Evaluation of Physical Properties of a Metakaolin-Based Alkali-Activated Binder Containing Waste Foam Glass
Abstract
:1. Introduction
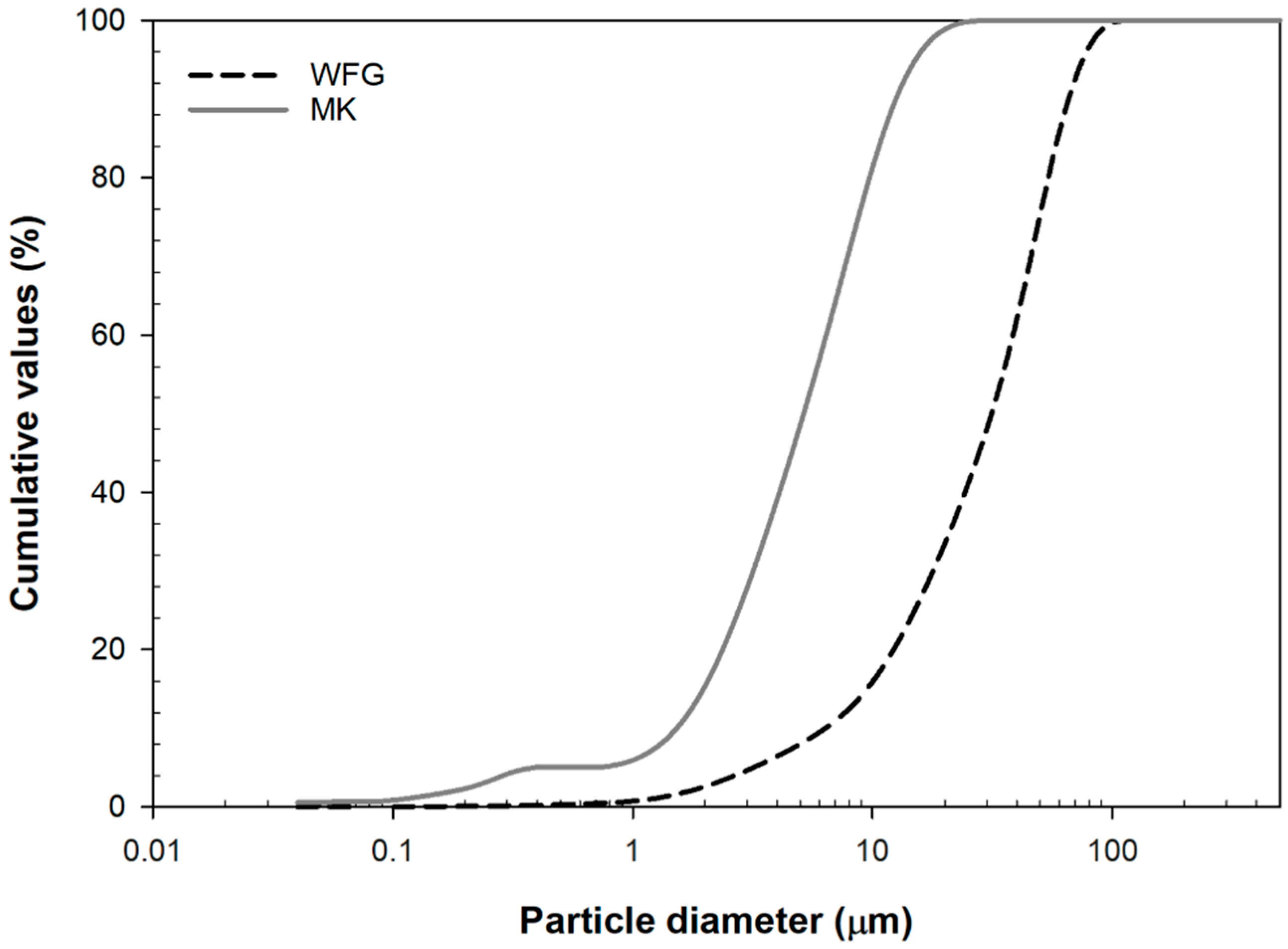
2. Materials and Methods
3. Results
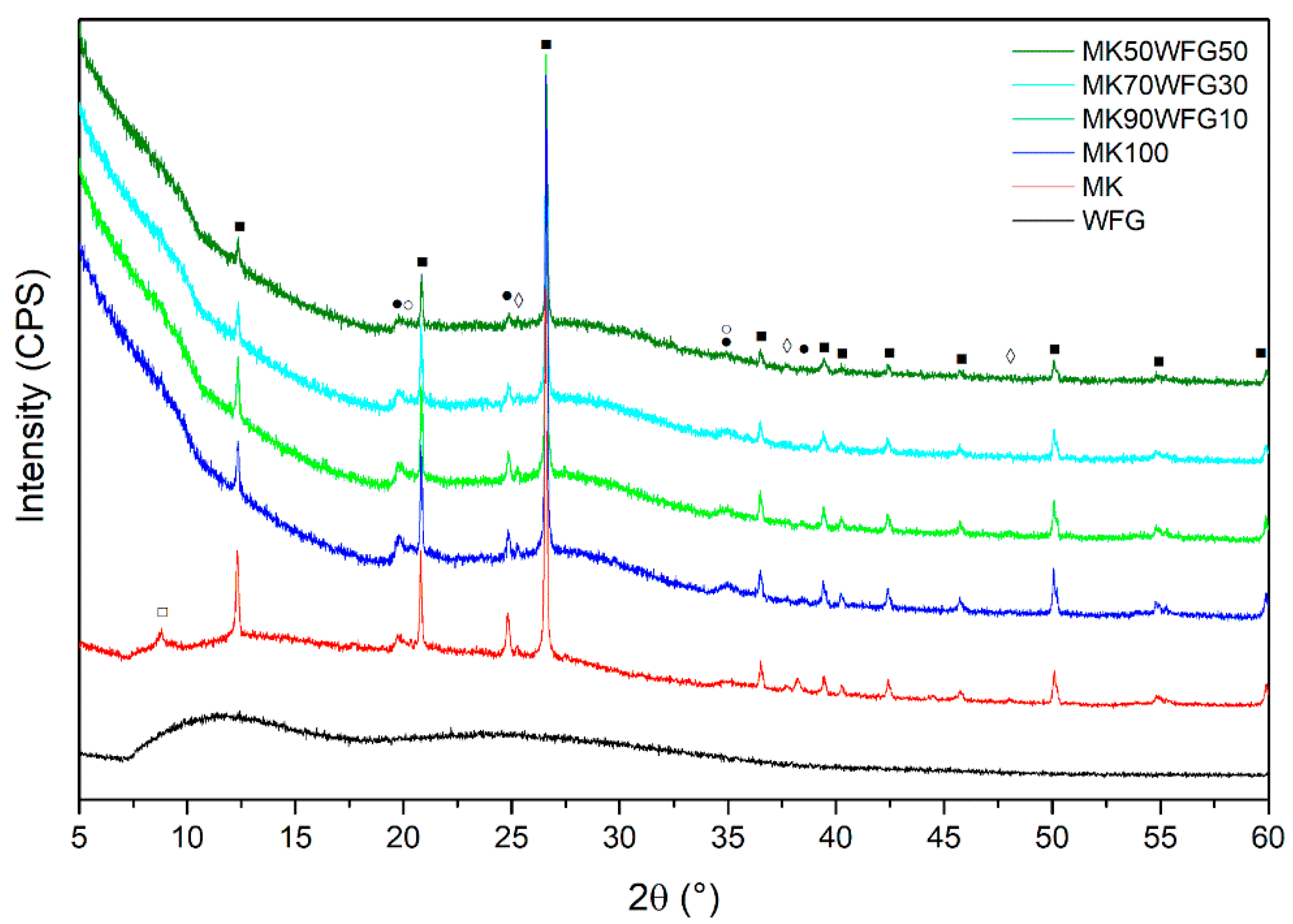
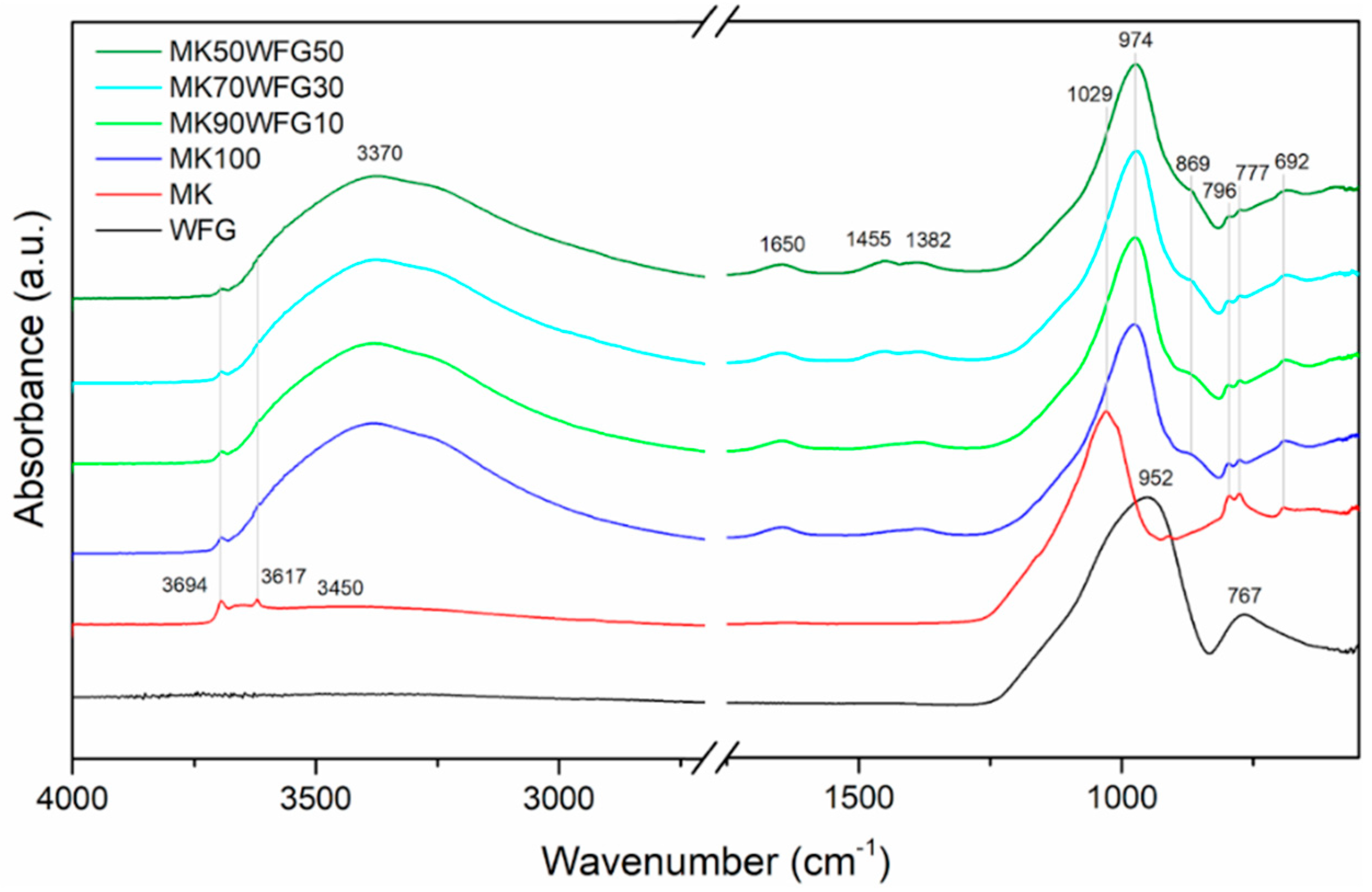
3.1. Phase Characterization
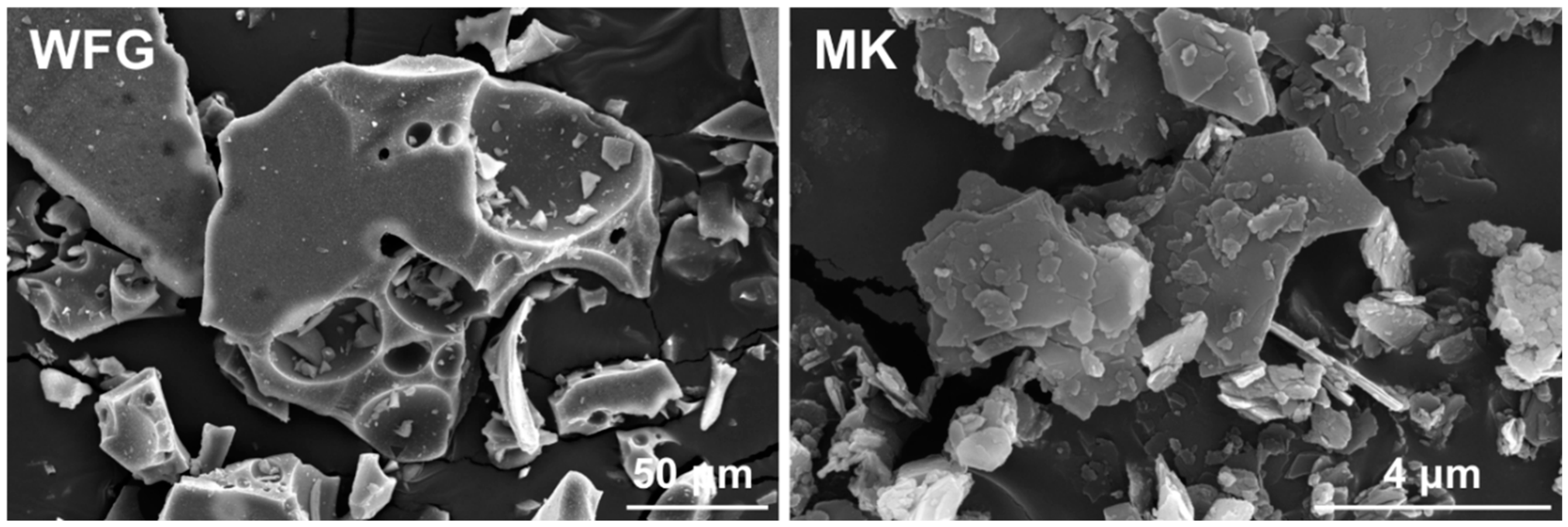
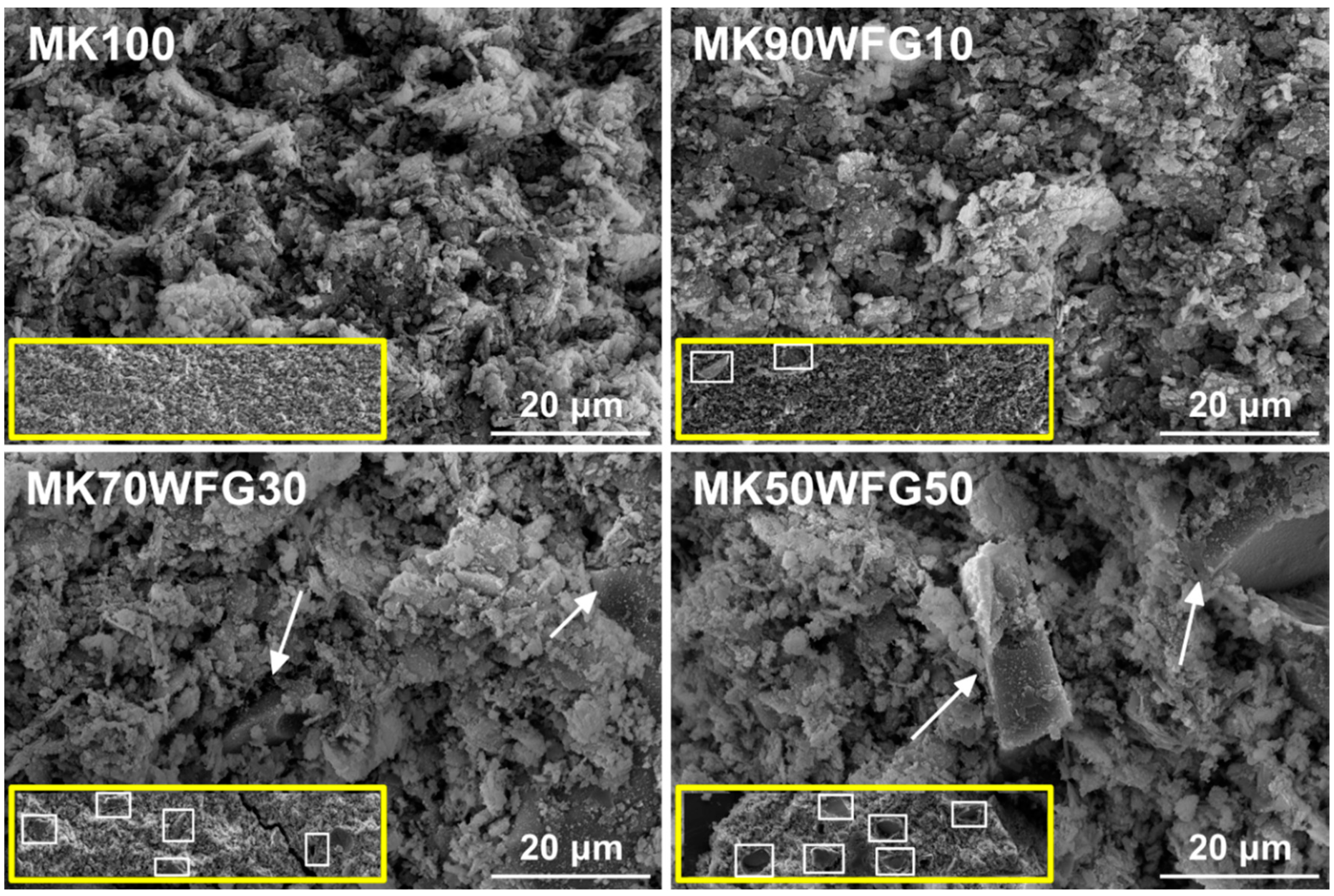
3.2. Morphological Characterization
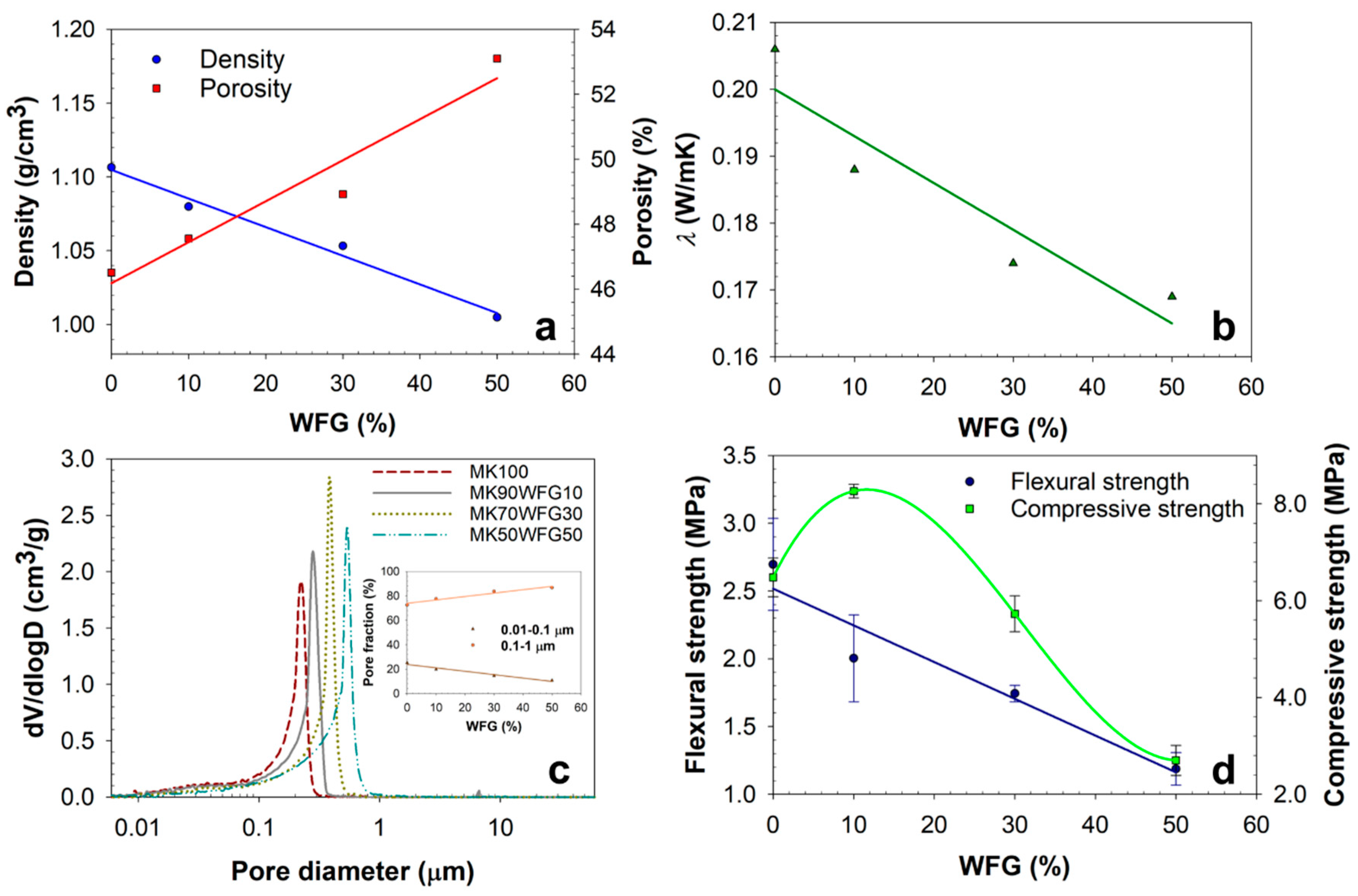
3.3. Physical and Mechanical Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cui, S.P.; Zhang, J.G.; Tian, Y.L.; Sun, S.B.; Wu, Z.W.; Liu, W.C. Generation review on the production line development of foam glass at home and abroad. Adv. Mater. Res. 2014, 915–916, 524–531. [Google Scholar] [CrossRef]
- El-Haggar, S.M. Sustainable Industrial Design and Waste Management; Elsevier Inc.: Burlington, MA, USA, 2007; ISBN 9780123736239. [Google Scholar]
