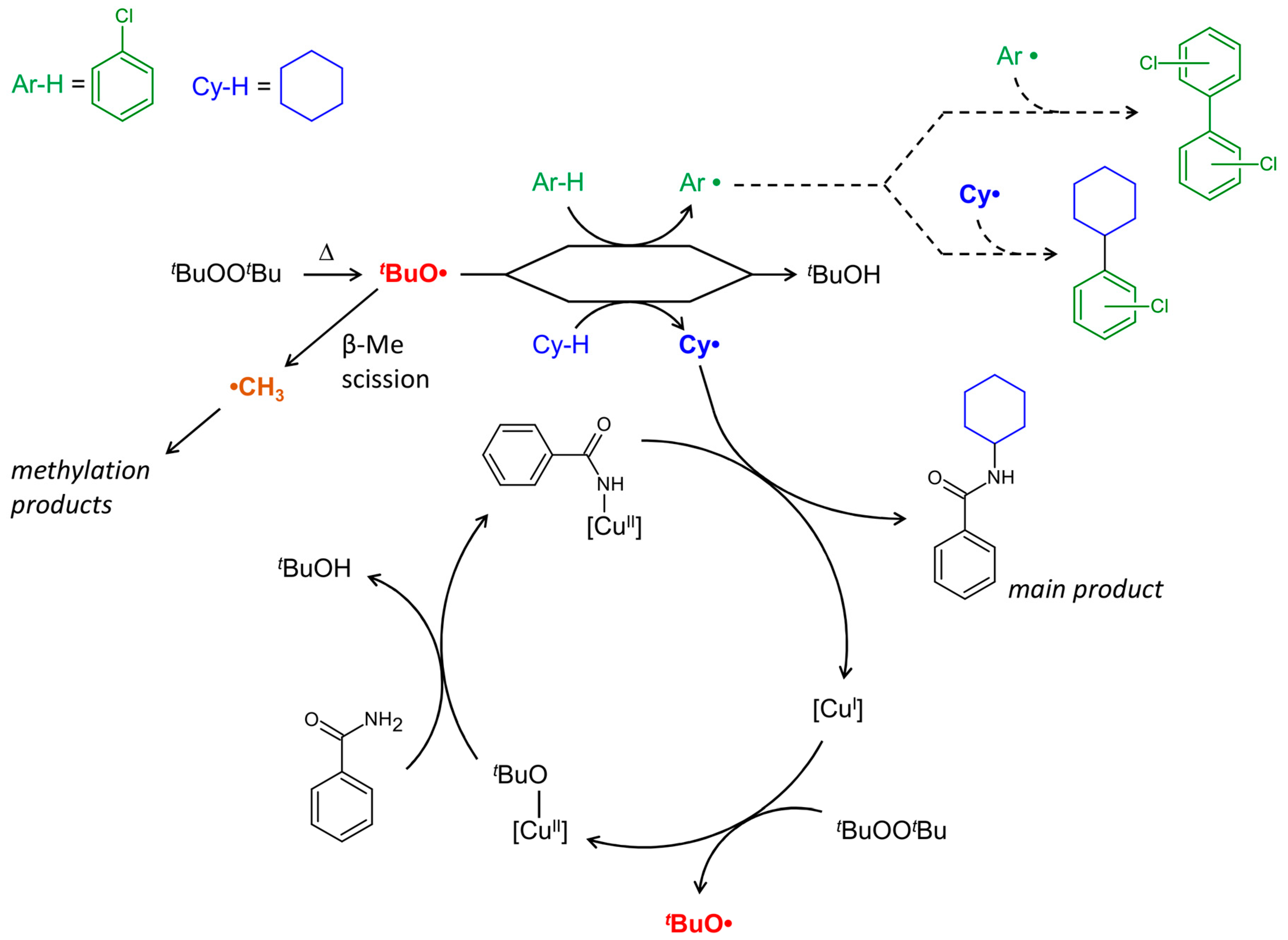
3.6. Catalytic Amidation of Cyclohexane
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Seoane, B.; Castellanos, S.; Dikhtiarenko, A.; Kapteijn, F.; Gascon, J. Multi-scale crystal engineering of metal organic frameworks. Coord. Chem. Rev. 2016, 307, 147–187. [Google Scholar] [CrossRef]
- Cui, Y.J.; Li, B.; He, H.J.; Zhou, W.; Chen, B.L.; Qian, G.D. Metal-Organic Frameworks as Platforms for Functional Materials. Acc. Chem. Res. 2016, 49, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Thorarinsdottir, A.E.; Harris, T.D. Metal-Organic Framework Magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Gimenez, V.; Tatay, S.; Marti-Gastaldo, C. Electrical conductivity and magnetic bistability in metal-organic frameworks and coordination polymers: Charge transport and spin crossover at the nanoscale. Chem. Soc. Rev. 2020, 49, 5601–5638. [Google Scholar] [CrossRef]
- Nesterov, D.S.; Nesterova, O.V.; Pombeiro, A.J.L. Homo- and heterometallic polynuclear transition metal catalysts for alkane C-H bonds oxidative functionalization: Recent advances. Coord. Chem. Rev. 2018, 355, 199–222. [Google Scholar] [CrossRef]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal-Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Liu, X.Q.; Jiang, H.L.; Sun, L.B. Metal-Organic Frameworks for Heterogeneous Basic Catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhao, S.N.; Zang, S.Q.; Li, J. Functional metal-organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef]
- Kirchon, A.; Feng, L.; Drake, H.F.; Joseph, E.A.; Zhou, H.C. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar] [CrossRef]
- Cai, X.C.; Xie, Z.X.; Li, D.D.; Kassymova, M.; Zang, S.Q.; Jiang, H.L. Nano-sized metal-organic frameworks: Synthesis and applications. Coord. Chem. Rev. 2020, 417, 21. [Google Scholar] [CrossRef]
- Pombeiro, A.J.L.; Guedes da Silva, M.F.C. Alkane Functionalization; Wiley: Hoboken, NJ, USA, 2019; pp. 1–15. [Google Scholar]
- Wang, V.C.C.; Maji, S.; Chen, P.R.Y.; Lee, H.K.; Yu, S.S.F.; Chan, S.I. Alkane Oxidation: Methane Monooxygenases, Related Enzymes, and Their Biomimetics. Chem. Rev. 2017, 117, 8574–8621. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.F. Evolution of C-H Bond Functionalization from Methane to Methodology. J. Am. Chem. Soc. 2016, 138, 2–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shul’pin, G.B. New Trends in Oxidative Functionalization of Carbon-Hydrogen Bonds: A Review. Catalysts 2016, 6, 50. [Google Scholar] [CrossRef] [Green Version]
- Kirillov, A.M.; Kirillova, M.V.; Pombeiro, A.J.L. Homogeneous Multicopper Catalysts for Oxidation and Hydrocarboxylation of Alkanes. Adv. Inorg. Chem. 2013, 65, 1–31. [Google Scholar] [CrossRef]
