Effect of Ga on the Oxide Film Structure and Oxidation Resistance of Sn–Bi–Zn Alloys as Heat Transfer Fluids
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Oxidation Kinetics
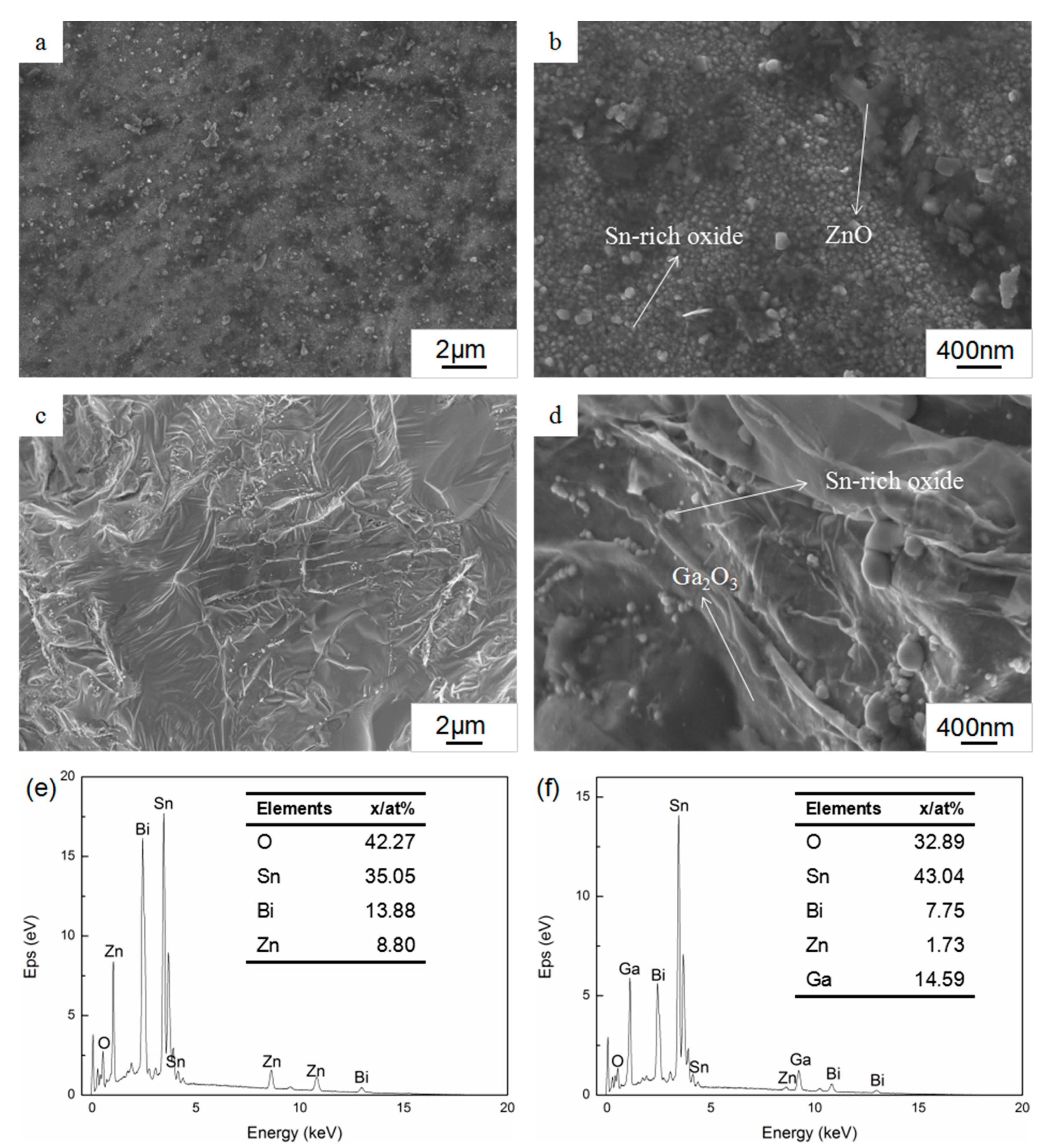
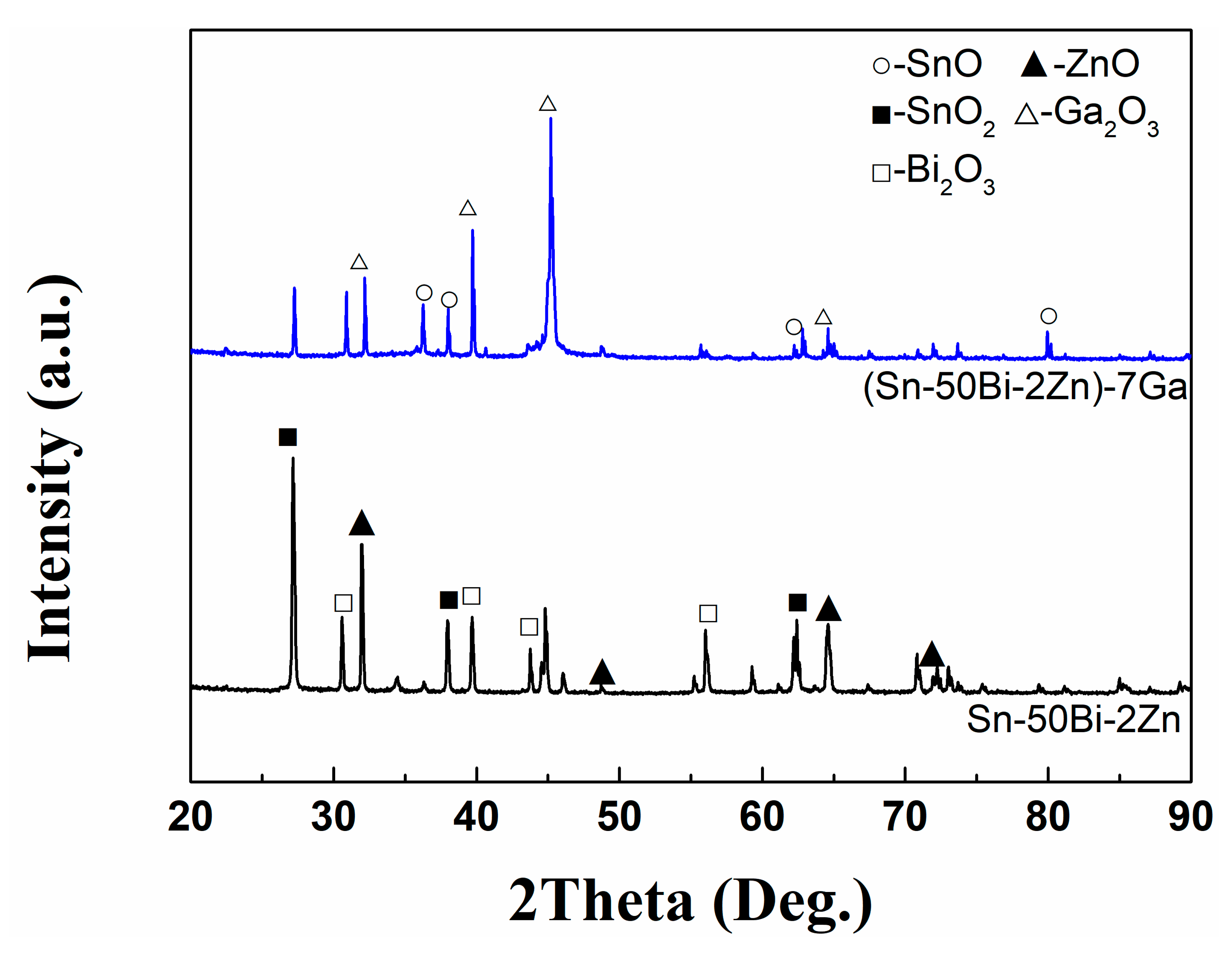
3.2. Surface and Cross-Sectional Morphology of Oxide Scale
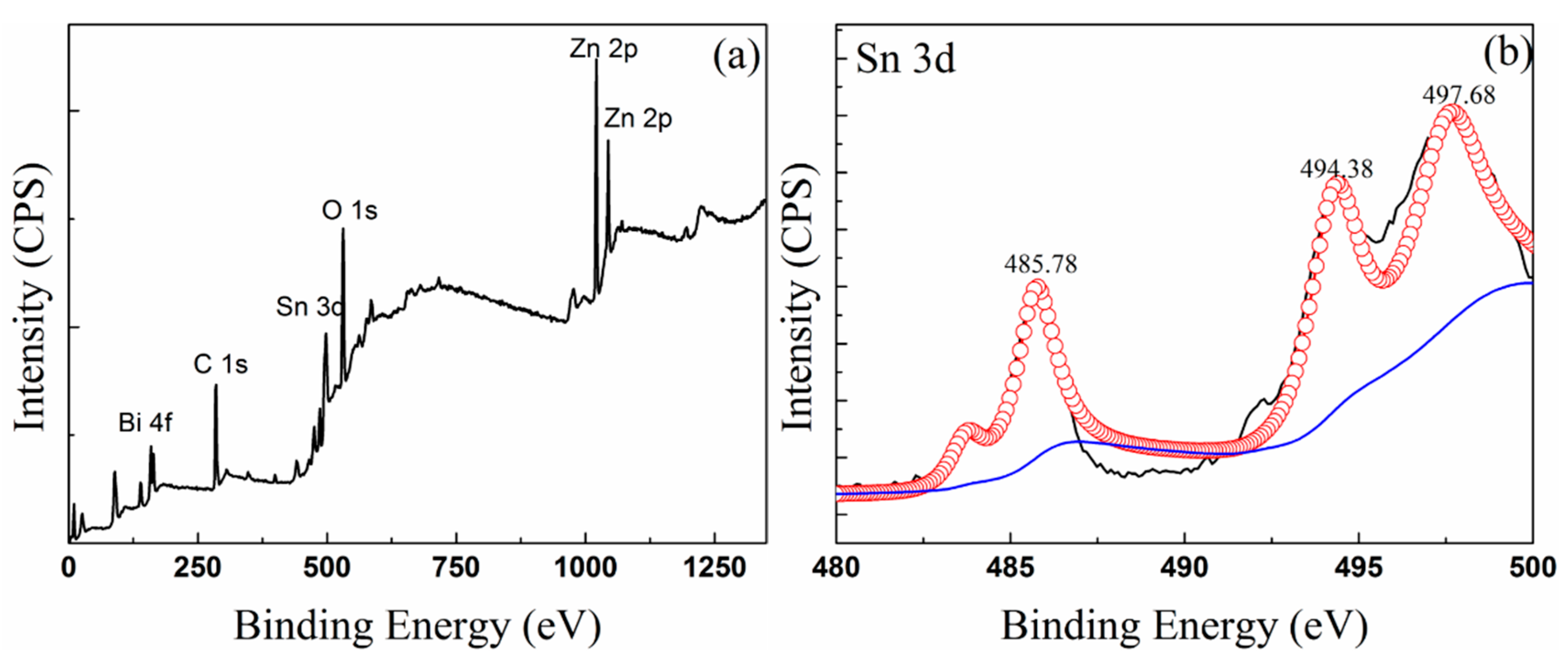
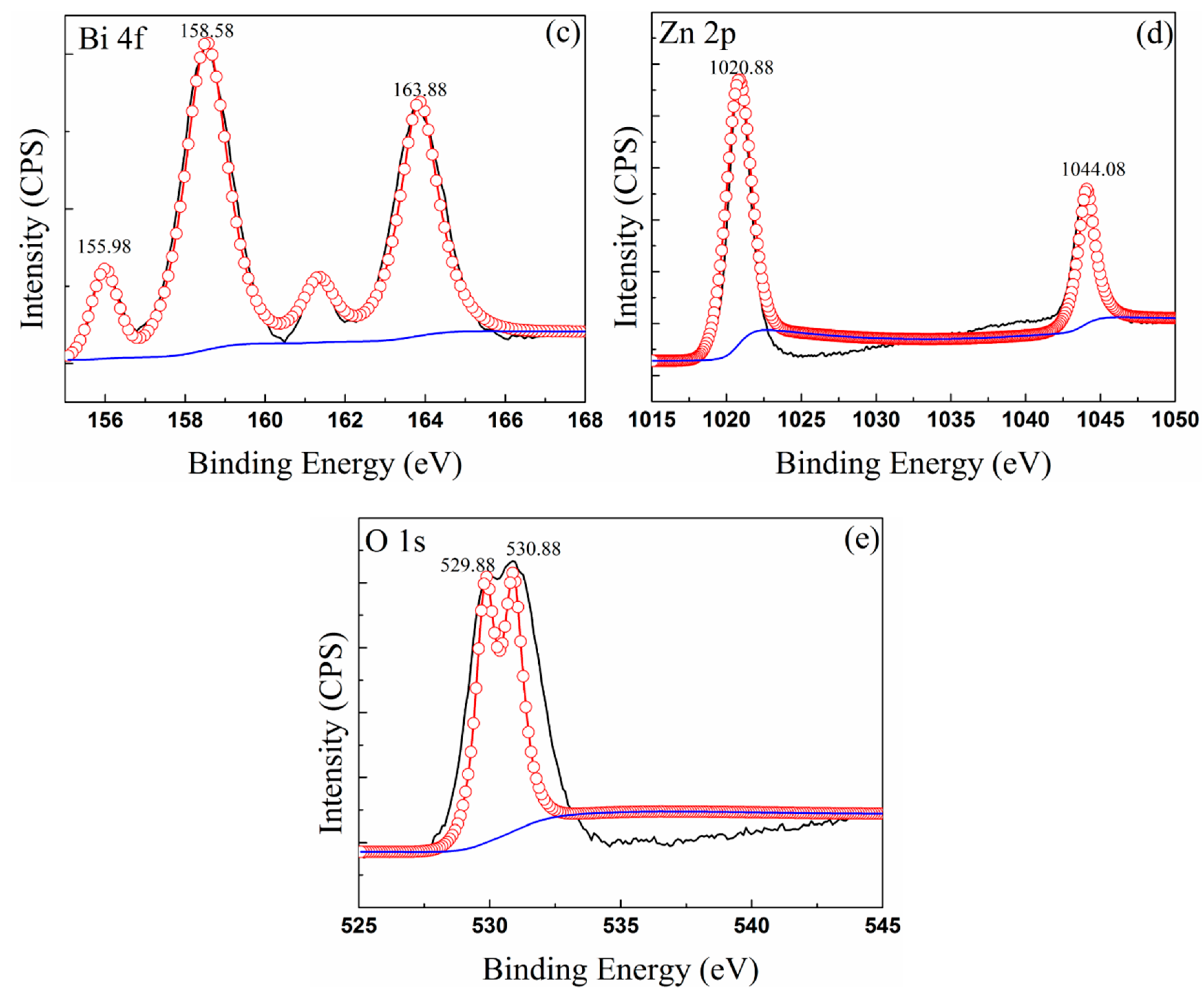
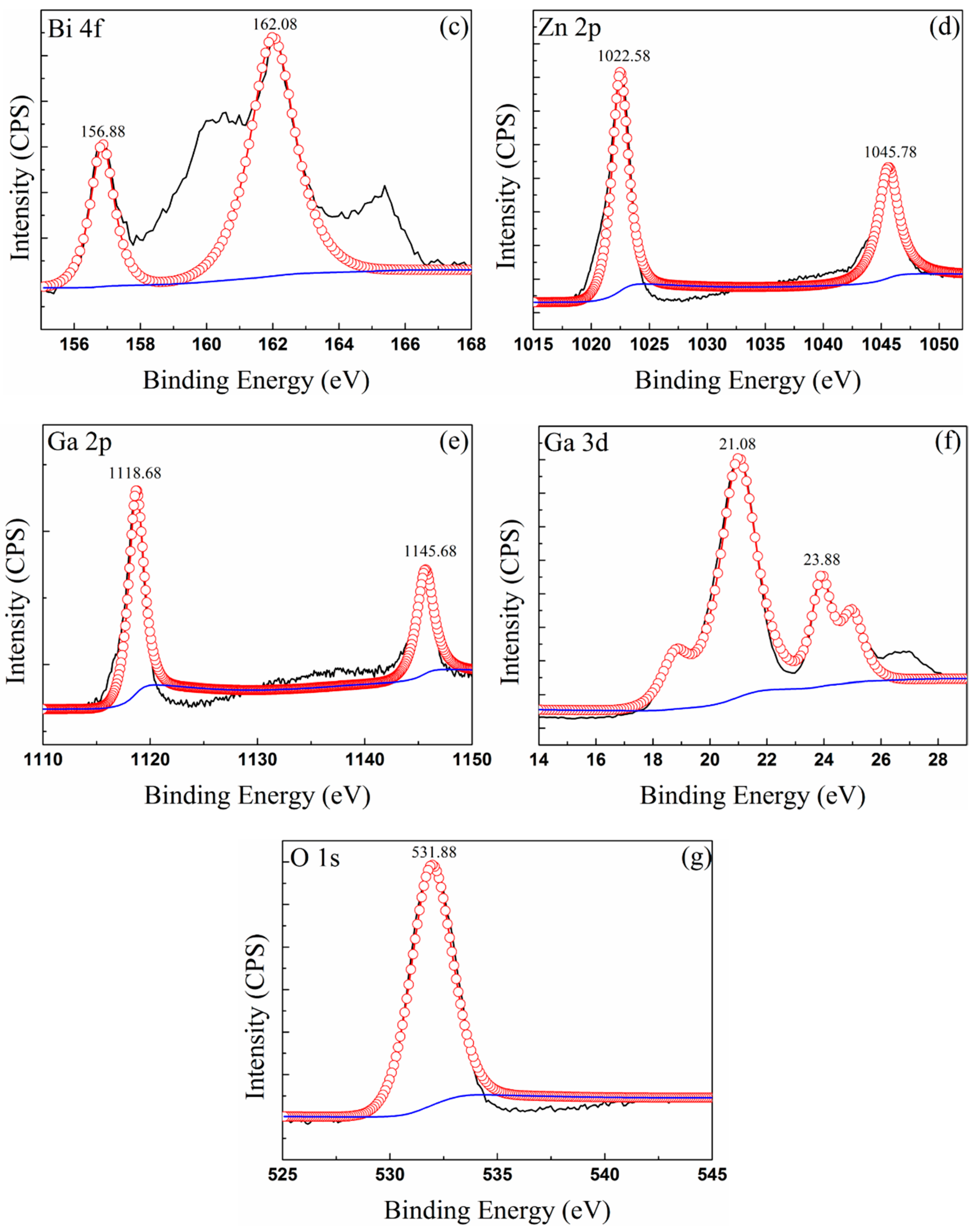
3.3. Composition of Oxide Scale
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takada, O.; Kamiyama, T.; Kiyanagi, Y. Study on phase transition of Pb–Bi eutectic alloy by neutron transmission spectroscopy. J. Nucl. Mater. 2010, 398, 129–131. [Google Scholar] [CrossRef] [Green Version]
- Prabu, T.; Sekar, C.A. Improving Heat Transfer in Hybrid Solar Systems Using Liquid Metal Alloys as a Coolant. J. Sol. Energy Eng. 2017, 139, 064501. [Google Scholar] [CrossRef]
- Popović, M.; Olmsted, D.; Bolind, A.; Asta, M.; Sohn, S.; Schroers, J.; Shao, R.; Hosemann, P. A study of the effects of minor additives to Pb–Bi eutectic: Designing novel Pb–Bi–X liquid alloys for heat transfer applications. Mater. Des. 2018, 159, 240–251. [Google Scholar] [CrossRef]
