Magnetic Properties and the Electronic Structure of the Gd0.4Tb0.6Co2 Compound
Abstract
:1. Introduction
2. Experimental and Computational Details
3. Results and Discussion
3.1. Crystal Structure
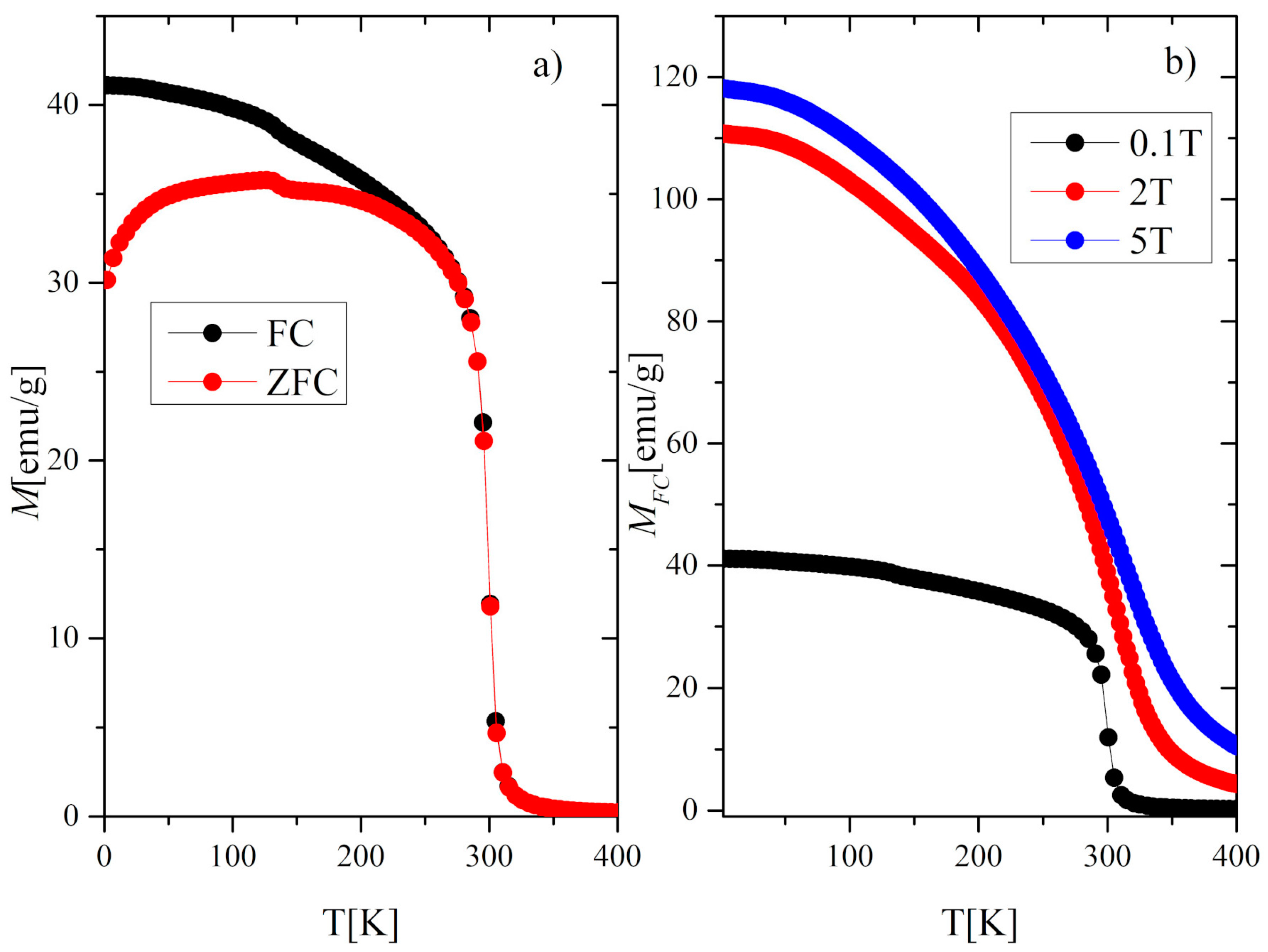
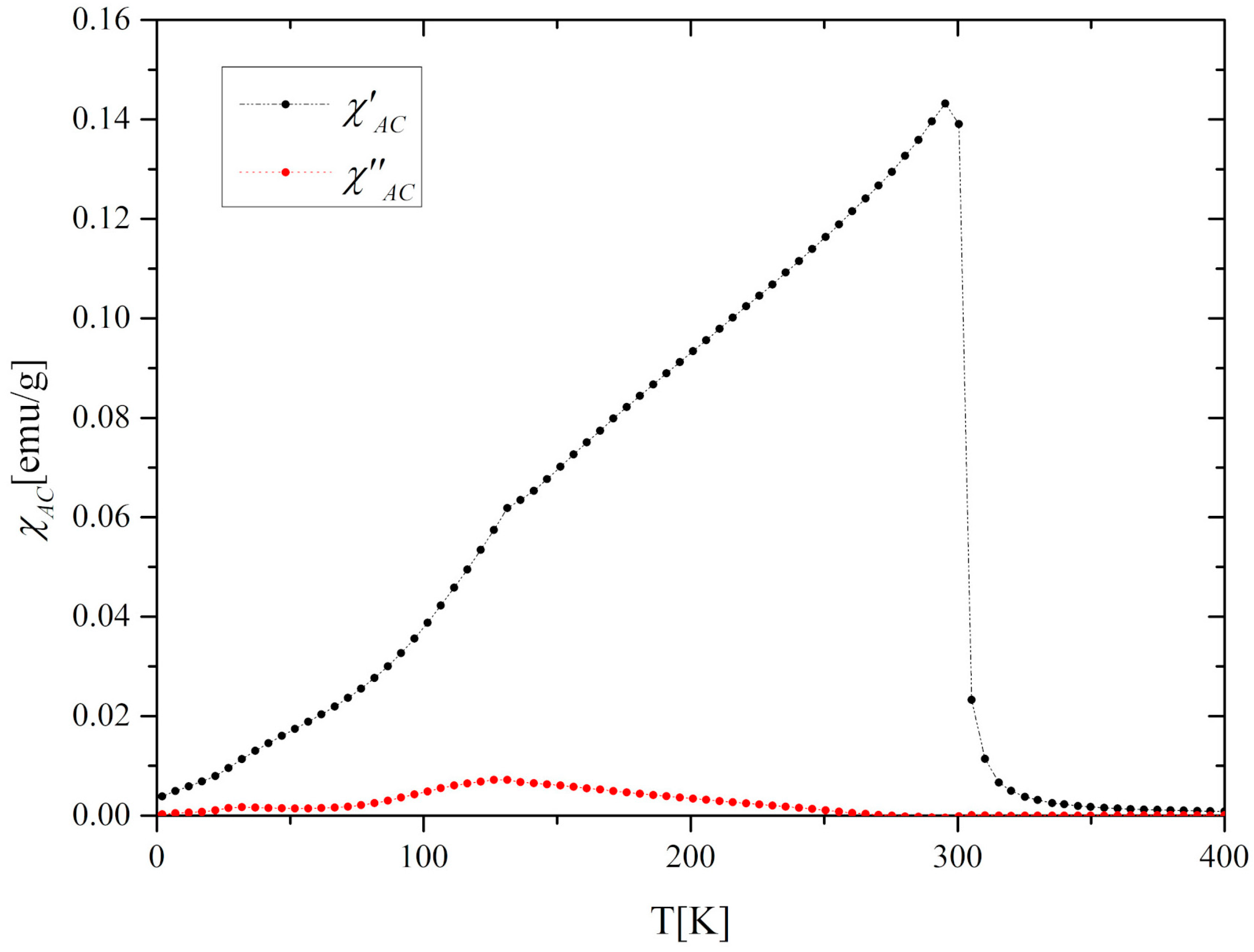
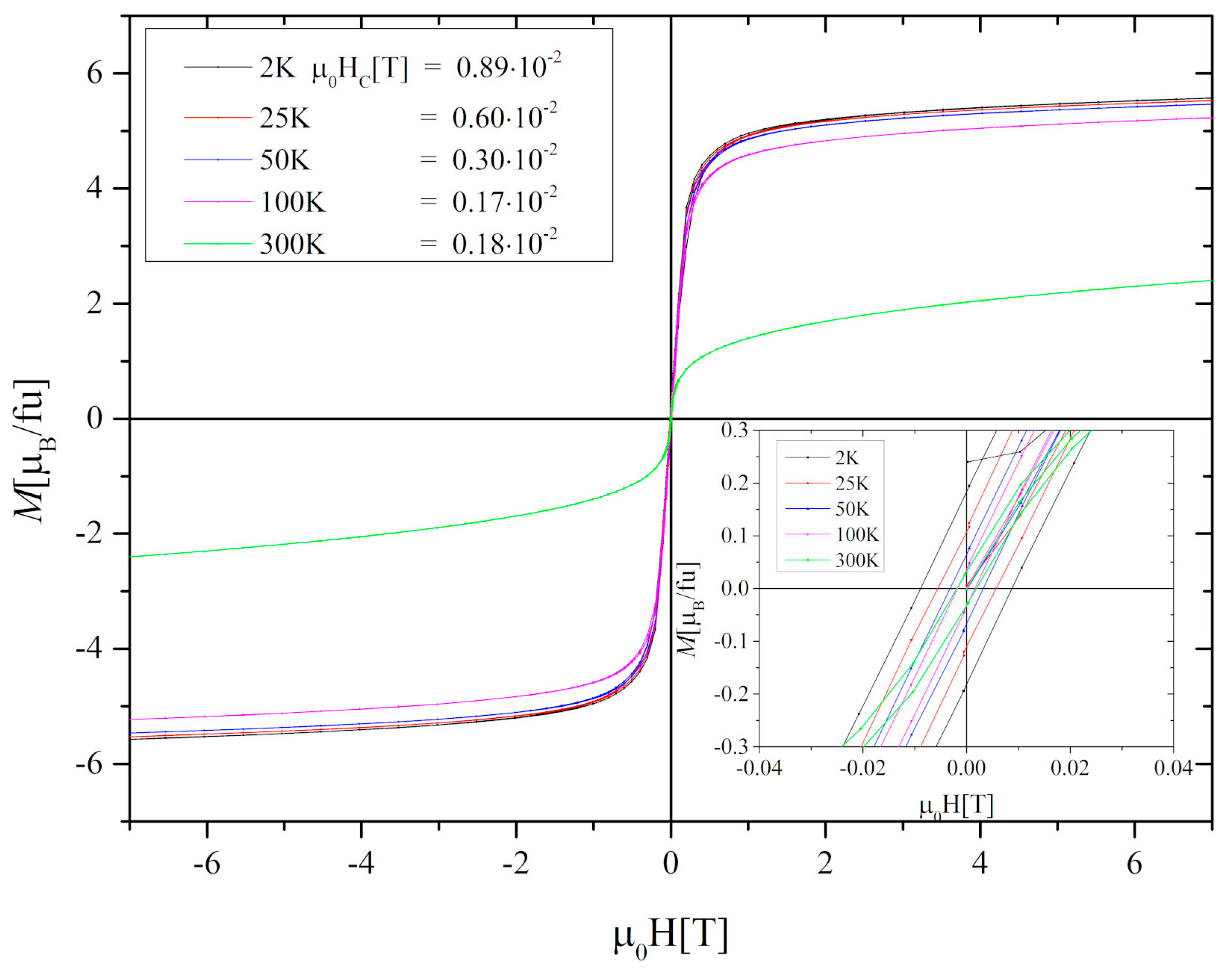
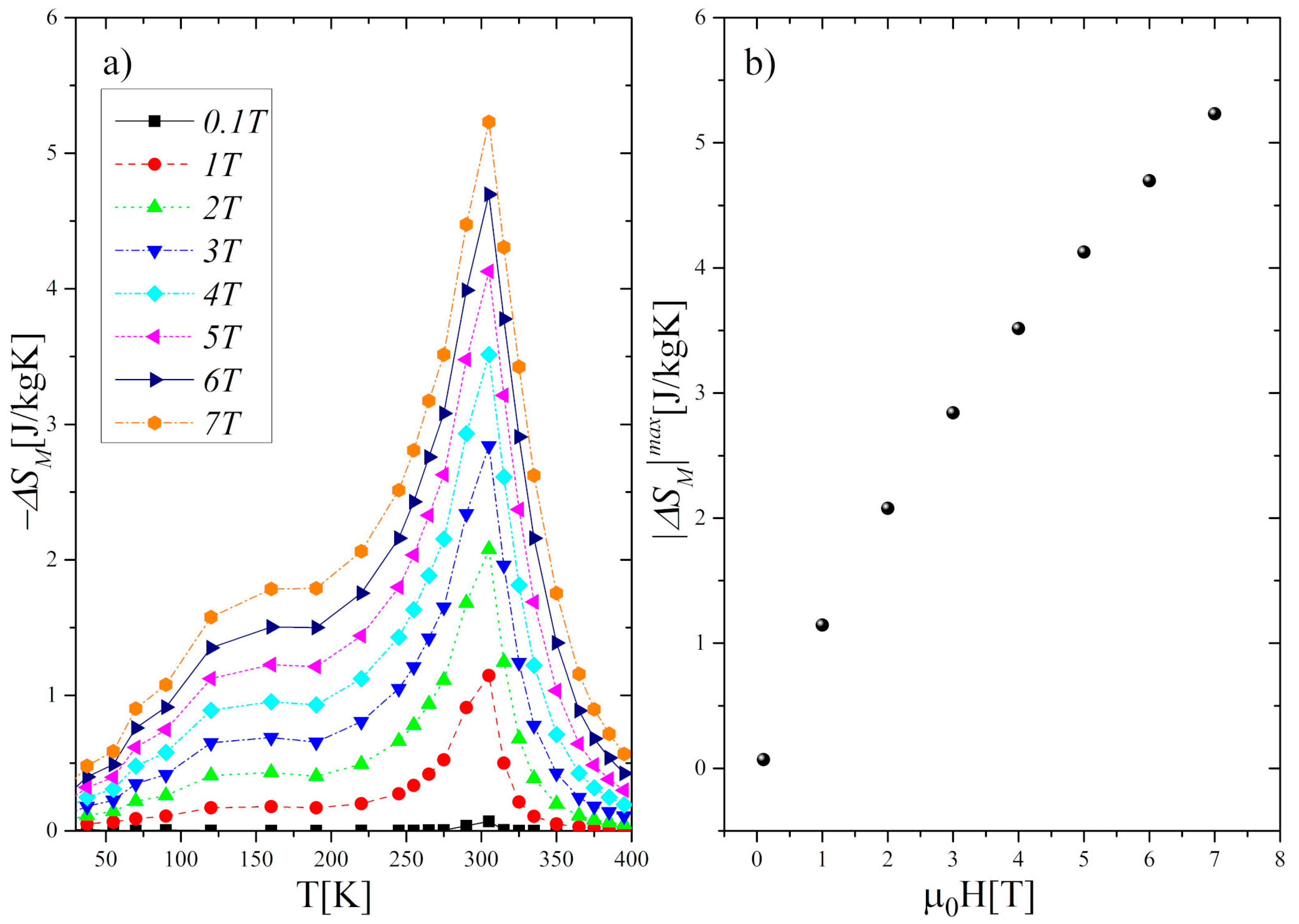
3.2. Magnetic and Magnetocaloric Properties
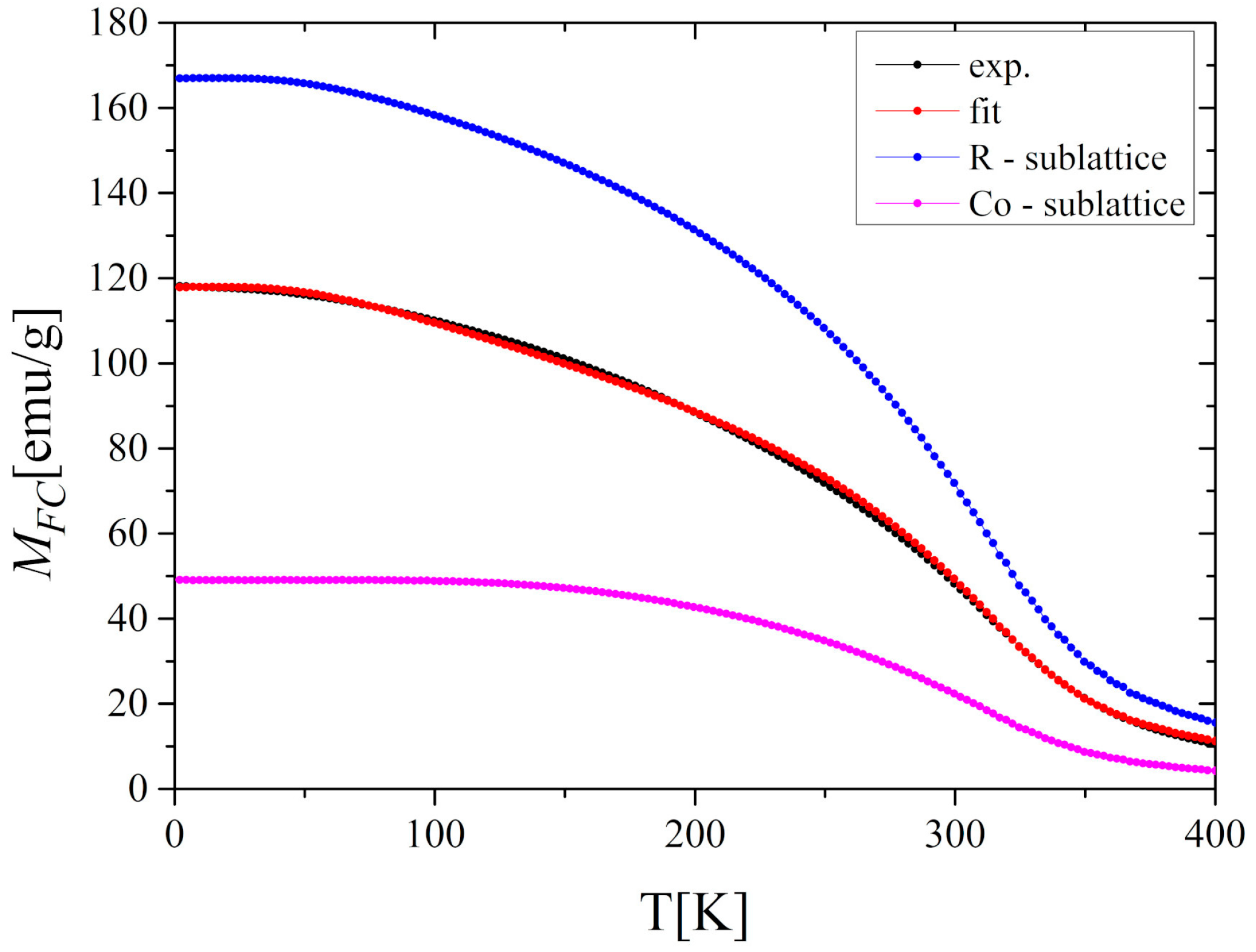
3.3. MFT Analysis
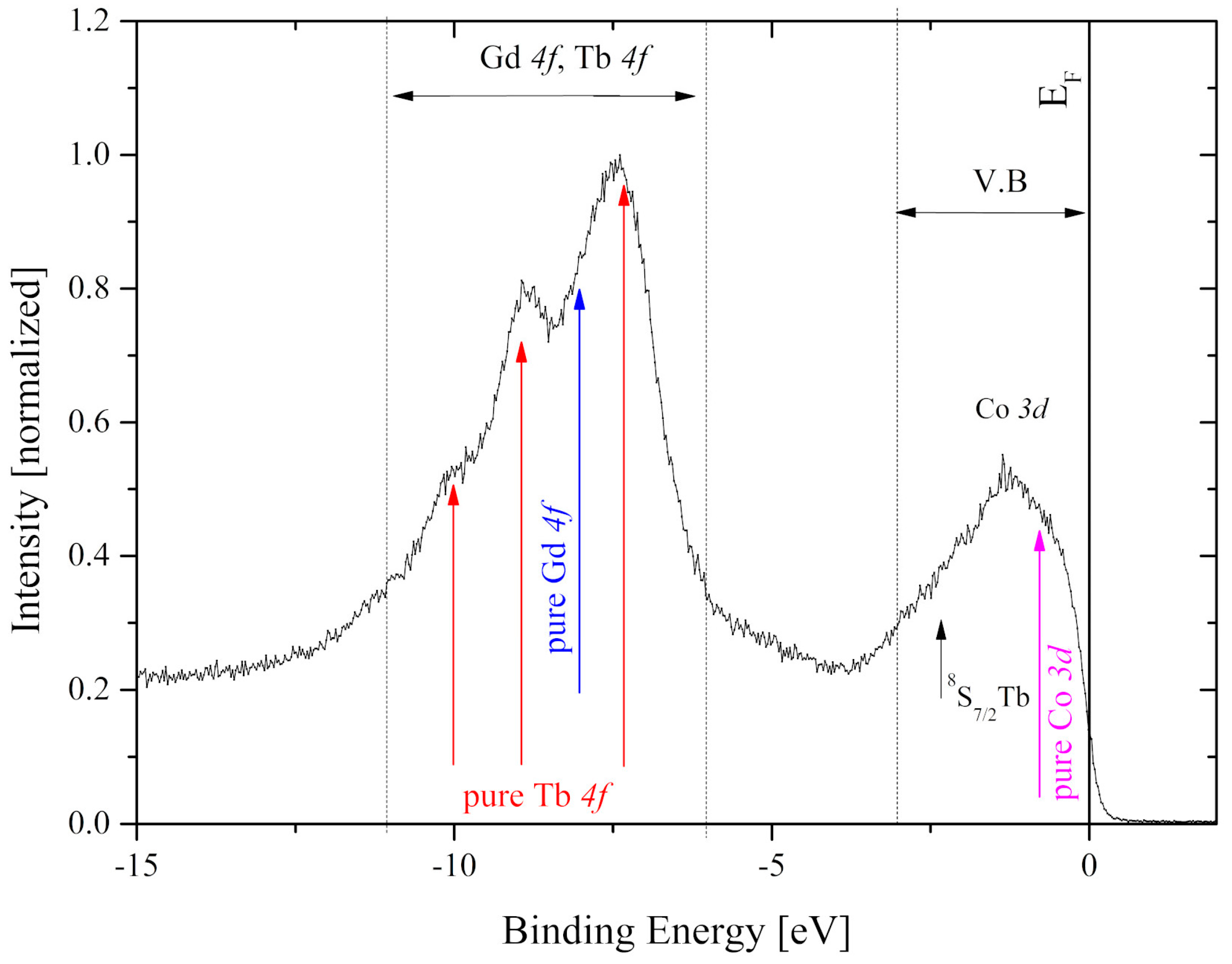