- Sharples, J.W.; Collison, D. The coordination chemistry and magnetism of some 3d-4f and 4f amino-polyalcohol compounds. Coord. Chem. Rev. 2014, 260, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Nesterova, O.V.; Nesterov, D.S. Polynuclear Cobalt Complexes as Catalysts for Light-Driven Water Oxidation: A Review of Recent Advances. Catalysts 2018, 8, 602. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Hamon, J.R. Recent developments in penta-, hexa- and heptadentate Schiff base ligands and their metal complexes. Coord. Chem. Rev. 2019, 389, 94–118. [Google Scholar] [CrossRef]
- Liu, X.; Manzur, C.; Novoa, N.; Celedon, S.; Carrillo, D.; Hamon, J.-R. Multidentate unsymmetrically-substituted Schiff bases and their metal complexes: Synthesis, functional materials properties, and applications to catalysis. Coord. Chem. Rev. 2018, 357, 144–172. [Google Scholar] [CrossRef]
- Nesterov, D.S.; Nesterova, O.V.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Catalytic behaviour of a novel Fe(III) Schiff base complex in the mild oxidation of cyclohexane. Catal. Sci. Technol. 2015, 5, 1801–1812. [Google Scholar] [CrossRef]
- Nesterova, O.V.; Nesterov, D.S.; Krogul-Sobczak, A.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Synthesis, crystal structures and catalytic activity of Cu(II) and Mn(III) Schiff base complexes: Influence of additives on the oxidation catalysis of cyclohexane and 1-phenylehanol. J. Mol. Catal. A 2017, 426, 506–515. [Google Scholar] [CrossRef]
- Nesterova, O.V.; Kasyanova, K.V.; Buvaylo, E.A.; Vassilyeva, O.Y.; Skelton, B.W.; Nesterov, D.S.; Pombeiro, A.J.L. Heterometallic CoIIIZnII Schiff Base Catalyst for Mild Hydroxylation of C(sp3)-H Bonds of Unactivated Alkanes: Evidence for Dual Mechanism Controlled by the Promoter. Catalysts 2019, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Nesterova, O.V.; Kasyanova, K.V.; Makhankova, V.G.; Kokozay, V.N.; Vassilyeva, O.Y.; Skelton, B.W.; Nesterov, D.S.; Pombeiro, A.J.L. Stereospecific sp3 C-H oxidation with m-CPBA: A CoIII Schiff base complex as pre-catalyst vs. its CoIIICdII heterometallic derivative. Appl. Catal. A 2018, 560, 171–184. [Google Scholar] [CrossRef]
- Nesterova, O.V.; Nesterov, D.S.; Jezierska, J.; Pombeiro, A.J.L.; Ozarowski, A. Copper(II) Complexes with Bulky N-Substituted Diethanolamines: High-Field Electron Paramagnetic Resonance, Magnetic, and Catalytic Studies in Oxidative Cyclohexane Amidation. Inorg. Chem. 2018, 57, 12384–12397. [Google Scholar] [CrossRef] [PubMed]