- Wang, Q.M.; Cheng, X.M.; Li, Y.Y.; Yu, G.M.; Liu, Z. Microstructures and thermal properties of Sn–Bi–Pb–Zn alloys as heat storage and transfer materials. Rare Met. 2019, 38, 350–358. [Google Scholar] [CrossRef]
- Xu, Z.; Utton, C.; Tsakiropoulos, P. A Study of the Effect of 2 at.% Sn on the Microstructure and Isothermal Oxidation at 800 and 1200 °C of Nb–24Ti–18Si-Based Alloys with Al and/or Cr Additions. Materials 2018, 11, 1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacio, J.; Wetzel, T. Corrigendum to “Assessment of liquid metal technology status and research paths for their use as efficient heat transfer fluids in solar central receiver systems”. Sol. Energy 2017, 93, 11–22. [Google Scholar] [CrossRef]
- Lorenzin, N.; Abánades, A. A review on the application of liquid metals as heat transfer fluid in Concentrated Solar Power technologies. Int. J. Hydrogen Energy 2016, 41, 6990–6995. [Google Scholar] [CrossRef]
- Song, J.-M.; Cheng, Y.-M.; Tsai, C.-H. Oxidation of liquid solders for die attachment. Corros. Sci. 2010, 52, 4011–4016. [Google Scholar] [CrossRef]
- Wu, X.D.; Liu, S.; Lin, F.; Weng, D. Nitrate storage behavior of Ba/MnOx–CeO2 catalyst and its activity for soot oxidation with heat transfer limitations. J. Hazard. Mater. 2010, 181, 722–728. [Google Scholar] [CrossRef]