- Zhang, H. Building Materials in Civil Engineering, 1st ed.; Woodhead Publishing Limited: Cambridge, UK, 2011; ISBN 9781845699550. [Google Scholar]
- Manevich, V.E.; Subbotin, K.Y. Foam glass and problems of energy conservation. Glas. Ceram. 2008, 65, 105–108. [Google Scholar] [CrossRef]
- Ferone, C.; Capasso, I.; Bonati, A.; Roviello, G.; Montagnaro, F.; Santoro, L.; Turco, R.; Cioffi, R. Sustainable management of water potabilization sludge by means of geopolymers production. J. Clean. Prod. 2019, 229, 1–9. [Google Scholar] [CrossRef]
- Yang, Z.; Mocadlo, R.; Zhao, M.; Sisson, R.D.; Tao, M.; Liang, J. Preparation of a geopolymer from red mud slurry and class F fly ash and its behavior at elevated temperatures. Constr. Build. Mater. 2019, 221, 308–317. [Google Scholar] [CrossRef]
- Sotiriadis, K.; Guzii, S.G.; Mácová, P.; Viani, A.; Dvořák, K.; Drdácký, M. Thermal Behavior of an Intumescent Alkaline Aluminosilicate Composite Material for Fire Protection of Structural Elements. J. Mater. Civ. Eng. 2019, 31, 1–9. [Google Scholar] [CrossRef]
