Antioxidants and Collagen-Crosslinking: Benefit on Bond Strength and Clinical Applicability
Abstract
:1. Introduction
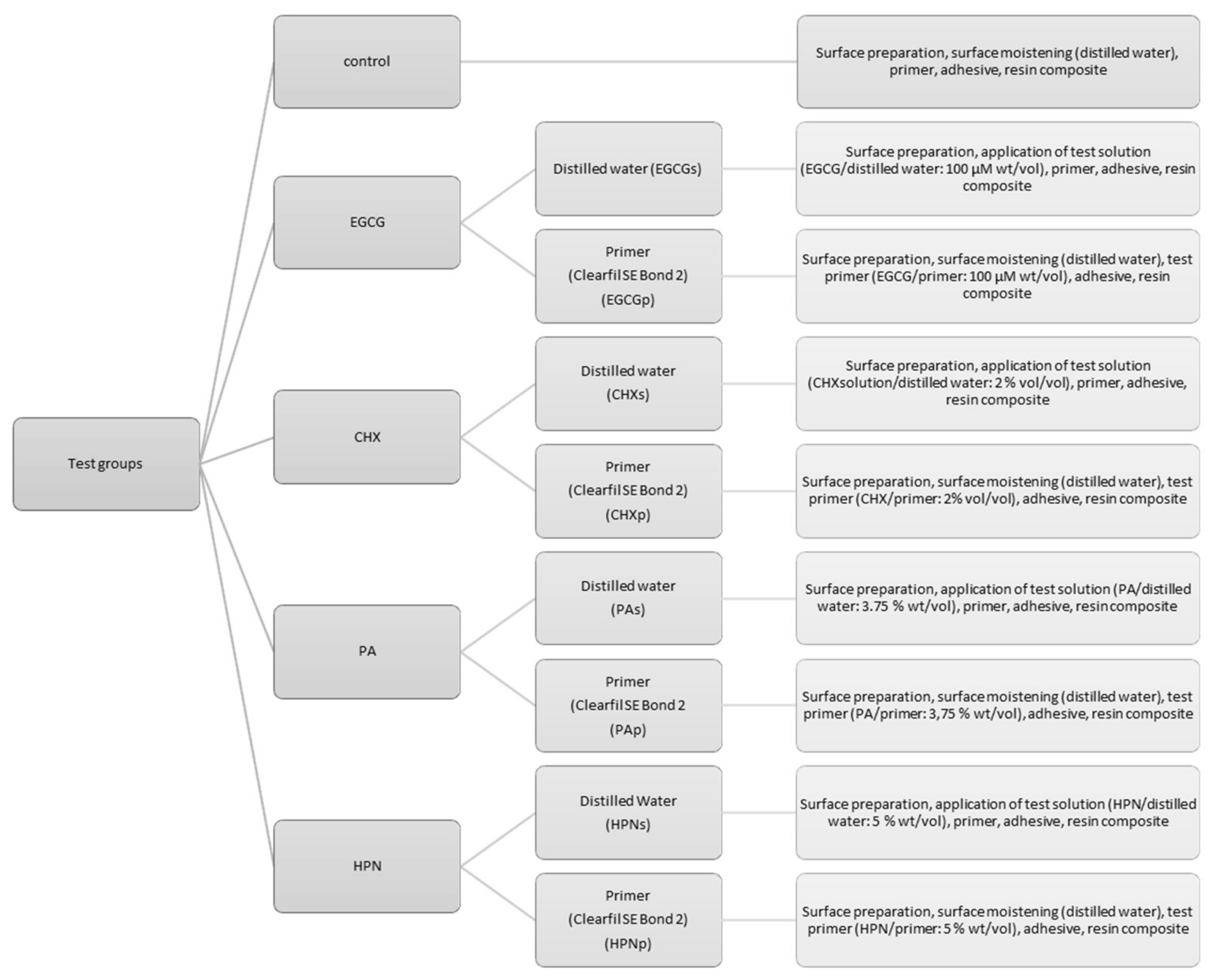
2. Materials and Methods
2.1. Dentin Substrate Preparation
2.2. Bonding Procedure
2.3. Fractography
2.4. Statistical Analysis
2.5. Ethical Approval
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simecek, J.W.; Diefenderfer, K.E.; Cohen, M.E. An evaluation of replacement rates for posterior resin-based composite and amalgam restorations in US Navy and Marine Corps recruits. J. Am. Dent. Assoc. 2009, 140, 200–209. [Google Scholar] [CrossRef]
- Levin, L.; Coval, M.; Geiger, S.B. Cross-sectional radiographic survey of amalgam and resin-based composite posterior restorations. Quintessence Int. 2007, 38, 511–514. [Google Scholar]
- Van Nieuwenhuysen, J.P.; D’Hoore, W.; Carvalho, J.; Qvist, V. Long-term evaluation of extensive restorations in permanent teeth. J. Dent. 2003, 31, 395–405. [Google Scholar] [CrossRef]
- Gaengler, P.; Hoyer, I.; Montag, R.; Gaebler, P. Micromorphological evaluation of posterior composite restorations—A 10-year report. J. Oral Rehabil. 2004, 31, 991–1000. [Google Scholar] [CrossRef]
- Spencer, P.; Ye, Q.; Park, J.; Topp, E.M.; Misra, A.; Marangos, O.; Wang, Y.; Bohaty, B.S.; Singh, V.; Sene, F.; et al. Adhesive/Dentin interface: The weak link in the composite restoration. Ann. Biomed. Eng. 2010, 38, 1989–2003. [Google Scholar] [CrossRef] [Green Version]
- Marshall, G.W., Jr.; Marshall, S.J.; Kinney, J.H.; Balooch, M. The dentin substrate: Structure and properties related to bonding. J. Dent. 1997, 25, 441–458. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Orgel, J.P.R.; Antipova, O.; Swain, M.V. The dentin organic matrix—limitations of restorative dentistry hidden on the nanometer scale. Acta Biomater. 2012, 8, 2419–2433. [Google Scholar] [CrossRef] [Green Version]
- Mjör, I.A.; Fejerskov, O. Human Oral Embryology and Histology, 1st ed.; Munksgaard: Copenhagen, Denmark, 1986. [Google Scholar]
- Jepsen, K.; Mansoura, M.; Kuhn, J.; Wu, H.; Jaenisch, R.; Bonadio, J.; Goldstein, S. An in vivo assessment ofthe contribution of type I collagen to the mechanical properties of cortical bone. In Proceedings of the 38th Annual Meeting, Orthopaedic Research Society, Washington, DC, USA, 17–20 February 1992. [Google Scholar]
- Ramachandran, G.N.; Kartha, G. Structure of Collagen. Nature 1955, 176, 593–595. [Google Scholar] [CrossRef]
- Traub, W.; Yonath, A.; Segal, D.M. On the Molecular Structure of Collagen. Nature 1969, 221, 914–917. [Google Scholar] [CrossRef] [PubMed]
- Kühn, K. Struktur und Biochemie des Kollagens. Chem. Unserer Zeit 1974, 8, 97–103. [Google Scholar] [CrossRef]
- Pashley, D.H. Dentin: A dynamic substrate—A review. Scanning Microsc. 1989, 3, 161–174, discussion 174–176. [Google Scholar]
- Pashley, D.H.; Carvalho, R.M. Dentine permeability and dentine adhesion. J. Dent. 1997, 25, 355–372. [Google Scholar] [CrossRef]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, composition and mineralization. Front. Biosci. 2011, 3, 711–735. [Google Scholar] [CrossRef]
- Nakajima, M.; Sano, H.; Burrow, M.F.; Tagami, J.; Yoshiyama, M.; Ebisu, S.; Ciucchi, B.; Russell, C.M.; Pashley, D.H. Tensile bond strength and SEM evaluation of caries-affected dentin using dentin adhesives. J. Dent. Res. 1995, 74, 1679–1688. [Google Scholar] [CrossRef]