- Nesterova, O.V.; Kopylovich, M.N.; Nesterov, D.S. Stereoselective oxidation of alkanes with m-CPBA as an oxidant and cobalt complex with isoindole-based ligands as catalysts. RSC Adv. 2016, 6, 93756–93767. [Google Scholar] [CrossRef]
- Nesterov, D.S.; Kokozay, V.N.; Dyakonenko, V.V.; Shishkin, O.V.; Jezierska, J.; Ozarowski, A.; Kirillov, A.M.; Kopylovich, M.N.; Pombeiro, A.J.L. An unprecedented heterotrimetallic Fe/Cu/Co core for mild and highly efficient catalytic oxidation of cycloalkanes by hydrogen peroxide. Chem. Commun. 2006, 4605–4607. [Google Scholar] [CrossRef] [PubMed]
- Bruker. APEX2 & SAINT; AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer (Version 17.5); University of Western Australia: Perth, Australia, 2020. [Google Scholar]
- Malrieu, J.P.; Caballol, R.; Calzado, C.J.; de Graaf, C.; Guihery, N. Magnetic Interactions in Molecules and Highly Correlated Materials: Physical Content, Analytical Derivation, and Rigorous Extraction of Magnetic Hamiltonians. Chem. Rev. 2014, 114, 429–492. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.; Cano, J.; Alvarez, S.; Alemany, P. Broken symmetry approach to calculation of exchange coupling constants for homobinuclear and heterobinuclear transition metal complexes. J. Comput. Chem. 1999, 20, 1391–1400. [Google Scholar] [CrossRef]
- Onofrio, N.; Mouesca, J.-M. Analysis of the Singlet-Triplet Splitting Computed by the Density Functional Theory-Broken-Symmetry Method: Is It an Exchange Coupling Constant? Inorg. Chem. 2011, 50, 5577–5586. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Exchange-Energy Approximation With Correct Asymptotic-Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-Functional Approximation for the Correlation-Energy of the Inhomogeneous Electron-Gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P. Correction. Phys. Rev. B 1986, 34, 7406. [Google Scholar] [CrossRef]
- Kendall, R.A.; Fruchtl, H.A. The impact of the resolution of the identity approximate integral method on modern ab initio algorithm development. Theor. Chem. Acc. 1997, 97, 158–163. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2017, 8, e1327. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminformatics 2012, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Noodleman, L.; Davidson, E.R. Ligand Spin Polarization and Antiferromagnetic Coupling in Transition-Metal Dimers. Chem. Phys. 1986, 109, 131–143. [Google Scholar] [CrossRef]
- Noodleman, L. Valence Bond Description of Anti-Ferromagnetic Coupling in Transition-Metal Dimers. J. Chem. Phys. 1981, 74, 5737–5743. [Google Scholar] [CrossRef]