4. X-ray Photoelectron Spectroscopy (XPS)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ćwik, J.; Koshkid’ko, Y.; de Oliveira, N.A.; Mikhailova, A.; Nenkov, K. Effect of composition changes on the structural, magnetic and thermodynamic properties in Tb1−xDyxNi2 intermetallic compounds. J. Alloys Compd. 2018, 769, 588–596. [Google Scholar] [CrossRef]
- Halder, M.; Yusuf, S.M.; Mukadam, M.D.; Shashikala, K. Magnetocaloric effect and critical behavior near the paramagnetic to ferromagnetic phase transition temperature in TbCo2−xFex. Phys. Rev. B 2010, 81, 174402. [Google Scholar] [CrossRef] [Green Version]
- Murtaza, A.; Zuo, W.; Yaseen, M.; Ghani, A.; Saeed, A.; Hao, C.; Mi, J.; Li, Y.; Chang, T.; Wang, L.; et al. Magnetocaloric effect in the vicinity of the magnetic phase transition in NdCo2−xFex compounds. Phys. Rev. B 2020, 101, 214427. [Google Scholar] [CrossRef]
- Zhou, C.; Ren, S.; Bao, H.; Yang, S.; Yao, Y.; Ji, Y.; Ren, X.; Matsushita, Y.; Katsuya, Y.; Tanaka, M.; et al. Inverse effect of morphotropic phase boundary on the magnetostriction of ferromagnetic Tb1−xGdxCo2. Phys. Rev. B 2014, 89, 100101. [Google Scholar] [CrossRef]