- Ferrari, M.; Tay, F.R. Technique sensitivity in bonding to vital, acid-etched dentin. Oper. Dent. 2003, 28, 3–8. [Google Scholar]
- Nair, P.; Hickel, R.; Ilie, N. Adverse effects of salivary contamination for adhesives in restorative dentistry. A literature review. Am. J. Dent. 2017, 30, 156–164. [Google Scholar]
- Suzuki, M.; Kato, H.; Wakumoto, S. Vibrational analysis by Raman spectroscopy of the interface between dental adhesive resin and dentin. J. Dent. Res. 1991, 70, 1092–1097. [Google Scholar] [CrossRef]
- Sano, H.; Shono, T.; Takatsu, T.; Hosoda, H. Microporous dentin zone beneath resin-impregnated layer. Oper. Dent. 1994, 19, 59–64. [Google Scholar]
- Wang, Y.; Spencer, P.; Yao, X.; Brenda, B. Effect of solvent content on resin hybridization in wet dentin bonding. J. Biomed. Mater. Res. A 2007, 82, 975–983. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Spencer, P.; Wang, Y.; Misra, A. Relationship of solvent to the photopolymerization process, properties, and structure in model dentin adhesives. J. Biomed. Mater. Res. A 2007, 80, 342–350. [Google Scholar] [CrossRef] [Green Version]
- Ferracane, J.L.; Berge, H.X.; Condon, J.R. In vitro aging of dental composites in water--effect of degree of conversion, filler volume, and filler/matrix coupling. J. Biomed. Mater. Res. 1998, 42, 465–472. [Google Scholar] [CrossRef]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef]
- Hashimoto, M.; Tay, F.R.; Ohno, H.; Sano, H.; Kaga, M.; Yiu, C.; Kumagai, H.; Kudou, Y.; Kubota, M.; Oguchi, H. SEM and TEM analysis of water degradation of human dentinal collagen. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 66, 287–298. [Google Scholar] [CrossRef]
- Tersariol, I.L.; Geraldeli, S.; Minciotti, C.L.; Nascimento, F.D.; Paakkonen, V.; Martins, M.T.; Carrilho, M.R.; Pashley, D.H.; Tay, F.R.; Salo, T.; et al. Cysteine cathepsins in human dentin-pulp complex. J. Endod. 2010, 36, 475–481. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Yiu, C.; Hashimoto, M.; Breschi, L.; Carvalho, R.M.; Ito, S. Collagen degradation by host-derived enzymes during aging. J. Dent. Res. 2004, 83, 216–221. [Google Scholar] [CrossRef]
- Vidal, C.M.; Tjaderhane, L.; Scaffa, P.M.; Tersariol, I.L.; Pashley, D.; Nader, H.B.; Nascimento, F.D.; Carrilho, M.R. Abundance of MMPs and cysteine cathepsins in caries-affected dentin. J. Dent. Res. 2014, 93, 269–274. [Google Scholar] [CrossRef]
- Tjaderhane, L.; Larjava, H.; Sorsa, T.; Uitto, V.J.; Larmas, M.; Salo, T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J. Dent. Res. 1998, 77, 1622–1629. [Google Scholar] [CrossRef]
- Turk, B.; Turk, D.; Turk, V. Lysosomal cysteine proteases: More than scavengers. Biochim. Biophys. Acta 2000, 1477, 98–111. [Google Scholar] [CrossRef]
- Mazzoni, A.; Pashley, D.H.; Nishitani, Y.; Breschi, L.; Mannello, F.; Tjaderhane, L.; Toledano, M.; Pashley, E.L.; Tay, F.R. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials 2006, 27, 4470–4476. [Google Scholar] [CrossRef]
- Nishitani, Y.; Yoshiyama, M.; Wadgaonkar, B.; Breschi, L.; Mannello, F.; Mazzoni, A.; Carvalho, R.M.; Tjaderhane, L.; Tay, F.R.; Pashley, D.H. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur. J. Oral Sci. 2006, 114, 160–166. [Google Scholar] [CrossRef]
- Tjaderhane, L.; Nascimento, F.D.; Breschi, L.; Mazzoni, A.; Tersariol, I.L.; Geraldeli, S.; Tezvergil-Mutluay, A.; Carrilho, M.; Carvalho, R.M.; Tay, F.R.; et al. Strategies to prevent hydrolytic degradation of the hybrid layer-A review. Dent. Mater. 2013, 29, 999–1011. [Google Scholar] [CrossRef] [Green Version]