- Ginsberg, A.P. Magnetic Exchange in Transition-Metal Complexes. 12. Calculation of Cluster Exchange Coupling-Constants With The X-Alpha-Scattered Wave Method. J. Am. Chem. Soc. 1980, 102, 111–117. [Google Scholar] [CrossRef]
- Bencini, A.; Gatteschi, D. X-Alpha-Sw Calculations of the Electronic-Structure and Magnetic-Properties of Weakly Coupled Transition-Metal Clusters-the [Cu2Cl6]2- Dimers. J. Am. Chem. Soc. 1986, 108, 5763–5771. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalyzed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Lu, J.; Han, L.W.; Lin, J.X.; Cao, R. Organic Complexes Built by Halogenated Molecules: Unexpected in Situ C-N Bond Formation in Metal-Free Solvothermal Conditions. Cryst. Growth. Des. 2011, 11, 2035–2038. [Google Scholar] [CrossRef]
- Heravi, M.M.; Ghavidel, M.; Mohammadkhani, L. Beyond a solvent: Triple roles of dimethylformamide in organic chemistry. RSC Adv. 2018, 8, 27832–27862. [Google Scholar] [CrossRef] [Green Version]
- Jad, Y.E.; Acosta, G.A.; Khattab, S.N.; de la Torre, B.G.; Govender, T.; Kruger, H.G.; El-Faham, A.; Albericio, F. Peptide synthesis beyond DMF: THF and ACN as excellent and friendlier alternatives. Org. Biomol. Chem. 2015, 13, 2393–2398. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, D.S.; Murray, J.; Sneddon, H.F.; Jamieson, C.; Watson, A.J.B. Evaluation of alternative solvents in common amide coupling reactions: Replacement of dichloromethane and N. N-dimethylformamide. Green Chem. 2013, 15, 596–600. [Google Scholar] [CrossRef] [Green Version]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Groom, C.R.; Allen, F.H. The Cambridge Structural Database in Retrospect and Prospect. Angew. Chem. Int. Ed. 2014, 53, 662–671. [Google Scholar] [CrossRef]
- CSD refcodes AVOJAX [45], AVOJEB [45], INEKIV (Lu, Y.-X.; Liu, C.-M.; Zou, Z.-G.; Xu, W.; Wang, J.-M.; Chen, M.-Q.; Huang, Y.-M. Bis(4-phenylpiperazin-1-yl)methane. /Acta Crystallogr. E/ 2003, /59/, o1960–o1961, doi:10.1107/S1600536803026011), MIHWIL (Kavitha, C.N.; Jasinski, J.P.; Anderson, B.J.; Yathirajan, H.S.; Kaur, M. Bis{4-[(1,3-benzodioxol-5-yl)methyl]piperazin-1-yl}methane. /Acta Crystallogr. E/ 2013, /69/, o1669, doi:10.1107/S1600536813028109), REHDUE (Ricken, S.; Schurmann, M.; Preut, H.; Eilbracht, P. Bis[4-(2-methyl-2-propenyl)piperazin-1-yl]methane. /Acta Crystallogr. E/ 2006, /62/, o1435–o1436, doi:10.1107/S1600536806008622), ULOZOM (Lian, Z.-M.; Juan, S. The crystal structure of bis(4-(2,4-dimethylphenyl)piperazin-1-yl)methane, C25H36N4. /Z. Kristallogr.-New Cryst. Struct./ 2016, /231/, 339–341, doi:10.1515/ncrs-2013-8055). The respective crystal structures can be retrieved using the free service provided by the CCDC at https://www.ccdc.cam.ac.uk/structures/.