- Duc, N.H.; Brommer, P.E. Formation of 3d-Moments and Spin Fluctuations in the Rare Earth—Transition Metal Intermetallics. In Handbook of Magnetic Materials; Duc, N.H., Ed.; Vietnam National University Press: Ho Chi Minh City, Vietnam, 1999; Volume 12. [Google Scholar]
- Goto, T.; Fukamichi, K.; Sakakibara, T.; Komatsu, H. Itinerant electron metamagnetism in YCo2. Solid State Commun. 1989, 72, 945–947. [Google Scholar] [CrossRef]
- Goto, T.; Sakakibara, T.; Murata, K.; Komatsu, H.; Fukamichi, K. Itinerant electron metamagnetism in YCo2 and LuCo2. J. Magn. Magn. Mater. 1990, 90, 700–702. [Google Scholar] [CrossRef]
- Baranov, N.; Yermakov, A.A.; Pirogov, A.N.; Proshkin, A.; Gvasaliya, S.N.; Podlesnyak, A.A. Irreversibility of the magnetic state of Tm1−xTbxCo2 revealed by specific heat, electrical resistivity, and neutron diffraction measurements. Phys. Rev. B 2006, 73, 104445. [Google Scholar] [CrossRef] [Green Version]
- Duc, N.H. Intersublattice Exchange Coupling in The Rare Earth–Transition Metal Intermetallics. In Handbook on the Physics and Chemistry of Rare Earths; Gschneidner, K.A., Jr., Eyring, L., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1997; Volume 24, pp. 339–398. [Google Scholar]
- Ćwik, J. Magnetism and magnetocaloric effect in multicomponent Laves-phase compounds: Study and comparative analysis. J. Solid State Chem. 2014, 209, 13–22. [Google Scholar] [CrossRef]
- Not Your Average Fridge Magnet: These High-Tech Magnets Will Keep Your Butter (and Beer) Cold. Available online: https://www.ge.com/news/reports/not-your-average-fridge-magnet (accessed on 24 November 2020).