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. Constr. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Krivenko, P. Why alkaline activation—60 years of the theory and practice of alkali-activated materials. J. Ceram. Sci. Technol. 2017, 8, 323–334. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si + Ca) and metakaolin (Si + Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Bobirică, C.; Shim, J.H.; Pyeon, J.H.; Park, J.Y. Influence of waste glass on the microstructure and strength of inorganic polymers. Ceram. Int. 2015, 41, 13638–13649. [Google Scholar] [CrossRef]
- Chokkha, S.; Phetnat, P.; Chandadi, W.; Srisitthigul, M. Use of waste glass as a reinforce material in calcined-kaolin based geopolymer. Key Eng. Mater. 2017, 751, 556–562. [Google Scholar] [CrossRef]
- Hao, H.; Lin, K.-L.; Wang, D.; Chao, S.-J.; Shiu, H.-S.; Cheng, T.-W.; Hwang, C.-L. Utilization of solar panel waste glass for metakaolinite-based geopolymer synthesis. Environ. Prog. Sustain. Energy 2013, 32, 797–803. [Google Scholar] [CrossRef]
- Novais, R.M.; Ascensão, G.; Seabra, M.P.; Labrincha, J.A. Waste glass from end-of-life fluorescent lamps as raw material in geopolymers. Waste Manag. 2016, 52, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, N.; Taveri, G.; Hurle, K.; Roether, J.A.; Ercole, P.; Dlouhý, I.; Boccaccini, A.R. Fly-Ash-Based geopolymers: How the sddition of recycled glass or red mud waste influences the structural and mechanical properties. J. Ceram. Sci. Technol. 2017, 8, 411–420. [Google Scholar] [CrossRef]
- Xiao, R.; Ma, Y.; Jiang, X.; Zhang, M.; Zhang, Y.; Wang, Y.; Huang, B.; He, Q. Strength, microstructure, efflorescence behavior and environmental impacts of waste glass geopolymers cured at ambient temperature. J. Clean. Prod. 2020, 252. [Google Scholar] [CrossRef]