- Han, J.; Su, B.; Meng, J.; Zhang, A.; Wu, Y. Microstructure and Composition Evolution of a Fused Slurry Silicide Coating on MoNbTaTiW Refractory High-Entropy Alloy in High-Temperature Oxidation Environment. Materials 2020, 13, 3592. [Google Scholar] [CrossRef]
- Yang, Y.; Kitashima, T.; Hara, T.; Hara, Y.; Yamabe-Mitarai, Y.; Hagiwara, M.; Iwasaki, S. Effects of Ga, Sn Addition and Microstructure on Oxidation Behavior of Near-α Ti Alloy. Oxid. Met. 2017, 88, 583–598. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Gao, Z.; Liu, Y.; Ma, Z.; Yu, L.; Li, H. Correlation between Zn-Rich Phase and Corrosion/Oxidation Behavior of Sn–8Zn–3Bi Alloy. Metals 2016, 6, 175. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.Q.; Yu, L.; Shen, Y.; Jia, Z.Q. Influence of Al Addition on Oxidation Behavior of Cu–Ni–Cr Alloys at 973–1073 K in 1.01 × 102 kPa Pure Oxygen. Oxid. Met. 2017, 88, 397–407. [Google Scholar]
- Wang, X.J.; Zhu, Q.S.; Liu, B.; Liu, N.; Wang, F.J. Effect of doping Al on the liquid oxidation of Sn–Bi–Zn solder. J. Mater. Sci. Mater. Electron. 2014, 25, 2297–2304. [Google Scholar] [CrossRef]