- Gendron, R.; Grenier, D.; Sorsa, T.; Mayrand, D. Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clin. Diagn. Lab. Immunol. 1999, 6, 437–439. [Google Scholar] [CrossRef] [Green Version]
- Hebling, J.; Pashley, D.H.; Tjaderhane, L.; Tay, F.R. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J. Dent. Res. 2005, 84, 741–746. [Google Scholar] [CrossRef]
- Scaffa, P.M.; Vidal, C.M.; Barros, N.; Gesteira, T.F.; Carmona, A.K.; Breschi, L.; Pashley, D.H.; Tjaderhane, L.; Tersariol, I.L.; Nascimento, F.D.; et al. Chlorhexidine inhibits the activity of dental cysteine cathepsins. J. Dent. Res. 2012, 91, 420–425. [Google Scholar] [CrossRef]
- Stanley, A.; Wilson, M.; Newman, H.N. The in vitro effects of chlorhexidine on subgingival plaque bacteria. J. Clin. Periodontol. 1989, 16, 259–264. [Google Scholar] [CrossRef]
- Liu, Z.; Li, F.; Zhang, L.; Yu, H.; Yu, F.; Chen, J. The effect of active components from citrus fruits on dentin MMPs. Arch. Oral Biol. 2017, 83, 111–117. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Menon, V.P. Effect of hesperidin on matrix metalloproteinases and antioxidant status during nicotine-induced toxicity. Toxicology 2007, 238, 90–98. [Google Scholar] [CrossRef]
- Kamaraj, S.; Anandakumar, P.; Jagan, S.; Ramakrishnan, G.; Devaki, T. Modulatory effect of hesperidin on benzo(a)pyrene induced experimental lung carcinogenesis with reference to COX-2, MMP-2 and MMP-9. Eur. J. Pharmacol. 2010, 649, 320–327. [Google Scholar] [CrossRef]
- Lee, K.H.; Yeh, M.H.; Kao, S.T.; Hung, C.M.; Liu, C.J.; Huang, Y.Y.; Yeh, C.C. The inhibitory effect of hesperidin on tumor cell invasiveness occurs via suppression of activator protein 1 and nuclear factor-kappaB in human hepatocellular carcinoma cells. Toxicol. Lett. 2010, 194, 42–49. [Google Scholar] [CrossRef]
- Hiraishi, N.; Sono, R.; Sofiqul, I.; Yiu, C.; Nakamura, H.; Otsuki, M.; Takatsuka, T.; Tagami, J. In vitro evaluation of plant-derived agents to preserve dentin collagen. Dent. Mater. 2013, 29, 1048–1054. [Google Scholar] [CrossRef]
- Hiraishi, N.; Sono, R.; Islam, M.S.; Otsuki, M.; Tagami, J.; Takatsuka, T. Effect of hesperidin in vitro on root dentine collagen and demineralization. J. Dent. 2011, 39, 391–396. [Google Scholar] [CrossRef]
- Islam, M.S.; Hiraishi, N.; Nassar, M.; Yiu, C.; Otsuki, M.; Tagami, J. Effect of hesperidin incorporation into a self-etching primer on durability of dentin bond. Dent. Mater. 2014, 30, 1205–1212. [Google Scholar] [CrossRef]
- Haslam, E. Natural Polyphenols (Vegetable Tannins) as Drugs: Possible Modes of Action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef]
- Fawzy, A.; Nitisusanta, L.; Iqbal, K.; Daood, U.; Beng, L.T.; Neo, J. Characterization of riboflavin-modified dentin collagen matrix. J. Dent. Res. 2012, 91, 1049–1054. [Google Scholar] [CrossRef]
- Lim, E.S.; Lim, M.J.; Min, K.S.; Kwon, Y.S.; Hwang, Y.C.; Yu, M.K.; Hong, C.U.; Lee, K.W. Effects of epicatechin, a crosslinking agent, on human dental pulp cells cultured in collagen scaffolds. J. Appl. Oral. Sci. 2016, 24, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Jaurequi, J.; Tang, B.W.; Nimni, M.E. Proanthocyanidin: A natural crosslinking reagent for stabilizing collagen matrices. J. Biomed. Mater. Res. A 2003, 65, 118–124. [Google Scholar] [CrossRef]
- Demeule, M.; Brossard, M.; Page, M.; Gingras, D.; Beliveau, R. Matrix metalloproteinase inhibition by green tea catechins. Biochim. Biophys. Acta 2000, 1478, 51–60. [Google Scholar] [CrossRef]
