The Effect of Microstructural Changes on Mechanical and Electrochemical Corrosion Properties of Duplex Stainless Steel Aged for Short Periods
Abstract
:1. Introduction
2. Materials and Methods
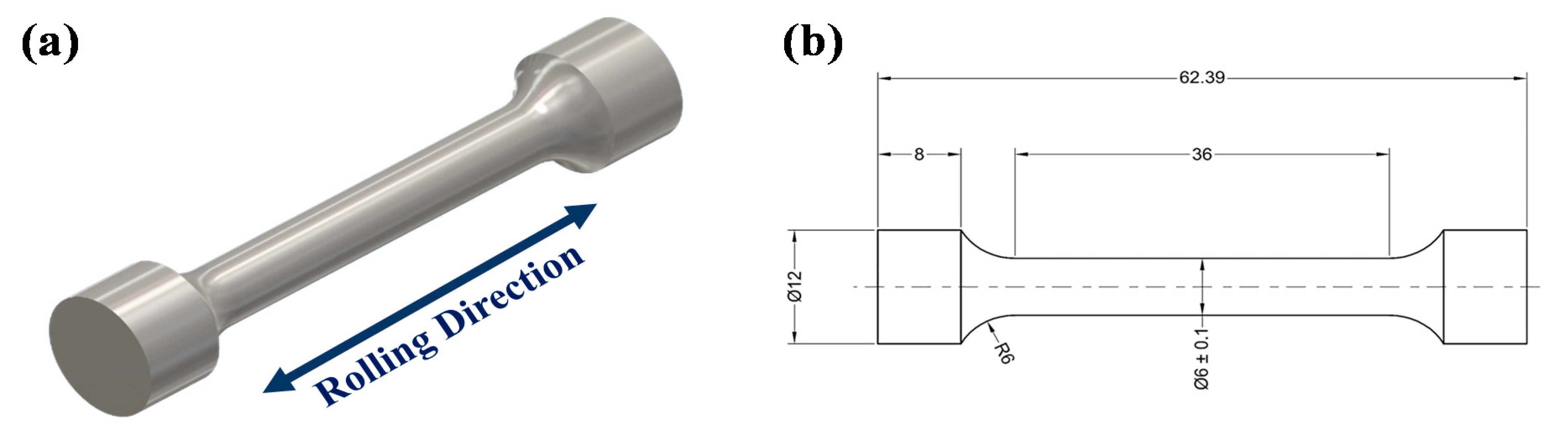
2.1. Heat Treatment
2.2. Structural, Morphological, and Mechanical Characterization
2.3. Electrochemical Corrosion Characterization
3. Results and Discussion
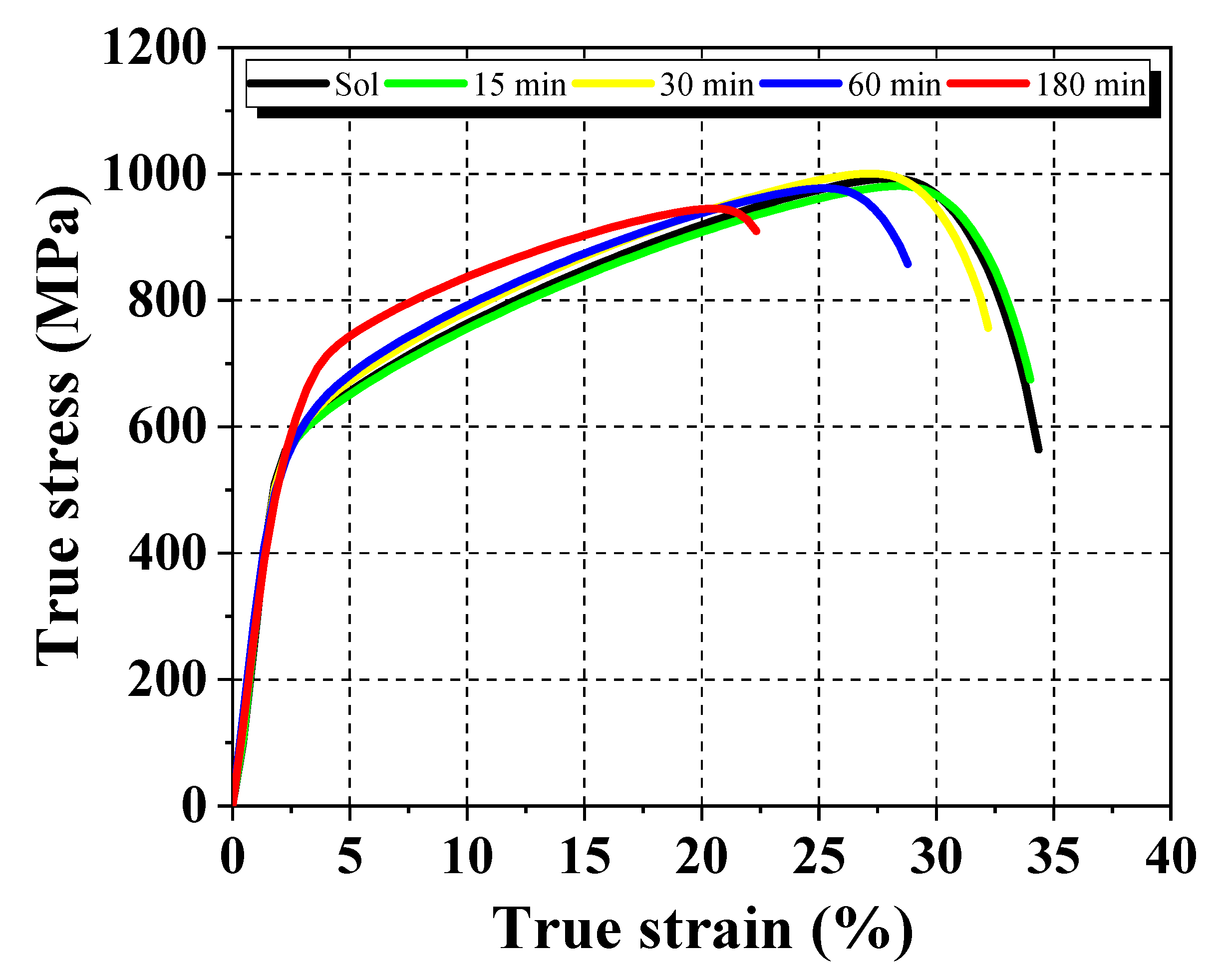
3.1. Structural, Morphological, and Mechanical Characterization
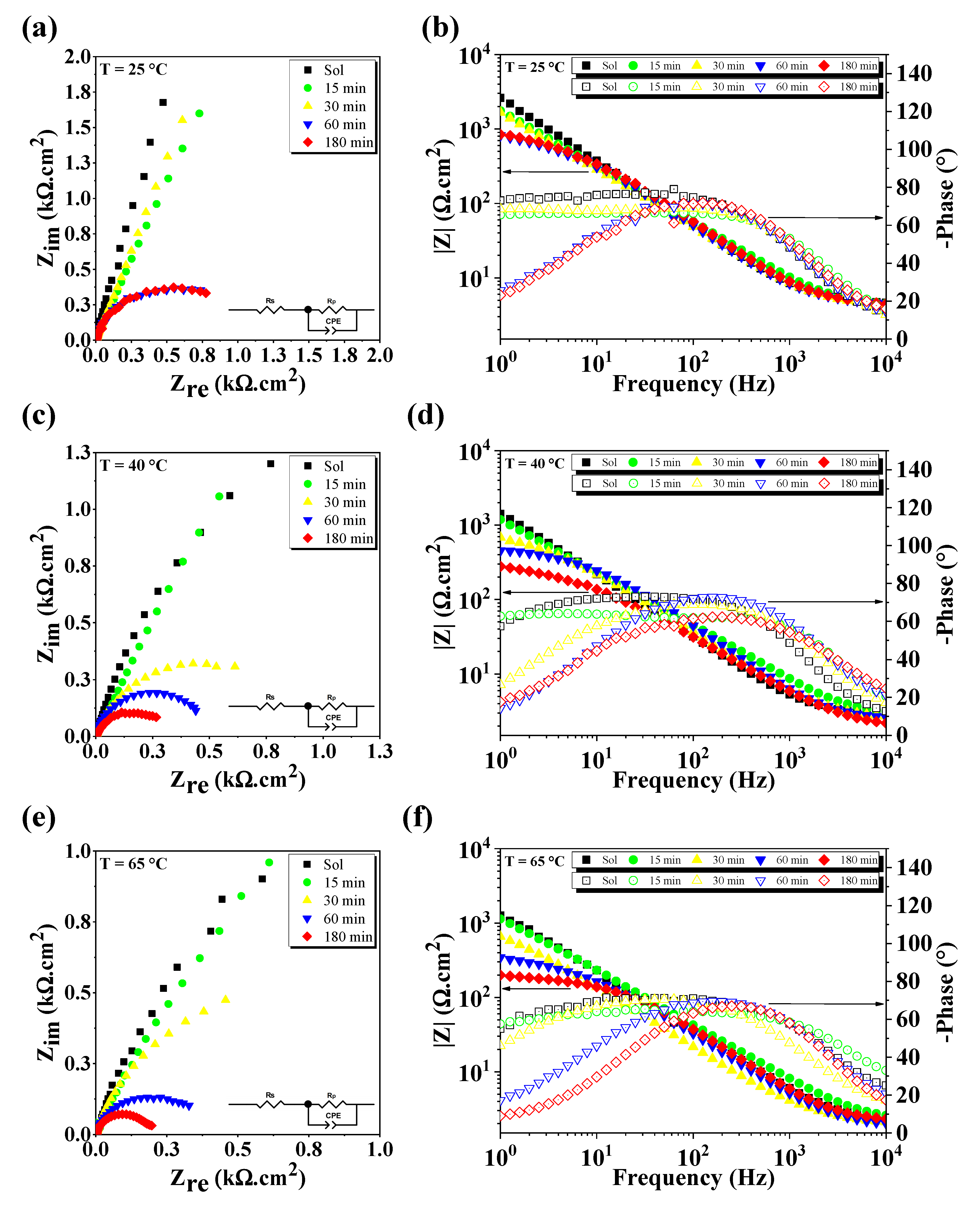
3.2. Electrochemical Corrosion Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gunn, R. Duplex Stainless Steels Microstructure Properties and Applications; Abington Publishing: Cambridge, UK, 2003. [Google Scholar]
- Chaves, R.; Costa, I.; de Melo, H.G.; Wolynec, S. Evaluation of selective corrosion in UNS S31803 duplex stainless steel with electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1842–1846. [Google Scholar] [CrossRef]
- Karlsson, L. Welding Duplex Stainless Steels—A Review Of Current Recommendations. Weld. World 2012, 56, 65–76. [Google Scholar] [CrossRef]
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. R Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Escobar, J.D.; Poplawsky, J.D.; Faria, G.A.; Rodriguez, J.; Oliveira, J.P.; Salvador, C.A.F.; Mei, P.R.; Babu, S.S.; Ramirez, A.J. Compositional analysis on the reverted austenite and tempered martensite in a Ti-stabilized supermartensitic stainless steel: Segregation, partitioning and carbide precipitation. Mater. Des. 2018, 140, 95–105. [Google Scholar] [CrossRef]