- Xu, Y.X.; Lu, J.T.; Yang, X.W.; Yan, J.B.; Li, W.Y. Effect and role of alloyed Nb on the air oxidation behaviour of Ni–Cr–Fe alloys at 1000 degrees C. Corros. Sci. 2017, 127, 10–20. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Ko, Y.G. Self-assembly of hierarchical N-heterocycles-inorganic materials into three-dimensional structure for superior corrosion protection. Chem. Eng. J. 2019, 356, 850–856. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Kim, M.J.; Kim, Y.G.; Ko, Y.G. Dual-functional crosslinked polymer-inorganic materials for robust electrochemical performance and antibacterial activity. Chem. Eng. J. 2020, 392, 123654. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Kamil, M.P.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent advances in hybrid organic-inorganic materials with spatial architecture for state-of-the-art applications. Prog. Mater. Sci. 2020, 112, 100663. [Google Scholar] [CrossRef]
- Wang, Q.M.; Cheng, X.M.; Li, Y.Y.; Yu, G.M.; Liu, Z. Microstructures and Thermal Properties of Sn–Bi–Zn–Ga Alloys as Heat Transfer and Heat Storage Materials. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019, 34, 676–683. [Google Scholar] [CrossRef]
- Cruchley, S.; Taylor, M.P.; Evans, H.E.; Hardy, M.C.; Child, D.J. Characterisation of subsurface oxidation damage in Ni based superalloy, RR1000. Mater. Sci. Technol. 2014, 30, 1884–1889. [Google Scholar] [CrossRef]