- Gschneidner, K.A.; Pecharsky, V.K. Magnetocaloric materials. Annu. Rev. Mater. Res. 2000, 30, 387–429. [Google Scholar] [CrossRef] [Green Version]
- Leary, A.; Ohodnicki, P.R.; McHenry, M.E. Soft magnetic materials in high-frequency, high-power conversion applications. JOM 2012, 64, 772–781. [Google Scholar] [CrossRef]
- Tishin, A.M.; Spichkin, Y. Recent progress in magnetocaloric effect: Mechanisms and potential applications. Int. J. Refrig. 2014, 37, 223–229. [Google Scholar] [CrossRef]
- Zhou, K.W.; Zhang, Y.H.; Li, J.Q.; Deng, J.Q.; Zhu, Q.M. Magnetocaloric effects in (Gd1-xTbx)Co2. Solid State Commun. 2006, 137, 275–277. [Google Scholar] [CrossRef]
- Singh, D.J.; Nordstrom, L. Planewaves, Pseudopotentials, and the LAPW Method, 2nd ed.; Springer Science: Berlin/Heidelberg, Germany, 2006; pp. 43–106. [Google Scholar]
- Blaha, P.; Schwarz, K.; Madsen, G.K.H.; Kvasnicka, D.; Luitz, J. WIEN2k, An Augmented Plane Wave + Local Orbitals Program for Calculating Crystal Properties; Karlheinz Schwarz, Technische Universität: Wien, Austria, 2018; ISBN 3-9501031-1-2. [Google Scholar]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin, L.A.; Zhou, X.; Burke, K. Restoring the Density-Gradient Expansion for Exchange in Solids and Surfaces. Phys. Rev. Lett. 2008, 100, 136406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anisimov, V.I.; Zaanen, J.; Andersen, O.K. Band theory and Mott insulators: Hubbard U instead of Stoner, I. Phys. Rev. B 1991, 44, 943–954. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, B.L.; Mund, H.S.; Sahariya, J.; Dashora, A.; Halder, M.; Yusuf, S.M.; Itou, M.; Sakurai, Y. Temperature dependent spin and orbital polarization in TbCo2: Magnetic Compton scattering and first-principles investigations. J. Alloys Compd. 2015, 633, 430–434. [Google Scholar] [CrossRef]
- Bajorek, A.; Deniszczyk, J.; Chrobak, A.; Chełkowska, G. Effect of In/Sn substitution on magnetism, crystal and electronic structure in Gd (In1-xSnx)3 system. Philos. Mag. 2015, 95, 3554–3576. [Google Scholar] [CrossRef]
- Lutterotti, L.; Ceccato, R.; Maschio, R.D.; Pagani, E. Quantitative Analysis of Silicate Glass in Ceramic Materials by the Rietveld Method. Mater. Sci. Forum 1998, 87–92. [Google Scholar] [CrossRef]
- Gialanella, S.; Lutterotti, L. On the measure of order in alloys. Prog. Mater. Sci. 1997, 42, 125–133. [Google Scholar] [CrossRef]
- Singh, N.K.; Suresh, K.G.; Rana, D.S.; Nigam, A.K.; Malik, S.K. Role of Fe substitution on the anomalous magnetocaloric and magnetoresistance behaviour in Tb (Ni1−xFex)2 compounds. J. Phys. Condens. Matter 2006, 18, 10775–10786. [Google Scholar] [CrossRef] [Green Version]
- Gratz, E.; Goremychkin, E.; Latroche, M.; Hilscher, G.; Rotter, M.; Muller, H.; Lindbaum, A.; Ichor, H.; Paul-Boncour, V.; Fernandez-Diaz, T. New magnetic phenomena in TbNi2. J. Phys. Condens. Matter 1999, 11, 7893–7905. [Google Scholar] [CrossRef]