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Naiya, S.; Biswas, C.; Drew, M.G.B.; Gomez-Garcia, C.J.; Clemente-Juan, J.M.; Ghosh, A. A Unique Example of Structural and Magnetic Diversity in Four Interconvertible Copper(II)-Azide Complexes with the Same Schiff Base Ligand: A Monomer, a Dimer, a Chain, and a Layer. Inorg. Chem. 2010, 49, 6616–6627. [Google Scholar] [CrossRef]
- Reger, D.L.; Pascui, A.E.; Smith, M.D.; Jezierska, J.; Ozarowski, A. Syntheses, Structural, Magnetic, and Electron Paramagnetic Resonance Studies of Monobridged Cyanide and Azide Dinuclear Copper(II) Complexes: Antiferromagnetic Superexchange Interactions. Inorg. Chem. 2015, 54, 1487–1500. [Google Scholar] [CrossRef] [PubMed]
- Buvaylo, E.A.; Kokozay, V.N.; Vassilyeva, O.Y.; Skelton, B.W.; Jezierska, J.; Brunel, L.C.; Ozarowski, A. A Cu-Zn-Cu-Zn heterometallomacrocycle shows significant antiferromagnetic coupling between paramagnetic centres mediated by diamagnetic metal. Chem. Commun. 2005, 4976–4978. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, M.L.; Pombeiro, A.J.L. Radical Formation in the [MeReO3]-Catalyzed Aqueous Peroxidative Oxidation of Alkanes: A Theoretical Mechanistic Study. Inorg. Chem. 2009, 48, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, M.V.; Kuznetsov, M.L.; Romakh, V.B.; Shul’pina, L.S.; Frausto da Silva, J.J.R.; Pombeiro, A.J.L.; Shul’pin, G.B. Mechanism of oxidations with H2O2 catalyzed by vanadate anion or oxovanadium(V) triethanolaminate (vanadatrane) in combination with pyrazine-2-carboxylic acid (PCA): Kinetic and DFT studies. J. Catal. 2009, 267, 140–157. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Carvalho, W.A.; Mandelli, D. Oxidation reactions catalyzed by osmium compounds. Part 4. Highly efficient oxidation of hydrocarbons and alcohols including glycerol by the H2O2/Os3(CO)12/pyridine reagent. RSC Adv. 2013, 3, 15065–15074. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Kudinov, A.R.; Mandelli, D. Extremely Efficient Alkane Oxidation by a New Catalytic Reagent H2O2/Os3(CO)12/Pyridine. Inorg. Chem. 2009, 48, 10480–10482. [Google Scholar] [CrossRef]
- Nesterova, O.V.; Kopylovich, M.N.; Nesterov, D.S. A Comparative Study of the Catalytic Behaviour of Alkoxy-1,3,5-Triazapentadiene Copper(II) Complexes in Cyclohexane Oxidation. Inorganics 2019, 7, 82. [Google Scholar] [CrossRef] [Green Version]