- Escobar, J.D.; Oliveira, J.P.; Salvador, C.A.F.; Faria, G.A.; Poplawsky, J.D.; Rodriguez, J.; Mei, P.R.; Babu, S.S.; Ramirez, A.J. Meta-equilibrium transition microstructure for maximum austenite stability and minimum hardness in a Ti-stabilized supermartensitic stainless steel. Mater. Des. 2018, 156, 609–621. [Google Scholar] [CrossRef]
- Pohl, M.; Storz, O.; Glogowski, T. Effect of Sigma-Phase Morphology on the Properties of Duplex Stainless Steels. Microsc. Microanal. 2005, 11, 230–231. [Google Scholar] [CrossRef] [Green Version]
- Practical Guidelines for the Fabrication of Duplex Stainless Steels, 3rd ed.; IMOA (International Molybdenum Association): London, UK, 2014; ISBN 9781907470097.
- Pareige, C.; Novy, S.; Saillet, S.; Pareige, P. Study of phase transformation and mechanical properties evolution of duplex stainless steels after long term thermal ageing (>20 years). J. Nucl. Mater. 2011, 411, 90–96. [Google Scholar] [CrossRef]
- Calliari, I.; Pellizzari, M.; Zanellato, M.; Ramous, E. The phase stability in Cr-Ni and Cr-Mn duplex stainless steels. J. Mater. Sci. 2011, 46, 6916–6924. [Google Scholar] [CrossRef]
- Børvik, T.; Lange, H.; Marken, L.A.; Langseth, M.; Hopperstad, O.S.; Aursand, M.; Rørvik, G. Pipe fittings in duplex stainless steel with deviation in quality caused by sigma phase precipitation. Mater. Sci. Eng. A 2010, 527, 6945–6955. [Google Scholar] [CrossRef]
- Ibrahim, O.H.; Ibrahim, I.S.; Khalifa, T.A.F. Effect of aging on the toughness of austenitic and duplex stainless steel weldments. J. Mater. Sci. Technol. 2010, 26, 810–816. [Google Scholar] [CrossRef]