- Zhu, L.J.; Zhu, S.L.; Wang, F.H.; Zhang, J.Q. Comparison of the cyclic oxidation behavior of low expansion Ni + Cr AlYSiN nanocomposite and a NiCrAlYSi coating. Corros. Sci 2014, 80, 393. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef] [PubMed]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of Photoelectron Spectroscopy; Perkin-Elmer Corporation: Waltham, MA, USA, 1992. [Google Scholar]
- Cho, S.; Yu, J.; Kang, S.K.; Shih, D.-Y. Oxidation study of pure tin and its alloys via electrochemical reduction analysis. J. Electron. Mater. 2005, 34, 635–642. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, M.; Wu, Z.; Xing, X.; Mo, G.; Wu, Z.; Liu, H. In-situ GISAXS study on the oxidation behavior of liquid Ga on Ni(Cu)/Si substrates. Nucl. Instrum. Methods Phys. Res. Sect. B 2015, 362, 133–137. [Google Scholar] [CrossRef]
- Denisov, V.M.; Denisova, L.T.; Osipovich, T.V.; Kirik, S.D. Influence of silver on oxidation of Ge–Bi melts. Russ. J. Non-Ferrous Met. 2010, 51, 49–51. [Google Scholar] [CrossRef]
- Landolt, B.; Börnstein, R. Thermodynamic Properties of Inorganic Material; Springer: Berlin, Germany, 1999; Volume 19A1. [Google Scholar]
- Zhang, S.; Zhang, Y.; Wang, H. Effect of oxide thickness of solder powders on the coalescence of SnAgCu lead-free solder pastes. J. Alloys Compd. 2009, 487, 682–686. [Google Scholar] [CrossRef]