- Buvaylo, E.A.; Kokozay, V.N.; Vassilyeva, O.Y.; Skelton, B.W.; Nesterova, O.V.; Pombeiro, A.J.L. Copper(II) complex of the 2-pyridinecarbaldehyde aminoguanidine Schiff base: Crystal structure and catalytic behaviour in mild oxidation of alkanes. Inorg. Chem. Commun. 2017, 78, 85–90. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kozlov, Y.N.; Shul’pina, L.S.; Lyakin, O.Y.; Kirillov, A.M.; Talsi, E.P.; Pombeiro, A.J.L.; Shul’pin, G.B. Remarkably fast oxidation of alkanes by hydrogen peroxide catalyzed by a tetracopper(II) triethanolaminate complex: Promoting effects of acid co-catalysts and water, kinetic and mechanistic features. J. Catal. 2009, 268, 26–38. [Google Scholar] [CrossRef]
- Gryca, I.; Czerwinska, K.; Machura, B.; Chrobok, A.; Shul’pina, L.S.; Kuznetsov, M.L.; Nesterov, D.S.; Kozlov, Y.N.; Pombeiro, A.J.L.; Varyan, I.A.; et al. High Catalytic Activity of Vanadium Complexes in Alkane Oxidations with Hydrogen Peroxide: An Effect of 8-Hydroxyquinoline Derivatives as Noninnocent Ligands. Inorg. Chem. 2018, 57, 1824–1839. [Google Scholar] [CrossRef]
- Astakhov, G.S.; Bilyachenko, A.N.; Korlyukov, A.A.; Levitsky, M.M.; Shul’pina, L.S.; Bantreil, X.; Lamaty, F.; Vologzhanina, A.V.; Shubina, E.S.; Dorovatovskii, P.V.; et al. High-Cluster Cu9 Cage Silsesquioxanes: Synthesis, Structure, and Catalytic Activity. Inorg. Chem. 2018, 57, 11524–11529. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B.; Nesterov, D.S.; Shul’pina, L.S.; Pombeiro, A.J.L. A hydroperoxo-rebound mechanism of alkane oxidation with hydrogen peroxide catalyzed by binuclear manganese(IV) complex in the presence of an acid with involvement of atmospheric dioxygen. Inorg. Chim. Acta. 2017, 455, 666–676. [Google Scholar] [CrossRef]
- Nesterova, O.V.; Nesterov, D.S.; Vranovicova, B.; Boca, R.; Pombeiro, A.J.L. Heterometallic CuIIFeIII and CuIIMnIII alkoxobridged complexes revealing a rare hexanuclear M6(µ-X)7(µ3-X)2 molecular core. Dalton Trans. 2018, 47, 10941–10952. [Google Scholar] [CrossRef] [PubMed]
- Bilyachenko, A.N.; Levitsky, M.M.; Yalymov, A.I.; Korlyukov, A.A.; Vologzhanina, A.V.; Kozlov, Y.N.; Shul’pina, L.S.; Nesterov, D.S.; Pombeiro, A.J.L.; Lamaty, F.; et al. A heterometallic Fe6Na8 cage-like silsesquioxane: Synthesis, structure, spin glass behavior and high catalytic activity. RSC Adv. 2016, 6, 48165–48180. [Google Scholar] [CrossRef]
- Tran, B.L.; Li, B.J.; Driess, M.; Hartwig, J.F. Copper-Catalyzed Intermolecular Amidation and Imidation of Unactivated Alkanes. J. Am. Chem. Soc. 2014, 136, 2555–2563. [Google Scholar] [CrossRef] [PubMed]
| 1 | 2 | |
|---|---|---|
| Empirical Formula | C26H41Cu2N15O4 | C27H38Cu2N12O3 |
| Formula Weight | 754.82 | 705.77 |
| Crystal System | Orthorhombic | Monoclinic |
| Space Group | Pbca | P 21/c |
| a/Å | 13.6181(10) | 11.3959(16) |
| b/Å | 19.6836(14) | 19.983(3) |
| c/Å | 25.2572(18) | 13.3403(17) |
| α/° | 90 | 90 |
| β/° | 90 | 90.335(6) |
| γ/° | 90 | 90 |
| V/Å3 | 6770.3(8) | 3037.8(7) |
| Z | 8 | 4 |
| Calculated Density/g cm−3 | 1.481 | 1.543 |
| T, K | 296(2) | 296(2) |