- Epasinghe, D.J.; Yiu, C.K.; Burrow, M.F.; Hiraishi, N.; Tay, F.R. The inhibitory effect of proanthocyanidin on soluble and collagen-bound proteases. J. Dent. 2013, 41, 832–839. [Google Scholar] [CrossRef]
- Cova, A.; Breschi, L.; Nato, F.; Ruggeri, A., Jr.; Carrilho, M.; Tjaderhane, L.; Prati, C.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; et al. Effect of UVA-activated riboflavin on dentin bonding. J. Dent. Res. 2011, 90, 1439–1445. [Google Scholar] [CrossRef] [Green Version]
- Castellan, C.S.; Bedran-Russo, A.K.; Antunes, A.; Pereira, P.N. Effect of dentin biomodification using naturally derived collagen cross-linkers: One-year bond strength study. Int. J. Dent. 2013, 2013, 918010. [Google Scholar] [CrossRef] [Green Version]
- Khamverdi, Z.; Rezaei-Soufi, L.; Rostamzadeh, T. The Effect of Epigallocatechin Gallate on the Dentin Bond Durability of Two Self-etch Adhesives. J. Dent. 2015, 16, 68–74. [Google Scholar]
- Liu, Y.; Dusevich, V.; Wang, Y. Proanthocyanidins rapidly stabilize the demineralized dentin layer. J. Dent. Res. 2013, 92, 746–752. [Google Scholar] [CrossRef] [Green Version]
- Montagner, A.F.; Sarkis-Onofre, R.; Pereira-Cenci, T.; Cenci, M.S. MMP Inhibitors on Dentin Stability: A Systematic Review and Meta-analysis. J. Dent. Res. 2014, 93, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Mine, A.; De Munck, J.; Cardoso, M.V.; Van Landuyt, K.L.; Poitevin, A.; Van Ende, A.; Matsumoto, M.; Yoshida, Y.; Kuboki, T.; Yatani, H.; et al. Dentin-smear remains at self-etch adhesive interface. Dent. Mater. 2014, 30, 1147–1153. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Swift, E.J., Jr. Dentin/enamel bonding. J. Esthet. Restor. Dent. 2010, 22, 157. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munck, J.; Van Landuyt, K.L. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef]
- Turk, V.; Stoka, V.; Vasiljeva, O.; Renko, M.; Sun, T.; Turk, B.; Turk, D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Biophys. Acta Proteins Proteom. 2012, 1824, 68–88. [Google Scholar] [CrossRef] [Green Version]
- Birkedal-Hansen, H.; Moore, W.G.; Bodden, M.K.; Windsor, L.J.; Birkedal-Hansen, B.; DeCarlo, A.; Engler, J.A. Matrix metalloproteinases: A review. Crit. Rev. Oral Biol. Med. 1993, 4, 197–250. [Google Scholar] [CrossRef] [Green Version]
- Palosaari, H.; Pennington, C.J.; Larmas, M.; Edwards, D.R.; Tjaderhane, L.; Salo, T. Expression profile of matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in mature human odontoblasts and pulp tissue. Eur. J. Oral Sci. 2003, 111, 117–127. [Google Scholar] [CrossRef]
- Nascimento, F.D.; Minciotti, C.L.; Geraldeli, S.; Carrilho, M.R.; Pashley, D.H.; Tay, F.R.; Nader, H.B.; Salo, T.; Tjaderhane, L.; Tersariol, I.L. Cysteine cathepsins in human carious dentin. J. Dent. Res. 2011, 90, 506–511. [Google Scholar] [CrossRef]
- Scaffa, P.M.; Breschi, L.; Mazzoni, A.; Vidal, C.M.; Curci, R.; Apolonio, F.; Gobbi, P.; Pashley, D.; Tjaderhane, L.; Tersariol, I.L.; et al. Co-distribution of cysteine cathepsins and matrix metalloproteases in human dentin. Arch. Oral Biol. 2017, 74, 101–107. [Google Scholar] [CrossRef]
- Sulkala, M.; Larmas, M.; Sorsa, T.; Salo, T.; Tjaderhane, L. The localization of matrix metalloproteinase-20 (MMP-20, enamelysin) in mature human teeth. J. Dent. Res. 2002, 81, 603–607. [Google Scholar] [CrossRef]