- Meier, H. Introduction to High Temperature Oxidation of Metals; Edward Arnold: London, UK, 1983; p. 88. [Google Scholar]
- Fontana, M.G.; Stactile, W. Corrosion Science and Technology; Plenum Press: London, UK, 1970; p. 149. [Google Scholar]




| Designed Compounds | XRF Results (wt.%) | |||
|---|---|---|---|---|
| Sn | Bi | Zn | Ga | |
| Sn–50Bi–2Zn | 48.29 | 49.57 | 2.14 | 0 |
| (Sn–50Bi–2Zn)–1Ga | 47.61 | 49.51 | 1.91 | 0.97 |
| (Sn–50Bi–2Zn)–3Ga | 46.65 | 48.57 | 1.87 | 2.91 |
| (Sn–50Bi–2Zn)–5Ga | 45.62 | 47.58 | 1.86 | 4.94 |
| (Sn–50Bi–2Zn)–7Ga | 44.73 | 46.54 | 1.81 | 6.92 |
| Alloys | k1 (10−3∙mg∙cm−2∙min−1) | Q (kJ∙mol−1) |
|---|---|---|
| Sn–50Bi–2Zn | 7.11 | 12.05 |
| (Sn–50Bi–2Zn)–1Ga | 3.71 | 15.69 |
| (Sn–50Bi–2Zn)–3Ga | 1.37 | 21.27 |
| (Sn–50Bi–2Zn)–5Ga | 1.11 | 22.45 |
| (Sn–50Bi–2Zn)–7Ga | 1.16 | 22.20 |
| Elements and Oxides | Sn 3d (eV) | Bi 4f (eV) | Zn 2p (eV) | Ga 2p (eV) | Ga 3d (eV) |
|---|---|---|---|---|---|
| Sn [23] | 484.9, 493.3 | ||||
| SnO [23] | 486.3 | ||||
| SnO2[25] | 487.3 | ||||
| Bi [24] | 157, 162.3 | ||||
| Bi2O3 [24,25] | 158.1–159.5 | ||||
| Zn [26] | 1021.8, 1044 | ||||
| ZnO [26] | 1021.7 | ||||
| Ga [26] | 1116.6, 1143.4 | ||||
| Ga2O3 [26] | 20.7 |