- Kristály, F.; Szabó, R.; Mádai, F.; Debreczeni, Á.; Mucsi, G. Lightweight composite from fly ash geopolymer and glass foam. J. Sustain. Cem. Mater. 2020, 1–22. [Google Scholar] [CrossRef]
- Cyr, M.; Idir, R.; Poinot, T. Properties of inorganic polymer (geopolymer) mortars made of glass cullet. J. Mater. Sci. 2012, 47, 2782–2797. [Google Scholar] [CrossRef]
- Rashidian-Dezfouli, H.; Rangaraju, P.R. Comparison of strength and durability characteristics of a geopolymer produced from fly ash, ground glass fiber and glass powder. Mater. Constr. 2017, 67. [Google Scholar] [CrossRef] [Green Version]
- Tho-In, T.; Sata, V.; Boonserm, K.; Chindaprasirt, P. Compressive strength and microstructure analysis of geopolymer paste using waste glass powder and fly ash. J. Clean. Prod. 2016, 172, 2892–2898. [Google Scholar] [CrossRef]
- Zhang, S.; Keulen, A.; Arbi, K.; Ye, G. Waste glass as partial mineral precursor in alkali-activated slag/fly ash system. Cem. Concr. Res. 2017, 102, 29–40. [Google Scholar] [CrossRef] [Green Version]
- El-Naggar, M.R.; El-Dessouky, M.I. Re-use of waste glass in improving properties of metakaolin-based geopolymers: Mechanical and microstructure examinations. Constr. Build. Mater. 2017, 132, 543–555. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Puertas, F. Waste glass in the geopolymer preparation. Mechanical and microstructural characterisation. J. Clean. Prod. 2015, 90, 397–408. [Google Scholar] [CrossRef]
- Toniolo, N.; Rincón, A.; Roether, J.A.; Ercole, P.; Bernardo, E.; Boccaccini, A.R. Extensive reuse of soda-lime waste glass in fly ash-based geopolymers. Constr. Build. Mater. 2018, 188, 1077–1084. [Google Scholar] [CrossRef]
- ÚNMZ Basic Analysis of Silicates—Common Regulations, ČSN 72 0100 2009; ÚNMZ: Prague, Czech Republic, 2009.