- Sulkala, M.; Tervahartiala, T.; Sorsa, T.; Larmas, M.; Salo, T.; Tjaderhane, L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch. Oral Biol. 2007, 52, 121–127. [Google Scholar] [CrossRef]
- Mazzoni, A.; Pashley, D.H.; Tay, F.R.; Gobbi, P.; Orsini, G.; Ruggeri, A., Jr.; Carrilho, M.; Tjaderhane, L.; Di Lenarda, R.; Breschi, L. Immunohistochemical identification of MMP-2 and MMP-9 in human dentin: Correlative FEI-SEM/TEM analysis. J. Biomed. Mater. Res. A 2009, 88, 697–703. [Google Scholar] [CrossRef]
- Cox, S.W.; Eley, B.M.; Kiili, M.; Asikainen, A.; Tervahartiala, T.; Sorsa, T. Collagen degradation by interleukin-1beta-stimulated gingival fibroblasts is accompanied by release and activation of multiple matrix metalloproteinases and cysteine proteinases. Oral Dis. 2006, 12, 34–40. [Google Scholar] [CrossRef]
- Hara, K.; Kominami, E.; Katunuma, N. Effect of proteinase inhibitors on intracellular processing of cathepsin B, H and L in rat macrophages. FEBS Lett. 1988, 231, 229–231. [Google Scholar] [CrossRef] [Green Version]
- Seseogullari-Dirihan, R.; Mutluay, M.M.; Vallittu, P.; Pashley, D.H.; Tezvergil-Mutluay, A. Effect of pretreatment with collagen crosslinkers on dentin protease activity. Dent. Mater. 2015, 31, 941–947. [Google Scholar] [CrossRef] [Green Version]
- Vidal, C.M.; Aguiar, T.R.; Phansalkar, R.; McAlpine, J.B.; Napolitano, J.G.; Chen, S.N.; Araujo, L.S.; Pauli, G.F.; Bedran-Russo, A. Galloyl moieties enhance the dentin biomodification potential of plant-derived catechins. Acta Biomater. 2014, 10, 3288–3294. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.; Hiraishi, N.; Nassar, M.; Yiu, C.; Otsuki, M.; Tagami, J. Effect of natural cross-linkers incorporation in a self-etching primer on dentine bond strength. J. Dent. 2012, 40, 1052–1059. [Google Scholar] [CrossRef]
- Hiraishi, N.; Maruno, T.; Tochio, N.; Sono, R.; Otsuki, M.; Takatsuka, T.; Tagami, J.; Kobayashi, Y. Hesperidin interaction to collagen detected by physico-chemical techniques. Dent. Mater. 2017, 33, 33–42. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Gil, M.I.; Tomás-Barberán, F.A.; Ferreres, F. Influence of Industrial Processing on Orange Juice Flavanone Solubility and Transformation to Chalcones under Gastrointestinal Conditions. J. Agric. Food Chem. 2003, 51, 3024–3028. [Google Scholar] [CrossRef]
- Ding, F.; Peng, W. Biological activity of natural flavonoids as impacted by protein flexibility: An example of flavanones. Mol. Biosyst. 2015, 11, 1119–1133. [Google Scholar] [CrossRef]
- Murray, N.J.; Williamson, M.P.; Lilley, T.H.; Haslam, E. Study of the interaction between salivary proline-rich proteins and a polyphenol by 1H-NMR spectroscopy. Eur. J. Biochem. 1994, 219, 923–935. [Google Scholar] [CrossRef]
- Sano, M.; Tabata, M.; Suzuki, M.; Degawa, M.; Miyase, T.; Maeda-Yamamoto, M. Simultaneous determination of twelve tea catechins by high-performance liquid chromatography with electrochemical detection. Analyst 2001, 126, 816–820. [Google Scholar] [CrossRef]
- Frazier, R.A.; Deaville, E.R.; Green, R.J.; Stringano, E.; Willoughby, I.; Plant, J.; Mueller-Harvey, I. Interactions of tea tannins and condensed tannins with proteins. J. Pharm. Biomed. Anal. 2010, 51, 490–495. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Butler, L.G. Determination of protein in tannin-protein precipitates. J. Agric. Food Chem. 1980, 28, 944–947. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Butler, L.G. The specificity of proanthocyanidin-protein interactions. J. Biol. Chem. 1981, 256, 4494–4497. [Google Scholar]