| Samples | Sn | Bi | Zn | Ga | O |
|---|---|---|---|---|---|
| Sn–50Bi–2Zn | 50.36 | 5.95 | 9.23 | — | 34.47 |
| (Sn–50Bi–2Zn)–7Ga | 24.14 | 5.33 | 13.14 | 33.4 | 32.24 |
| Oxidation Reaction | ΔGθ/(J·mol−1) | |||
|---|---|---|---|---|
| 298 K | 400 K | 600 K | 800 K | |
| Sn–SnO | −302.61 | −309.06 | −325.03 | −344.30 |
| Sn–SnO2 | −596.33 | −602.56 | −619.43 | −641.61 |
| Bi–Bi2O3 | −615.89 | −633.08 | −675.29 | −725.87 |
| Zn–ZnO | −361.08 | −366.18 | −379.44 | −396.05 |
| Ga–Ga2O3 | −1114.43 | −1124.63 | −1152.28 | −1187.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Cheng, X.; Liu, Z.; Lv, Z.; Cheng, Q. Effect of Ga on the Oxide Film Structure and Oxidation Resistance of Sn–Bi–Zn Alloys as Heat Transfer Fluids. Materials 2020, 13, 5461. https://doi.org/10.3390/ma13235461
Wang Q, Cheng X, Liu Z, Lv Z, Cheng Q. Effect of Ga on the Oxide Film Structure and Oxidation Resistance of Sn–Bi–Zn Alloys as Heat Transfer Fluids. Materials. 2020; 13(23):5461. https://doi.org/10.3390/ma13235461
Chicago/Turabian StyleWang, Qingmeng, Xiaomin Cheng, Zhi Liu, Zean Lv, and Qianju Cheng. 2020. "Effect of Ga on the Oxide Film Structure and Oxidation Resistance of Sn–Bi–Zn Alloys as Heat Transfer Fluids" Materials 13, no. 23: 5461. https://doi.org/10.3390/ma13235461