- ISO [International Organization for Standardization]. Determination of the Specific Surface Area of Solids by Gas Adsorption—BET Method; ISO 9277:2010(E); International Organization for Standardization: Geneva, Switzerland, 2010. [Google Scholar]
- Bednařík, V.; Vondruška, M. Conductometric analysis of water glass. Chem. List. 2008, 102, 444–446. [Google Scholar]
- Bai, C.; Li, H.; Bernardo, E.; Colombo, P. Waste-to-resource preparation of glass-containing foams from geopolymers. Ceram. Int. 2019, 45, 7196–7202. [Google Scholar] [CrossRef]
- Sotiriadis, K.; Guzii, S.; Kumpová, I.; Mácová, P.; Viani, A. The effect of firing temperature on the composition and microstructure of a geocement-based binder of sodium water-glass. Solid State Phenom. 2017, 267, 58–62. [Google Scholar] [CrossRef]
- Tchakoute Kouamo, H.; Elimbi, A.; Mbey, J.A.; Ngally Sabouang, C.J.; Njopwouo, D. The effect of adding alumina-oxide to metakaolin and volcanic ash on geopolymer products: A comparative study. Constr. Build. Mater. 2012, 35, 960–969. [Google Scholar] [CrossRef]
- Provis, J.L.; Yong, S.L.; Van Deventer, J.S.J. Characterising the reaction of metakaolin in an alkaline environment by XPS, and time- and spatially-resolved FTIR spectroscopy. In Calcined Clays for Sustainable Concrete; Scrivener, K., Favier, A., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 299–304. ISBN 9789401799393. [Google Scholar]
- White, C.E.; Provis, J.L.; Proffen, T.; Riley, D.P.; Van Deventer, J.S.J. Combining density functional theory (DFT) and pair distribution function (PDF) analysis to solve the structure of metastable materials: The case of metakaolin. Phys. Chem. Chem. Phys. 2010, 12, 3239–3245. [Google Scholar] [CrossRef] [PubMed]
- Kljajević, L.M.; Nenadović, S.S.; Nenadović, M.T.; Bundaleski, N.K.; Todorović, B.; Pavlović, V.B.; Rakočević, Z.L. Structural and chemical properties of thermally treated geopolymer samples. Ceram. Int. 2017, 43, 6700–6708. [Google Scholar] [CrossRef]
- Yunsheng, Z.; Wei, S.; Zongjin, L. Composition design and microstructural characterization of calcined kaolin-based geopolymer cement. Appl. Clay Sci. 2010, 47, 271–275. [Google Scholar] [CrossRef]
- Allahverdi, A.; Najafi Kani, E.; Hossain, K.M.A.; Lachemi, M. Methods to control efflorescence in alkali-activated cement-based materials. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Pacheco-Torgal, F., Labrincha, J.A., Leonelli, C., Palomo, A., Chindaprasirt, P., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2015; pp. 463–483. ISBN 9781782422884. [Google Scholar]
- Gong, X.Z.; Tian, Y.L.; Zhang, L.J. A comparative life cycle assessment of typical foam glass production. Mater. Sci. Forum 2018, 913, 1054–1061. [Google Scholar] [CrossRef]
- Yan, D.; Xie, L.; Qian, X.; Ruan, S.; Zeng, Q. Compositional dependence of pore structure, strengthand freezing-thawing resistance of metakaolin-based geopolymers. Materials 2020, 13, 2973. [Google Scholar] [CrossRef] [PubMed]
- Pouhet, R.; Cyr, M.; Bucher, R. Influence of the initial water content in flash calcined metakaolin-based geopolymer. Constr. Build. Mater. 2019, 201, 421–429. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Wang, Y.; Feng, J. Preparation and properties of alkali activated metakaolin-based geopolymer. Materials 2016, 9, 767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, V.F.F.; MacKenzie, K.J.D.; Thaumaturgo, C. Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: Sodium polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Engineering ToolBox Thermal Conductivity of Selected Materials and Gases. Available online: https://www.engineeringtoolbox.com/thermal-conductivity-d_429.html (accessed on 8 October 2020).