- Muenzer, J.; Bildstein, C.; Gleason, M.; Carlson, D.M. Properties of proline-rich proteins from parotid glands of isoproterenol-treated rats. J. Biol. Chem. 1979, 254, 5629–5634. [Google Scholar]
- Ramachandran, G.N.; Ramakrishnan, C. Molecular Structure. In Biochemistry of Collagen; Ramachandran, G.N., Reddi, A.H., Eds.; Springer US: Boston, MA, USA, 1976; pp. 45–84. ISBN 978-1-4757-4602-0. [Google Scholar]
- Charlton, A.J.; Haslam, E.; Williamson, M.P. Multiple conformations of the proline-rich protein/epigallocatechin gallate complex determined by time-averaged nuclear Overhauser effects. J. Am. Chem. Soc. 2002, 124, 9899–9905. [Google Scholar] [CrossRef]
- Garnero, P.; Borel, O.; Byrjalsen, I.; Ferreras, M.; Drake, F.H.; McQueney, M.S.; Foged, N.T.; Delmas, P.D.; Delaisse, J.M. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J. Biol. Chem. 1998, 273, 32347–32352. [Google Scholar] [CrossRef] [Green Version]
- Lecaille, F.; Choe, Y.; Brandt, W.; Li, Z.; Craik, C.S.; Bromme, D. Selective inhibition of the collagenolytic activity of human cathepsin K by altering its S2 subsite specificity. Biochemistry 2002, 41, 8447–8454. [Google Scholar] [CrossRef] [Green Version]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [Green Version]
- Bode, W.; Maskos, K. Structural basis of the matrix metalloproteinases and their physiological inhibitors, the tissue inhibitors of metalloproteinases. Biol. Chem. 2003, 384, 863–872. [Google Scholar] [CrossRef]
- Sarni-Manchado, P.; Deleris, A.; Avallone, S.; Cheynier, V.; Moutounet, M. Analysis and Characterization of Wine Condensed Tannins Precipitated by Proteins Used as Fining Agent in Enology. Am. J. Enol. Vitic 1999, 50, 81. [Google Scholar]
- Hagerman, A.E.; Butler, L.G. Protein precipitation method for the quantitative determination of tannins. J. Agric. Food Chem. 1978, 26, 809–812. [Google Scholar] [CrossRef]
- Highberger, J.H. The Isoelectric Point of Collagen. J. Am. Chem. Soc. 1939, 61, 2302–2303. [Google Scholar] [CrossRef]
- Hiraishi, N.; Yiu, C.K.; King, N.M.; Tay, F.R. Effect of chlorhexidine incorporation into a self-etching primer on dentine bond strength of a luting cement. J. Dent. 2010, 38, 496–502. [Google Scholar] [CrossRef]
- Sodhi, R.N.; Grad, H.A.; Smith, D.C. Examination by X-ray photoelectron spectroscopy of the adsorption of chlorhexidine on hydroxyapatite. J. Dent. Res. 1992, 71, 1493–1497. [Google Scholar] [CrossRef]
- Yoshida, Y.; Nagakane, K.; Fukuda, R.; Nakayama, Y.; Okazaki, M.; Shintani, H.; Inoue, S.; Tagawa, Y.; Suzuki, K.; De Munck, J.; et al. Comparative study on adhesive performance of functional monomers. J. Dent. Res. 2004, 83, 454–458. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Peumans, M.; Poitevin, A.; Mine, A.; Van Ende, A.; Neves, A.; De Munck, J. Relationship between bond-strength tests and clinical outcomes. Dent. Mater. 2010, 26, e100–e121. [Google Scholar] [CrossRef]
- Braga, R.R.; Meira, J.B.; Boaro, L.C.; Xavier, T.A. Adhesion to tooth structure: A critical review of “macro” test methods. Dent. Mater. 2010, 26, e38–e49. [Google Scholar] [CrossRef]
- De Munck, J.; Mine, A.; Poitevin, A.; Van Ende, A.; Cardoso, M.V.; Van Landuyt, K.L.; Peumans, M.; Van Meerbeek, B. Meta-analytical review of parameters involved in dentin bonding. J. Dent. Res. 2012, 91, 351–357. [Google Scholar] [CrossRef]