- Coey, J.M.D. Magnetism and Magnetic Materials; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Franco, V.; Blazquez, J.S.; Ipus, J.J.; Law, J.Y.; Moreno-Ramirez, L.M.; Conde, A. Magnetocaloric effect: From materials research to refrigeration devives. Prog. Mater. Sci. 2018, 93, 112–232. [Google Scholar] [CrossRef]
- Gschneidner Jr, K.A.; Pecharsky, V.K.; Tsokol, A.O. Recent developments in magnetocaloric materials. Rep. Prog. Phys. 2005, 68, 1479–1539. [Google Scholar] [CrossRef]
- Mozharivskyj, Y. Magnetocaloric Effect and Magnetocaloric Materials; Elsevier B.V.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Shen, B.G.; Sun, J.R.; Hu, F.X.; Zhang, H.W.; Cheng, Z.H. Recent progress in exploring magnetocaloric materials. Adv. Mater. 2009, 21, 4545–4564. [Google Scholar] [CrossRef] [Green Version]
- Chrobak, A.; Bajorek, A.; Chełkowska, G. Effect of Tb/Gd substitution on crystal structure and exchange interactions in Gd1-xTbxNi3 intermetallic. Compd. Acta Phys. Pol. A 2012, 121, 1132–1135. [Google Scholar] [CrossRef]
- Danh, T.; Duc, N.; Thuy, N. Exchange interactions in amorphous Gd–Fe alloys. J. Magn. Magn. Mater. 1998, 185, 105–108. [Google Scholar] [CrossRef]
- Duc, N.H. An evaluation of the R-T spin coupling parameter in the rare earth–transition metal intermetallics. Phys. Status solidi B 1991, 164, 545–552. [Google Scholar] [CrossRef]
- Lang, J.K.; Baer, Y.; Cox, P.A. Study of the 4f and valence band density of states in rare-earth metals: II. Experiment and results. J. Phys. F Met. Phys. 1981, 11, 121–138. [Google Scholar] [CrossRef]
- Saini, S.M.; Singh, N.; Nautiyal, T.; Auluck, S. Comparative study of optical and magneto-optical properties of GdFe2and GdCo2. J. Phys. Condens. Matter 2007, 19, 176203. [Google Scholar] [CrossRef]
- Shabanova, I.N.; Keller, N.V. X-ray photoelectron studies of spin-state changes in 3d metal systems. Surf. Interface Anal. 2001, 32, 114–116. [Google Scholar] [CrossRef]
- Doniach, S.; Sunjic, M. Many-electron singularity in X-ray photoemission and X-ray line spectra from metals. J. Phys. C Solid State Phys. 1970, 3, 285–291. [Google Scholar] [CrossRef]
- Tougaard, S. Practical algorithm for background subtraction. Surf. Sci. 1989, 216, 343–360. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikora, M.; Bajorek, A.; Chrobak, A.; Deniszczyk, J.; Ziółkowski, G.; Chełkowska, G. Magnetic Properties and the Electronic Structure of the Gd0.4Tb0.6Co2 Compound. Materials 2020, 13, 5481. https://doi.org/10.3390/ma13235481
Sikora M, Bajorek A, Chrobak A, Deniszczyk J, Ziółkowski G, Chełkowska G. Magnetic Properties and the Electronic Structure of the Gd0.4Tb0.6Co2 Compound. Materials. 2020; 13(23):5481. https://doi.org/10.3390/ma13235481
Chicago/Turabian StyleSikora, Marcin, Anna Bajorek, Artur Chrobak, Józef Deniszczyk, Grzegorz Ziółkowski, and Grażyna Chełkowska. 2020. "Magnetic Properties and the Electronic Structure of the Gd0.4Tb0.6Co2 Compound" Materials 13, no. 23: 5481. https://doi.org/10.3390/ma13235481