- Kryvenko, P.; Kyrychok, V.; Guzii, S. Influence of the ratio of oxides and temperature on the structure formation of alkaline hydro-aluminosilicates. East. Eur. J. Enterp. Technol. 2016, 5, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Hajimohammadi, A.; Ngo, T.; Kashani, A. Glass waste versus sand as aggregates: The characteristics of the evolving geopolymer binders. J. Clean. Prod. 2018, 193, 593–603. [Google Scholar] [CrossRef]

| Raw Material | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | MnO | Na2O | K2O | P2O5 | TiO2 | SO3 | LOI | Humidity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MK | 56.90 | 38.12 | 0.72 | 0.72 | 0.30 | 0.01 | 0.19 | 0.67 | 0.05 | 0.42 | 0.02 | 1.80 | 0.50 |
| WFG | 64.03 | 7.37 | 3.99 | 4.76 | 1.99 | 0.42 | 14.89 | 1.45 | 0.32 | 0.61 | 0.17 | 0.90 | 0.30 |
| Code | MK (g) | WFG (g) | Water Glass (g) | NaOH (g) | H2O (g) |
|---|---|---|---|---|---|
| MK100 | 324.0 | 0 | 207.93 | 40.48 | 147.60 |
| MK90WFG10 | 291.6 | 32.4 | 207.93 | 40.48 | 147.60 |
| MK70WFG30 | 226.8 | 97.2 | 207.93 | 40.48 | 147.60 |
| MK50WFG50 | 162.0 | 162.0 | 207.93 | 40.48 | 147.60 |
| WFG (%) | Density (g/cm3) | Porosity (%) | λ (W/m·K) | Flexural Strength (MPa) | Compressive Strength (MPa) |
|---|---|---|---|---|---|
| 0 | 1.11 | 46.5 | 0.206 | 2.70 | 6.48 |
| 10 | 1.08 | 47.6 | 0.188 | 2.00 | 8.27 |
| 30 | 1.05 | 48.9 | 0.174 | 1.74 | 5.73 |
| 50 | 1.01 | 53.1 | 0.169 | 1.19 | 2.70 |
| WFG (%) | Total Intrusion Volume (mL/g) | Total Pore Area (m2/g) | Average Pore Diameter (Å) | Bulk Density At 0.53 Psia (g/mL) |
|---|---|---|---|---|
| 0 | 0.42 | 19.8 | 1881 | 1.13 |
| 10 | 0.43 | 17.6 | 2479 | 1.09 |
| 30 | 0.47 | 13.8 | 3484 | 1.05 |
| 50 | 0.51 | 11.4 | 4372 | 1.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mácová, P.; Sotiriadis, K.; Slížková, Z.; Šašek, P.; Řehoř, M.; Závada, J. Evaluation of Physical Properties of a Metakaolin-Based Alkali-Activated Binder Containing Waste Foam Glass. Materials 2020, 13, 5458. https://doi.org/10.3390/ma13235458
Mácová P, Sotiriadis K, Slížková Z, Šašek P, Řehoř M, Závada J. Evaluation of Physical Properties of a Metakaolin-Based Alkali-Activated Binder Containing Waste Foam Glass. Materials. 2020; 13(23):5458. https://doi.org/10.3390/ma13235458
Chicago/Turabian StyleMácová, Petra, Konstantinos Sotiriadis, Zuzana Slížková, Petr Šašek, Michal Řehoř, and Jaroslav Závada. 2020. "Evaluation of Physical Properties of a Metakaolin-Based Alkali-Activated Binder Containing Waste Foam Glass" Materials 13, no. 23: 5458. https://doi.org/10.3390/ma13235458
APA StyleMácová, P., Sotiriadis, K., Slížková, Z., Šašek, P., Řehoř, M., & Závada, J. (2020). Evaluation of Physical Properties of a Metakaolin-Based Alkali-Activated Binder Containing Waste Foam Glass. Materials, 13(23), 5458. https://doi.org/10.3390/ma13235458