| Commercial Name, Manufacturer, LOT Number | Type of Material | Main Components | Instructions for Use |
|---|---|---|---|
| Clearfil SE Bond 2,Kuraray Noritake,LOT 000031 | Two-step self-etch adhesive | Primer:
| Apply primer to the entire cavity wall for 20 s and dry with mild air for more than 5 s until the PRIMER does not move; |
Adhesive:
| Apply bond to the entire cavity wall and make a uniform bond film using a gentle air flow;Light-cure bond with a dental curing unit for 10 s | ||
| Admira Fusion xtra, VOCO, LOT 1537600 | Nanohybrid-ORMOCER bulk-fillresin-composite | Matrix:ORMOCERFillers:Based on silicon oxide (84 wt.%) | Apply in ≤ 4 mm increments;Light cure for 40 s |
| Bond Strength (MPa) | EGCG 100 M (wt/vol) | CHX 2% (vol/vol) | PA 3.75% (wt/vol) | HPN 5% (wt/vol) | Control (No Additive) | ANOVA | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Primer | Solution | Primer | Solution | Primer | Solution | Primer | Solution | |||
| One week | 12.68 (4.31) a | 12.53 (4.27) a | 11.41 (3.84) ab | 13.18 (4.47) a | 12.49 (4.06) a | 12.66 (4.28) a | 15.12 (3.92) a | 12.10 (4.77) a | 14.10 (3.40) ab | p = 0.199 |
| One month | 11.78 (3.41) ab | 10.84 (4.28) a | 13.80 (5.84) a | 15.59 (3.94) a | 17.03 (4.08) bc | 13.53 (4.16) a | 16.68 (5.72) a | 15.35 (3.65) a | 17.66 (5.89) a | p < 0.001 |
| Three months | 12.30 (5.02) a | 13.29 (5.37) a | 11.50 (3.25) ab | 15.84 (3.23) a | 19.23 (2.37) c | 15.43 (5.03) a | 16.42 (5.39) a | 12.59 (4.27) a | 14.29 (4.14) ab | p < 0.001 |
| Six months | 12.11 (3.52) a | 10.56 (3.82) a | 9.69 (3.81) b | 12.18 (4.71) a | 15.08 (3.16) ab | 14.85 (4.15) a | 10.10 (4.47) b | 12.68 (4.19) a | 14.49 (5.06) ab | p < 0.001 |
| One year | 8.42 (3.83) b | 13.35 (5.06) a | 10.41 (3.92) ab | 14.63 (5.30) a | 14.36 (2.95) ab | 15.17 (2.98) a | 13.12 (4.92) ab | 14.41 (3.93) a | 13.58 (3.66) b | p < 0.001 |
| ANOVA | p = 0.009 | p = 0.159 | p = 0.027 | p = 0.043 | p < 0.001 | p = 0.178 | p < 0.001 | p = 0.074 | p = 0.046 | |
| Weibull Modulus m, Confidence Interval (95%), R2 and σθ (MPa) |
EGCG 100 M (wt./vol) | CHX 2% (vol/vol) | PA 3.75% (wt./vol) | HPN 5% (wt/vol) | Control (No Additive) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Primer | Solution | Primer | Solution | Primer | Solution | Primer | Solution | ||
| One week | 3.30 (0.24) R2 = 0.98 σ0 = 14.15 | 2.66 (0.37) R2 = 0.92 σ0 = 14.35 | 3.15 (0.44) R2 = 0.92 σ0 = 12.82 | 3.46 (0.46) R2 = 0.92 σ0 = 14.67 | 3.00 (0.28) R2 = 0.96 σ0 = 14.10 | 2.81 (0.32) R2 = 0.94 σ0 = 14.37 | 4.35 (0.25) R2 = 0.98 σ0 = 16.61 | 2.32 (0.26) R2 = 0.95 σ0 = 13.88 | 4.67 (0.39) R2 = 0.97 σ0 = 15.43 |
| One month | 3.59 (0.37) R2 = 0.95 σ0 = 13.13 | 2.65 (0.16) R2 = 0.98 σ0 = 12.26 | 2.46 (0.21) R2 = 0.97 σ0 = 15.63 | 4.06 (0.47) R2 = 0.94 σ0 = 17.25 | 5.03 (0.62) R2 = 0.93 σ0 = 18.54 | 2.89 (0.44) R2 = 0.90 σ0 = 15.41 | 2.77 (0.29) R2 = 0.95 σ0 = 18.94 | 4.82 (0.44) R2 = 0.96 σ0 = 16.76 | 3.24 (0.36) R2 = 0.94 σ0 = 19.76 |
| Three months | 2.61 (0.15) R2 = 0.98 σ0 = 13.89 | 2.95 (0.36) R2 = 0.93 σ0 = 14.89 | 3.75 (0.41) R2 = 0.95 σ0 = 12.79 | 5.66 (0.44) R2 = 0.97 σ0 = 17.13 | 9.41 (0.99) R2 = 0.95 σ0 = 20.26 | 3.23 (0.17) R2 = 0.99 σ0 = 17.28 | 2.46 (0.39) R2 = 0.89 σ0 = 19.01 | 3.30 (0.29) R2 = 0.97 σ0 = 14.05 | 2.27 (0.30) R2 = 0.76 σ0 = 17.02 |