| μ(Mo-Kα)/mm−1 | 1.313 | 1.452 |
| F(000) | 3136 | 1464 |
| Reflections Collected/Unique | 44613/5951 | 21704/5812 |
| Rint | 0.1175 | 0.1057 |
| Reflections with F2 > 2σ(F2) | 3586 | 2005 |
| Θmin, Θmax/° | 2.199, 25.014 | 3.220, 26.373 |
| R1, F2 > 2σ(F2) | 0.0743 | 0.0486 |
| wR2 (all data) | 0.2190 | 0.1192 |
| GoF | 1.096 | 0.729 |
| Radiation | Mo Kα | Mo Kα |
| CCDC numbers | 2036334 | 2036341 |
| Cu1–O1 | 1.940(6) | Cu2–O2 | 1.927(6) |
| Cu1–N2 | 2.101(6) | Cu2–N8 | 2.088(6) |
| Cu1–N3 | 1.967(6) | Cu2–N9 | 1.962(7) |
| Cu1–N4 | 1.996(7) | Cu2–N10 | 1.949(8) |
| Cu1–N13 | 2.329(7) | Cu2–N13 | 2.390(7) |
| O1–Cu1–N2 | 172.9(3) | O2–Cu2–N8 | 174.0(3) |
| O1–Cu1–N3 | 92.2(3) | O2–Cu2–N9 | 92.4(3) |
| O1–Cu1–N4 | 92.9(3) | O2–Cu2–N10 | 91.8(3) |
| O1–Cu1–N13 | 94.0(2) | O2–Cu2–N13 | 92.9(2) |
| N2–Cu1–N3 | 82.9(3) | N8–Cu2–N9 | 83.8(3) |
| N2–Cu1–N4 | 90.0(3) | N8–Cu2–N10 | 90.0(3) |
| N2–Cu1–N13 | 91.9(3) | N8–Cu2–N13 | 91.6(2) |
| N3–Cu1–N4 | 158.9(3) | N9–Cu2–N10 | 157.7(4) |
| N3–Cu1–N13 | 101.2(3) | N9–Cu2–N13 | 88.8(3) |
| N4–Cu1–N13 | 98.9(3) | N10–Cu2–N13 | 112.8(4) |
| Cu1–O1 | 1.905(4) | Cu2–O2 | 1.890(4) |
| Cu1–N1 | 1.924(5) | Cu2–N7 | 1.943(5) |
| Cu1–N2 | 2.080(4) | Cu2–N8 | 2.077(4) |
| Cu1–N4 | 1.949(4) | Cu2–N10 | 1.953(5) |
| O1–Cu1–N1 | 92.36(19) | O2–Cu2–N7 | 92.4(2) |
| O1–Cu1–N2 | 175.51(18) | O2–Cu2–N8 | 176.22(17) |
| O1–Cu1–N4 | 92.32(18) | O2–Cu2–N10 | 90.9(2) |
| N1–Cu1–N2 | 84.24(19) | N7–Cu2–N8 | 84.53(19) |
| N1–Cu1–N4 | 173.6(2) | N7–Cu2–N10 | 171.2(2) |
| N2–Cu1–N4 | 91.30(18) | N8–Cu2–N10 | 91.90(19) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nesterova, O.V.; Pombeiro, A.J.L.; Nesterov, D.S. Novel H-Bonded Synthons in Copper Supramolecular Frameworks with Aminoethylpiperazine-Based Ligands. Synthesis, Structure and Catalytic Activity. Materials 2020, 13, 5435. https://doi.org/10.3390/ma13235435
Nesterova OV, Pombeiro AJL, Nesterov DS. Novel H-Bonded Synthons in Copper Supramolecular Frameworks with Aminoethylpiperazine-Based Ligands. Synthesis, Structure and Catalytic Activity. Materials. 2020; 13(23):5435. https://doi.org/10.3390/ma13235435
Chicago/Turabian StyleNesterova, Oksana V., Armando J. L. Pombeiro, and Dmytro S. Nesterov. 2020. "Novel H-Bonded Synthons in Copper Supramolecular Frameworks with Aminoethylpiperazine-Based Ligands. Synthesis, Structure and Catalytic Activity" Materials 13, no. 23: 5435. https://doi.org/10.3390/ma13235435
APA StyleNesterova, O. V., Pombeiro, A. J. L., & Nesterov, D. S. (2020). Novel H-Bonded Synthons in Copper Supramolecular Frameworks with Aminoethylpiperazine-Based Ligands. Synthesis, Structure and Catalytic Activity. Materials, 13(23), 5435. https://doi.org/10.3390/ma13235435