| Six months | 2.93 (0.47) R2 = 0.89 σ0 = 13.83 | 3.15 (0.18) R2 = 0.99 σ0 = 11.79 | 2.88 (0.20) R2 = 0.98 σ0 = 10.87 | 2.63 (0.13) R2 = 0.99 σ0 = 13.79 | 5.50 (0.40) R2 = 0.98 σ0 = 17.16 | 3.95 (0.21) R2 = 0.99 σ0 = 16.41 | 2.35 (0.24) R2 = 0.95 σ0 = 11.46 | 3.35 (0.35) R2 = 0.95 σ0 = 14.15 | 2.79 (0.28) R2 = 0.95 σ0 = 16.42 |
| One year | 2.19 (0.16) R2 = 0.98 σ0 = 9.60 | 3.18 (0.38) R2 = 0.94 σ0 = 14.90 | 2.57 (0.28) R2 = 0.95 σ0 = 11.56 | 3.31 (0.58) R2 = 0.87 σ0 = 16.35 | 5.72 (0.60) R2 = 0.95 σ0 = 15.52 | 6.12 (0.86) R2 = 0.91 σ0 = 16.34 | 2.68 (0.17) R2 = 0.98 σ0 = 14.85 | 3.91 (0.35) R2 = 0.96 σ0 = 15.96 | 4.00 (0.25) R2 = 0.98 σ0 = 14.98 |
| Fracture Analysis |
EGCG 100 M (wt/vol) | CHX 2% (vol/vol) | PA 3.75% (wt/vol) | HPN 5% (wt/vol) | Control (No Additive) | In Total | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Primer | Solution | Primer | Solution | Primer | Solution | Primer | Solution | |||
| 1 week | 70% (14) a 30% (6) m 0% (0) c | 55% (11) a 45% (9) m 0% (0) c | 80% (16) a 20% (4) m 0% (0) c | 45% (9) a 55% (11) m 0% (0) c | 45% (9) a 55% (11) m 0% (0) c | 60% (12) a 40% (8) m 0% (0) c | 30% (6) a 60% (12) m 10% (2) c | 45% (9) a 55% (11) m 0% (0) c | 55% (11) a 45% (9) m 0% (0) c | 52.8% (95) a 46.1% (83) m 2.2% (2) c |
| 1 month | 65% (13) a 35% (7) m 0% (0) c | 60% (12) a 40% (8) m 0% (0) c | 75% (15) a 25% (5) m 0% (0) c | 30% (6) a 65% (13) m 5% (1) c | 35% (7) a 65% (13) m 0% (0) c | 60% (12) a 40% (8) m 0% (0) c | 30% (6) a 65% (13) m 5% (1) c | 35% (7) a 55% (11) m 10% (2) c | 15% (3) a 85% (17) m 0% (0) c | 45% (81) a 52.8% (95) m 2.2% (4) c |
| 3 months | 75% (15) a 25% (5) m 0% (0) c | 85% (17) a 15% (3) m 0% (0) c | 85% (17) a 15% (3) m 0% (0) c | 60% (12) a 40% (8) m 0% (0) c | 40% (8) a 60% (12) m 0% (0) c | 60% (12) a 35% (7) m 5% (1) c | 55% (11) a 35% (7) m 10% (2) c | 50% (10) a 45% (9) m 5% (1) c | 60% (12) a 40% (8) m 0% (0) c | 63.3% (114) a 34.4% (62) m 2.2% (4) c |
| 6 months | 55% (11) a 45% (9) m 0% (0) c | 70% (14) a 30% (6) m 0% (0) c | 90% (18) a 10% (2) m 0% (0) c | 60% (12) a 35% (7) m 5% (1) c | 45% (9) a 50% (10) m 5% (1) c | 70% (14) a 30% (6) m 0% (0) c | 65% (13) a 35% (7) m 0% (0) c | 50% (10) a 50% (10) m 0% (0) c | 70% (14) a 30% (6) m 0% (0) c | 63.9% (115) a 35% (63) m 1.1% (2) c |
| 1 year | 75% (15) a 25% (5) m 0% (0) c | 50% (10) a 50% (10) m 0% (0) c | 60% (12) a 35% (7) m 5% (1) c | 45% (9) a 55% (11) m 0% (0) c | 45% (9) a 55% (11) m 0% (0) c | 45% (9) a 45% (9) m 10% (2) c | 40% (8) a 55% (11) m 5% (1) c | 40% (8) a 60% (12) m 0% (0) c | 35% (7) a 65% (13) m 0% (0) c | 48.3% (87) a 49.4% (89) m 2.2% (4) c |
| In total | 68% (68) a 32% (32) m 0% (0) c | 62% (62) a 38% (38) m 0% (0) c | 78% (78) a 21% (21) m 1% (1) c | 48% (48) a 50% (50) m 2% (2) c | 42% (42) a 57% (57) m 1% (1) c | 59% (59) a 38% (38) m 3% (3) c | 44% (44) a 50% (50) m 6% (6) c | 44% (44) a 53% (53) m 3% (3) c | 47% (47) a 53% (53) m 0% (0) c | 54.7% (492) a 43.6% (392) m 1.8% (16) c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beck, F.; Ilie, N. Antioxidants and Collagen-Crosslinking: Benefit on Bond Strength and Clinical Applicability. Materials 2020, 13, 5483. https://doi.org/10.3390/ma13235483
Beck F, Ilie N. Antioxidants and Collagen-Crosslinking: Benefit on Bond Strength and Clinical Applicability. Materials. 2020; 13(23):5483. https://doi.org/10.3390/ma13235483
Chicago/Turabian StyleBeck, Franziska, and Nicoleta Ilie. 2020. "Antioxidants and Collagen-Crosslinking: Benefit on Bond Strength and Clinical Applicability" Materials 13, no. 23: 5483. https://doi.org/10.3390/ma13235483