- Lo, K.H.; Kwok, C.T.; Chan, W.K.; Zeng, D. Corrosion resistance of duplex stainless steel subjected to long-term annealing in the spinodal decomposition temperature range. Corros. Sci. 2012, 55, 267–271. [Google Scholar] [CrossRef]
- Escriba, D.M.; Materna-Morris, E.; Plaut, R.L.; Padilha, A.F. Chi-phase precipitation in a duplex stainless steel. Mater. Charact. 2009, 60, 1214–1219. [Google Scholar] [CrossRef]
- Magnabosco, R. Kinetics of sigma phase formation in a duplex stainless steel. Mater. Res. 2009, 12, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, D.C.; Magnabosco, R.; De Moura-Neto, C. Influence of sigma phase formation on pitting corrosion of an aged uns s31803 duplex stainless steel. Corrosion 2013, 69, 900–911. [Google Scholar] [CrossRef]
- Ferro, P.; Bonollo, F. A Semiempirical Model for Sigma-Phase Precipitation in Duplex and Superduplex Stainless Steels. Metall. Mater. Trans. A 2012, 43, 1109–1116. [Google Scholar] [CrossRef]
- dos Santos, D.C.; Magnabosco, R. Kinetic Study to Predict Sigma Phase Formation in Duplex Stainless Steels. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2016, 47, 1554–1565. [Google Scholar] [CrossRef]
- Långberg, M.; Zhang, F.; Grånäs, E.; Örnek, C.; Cheng, J.; Liu, M.; Wiemann, C.; Gloskovskii, A.; Keller, T.F.; Schlueter, C.; et al. Lateral variation of the native passive film on super duplex stainless steel resolved by synchrotron hard X-ray photoelectron emission microscopy. Corros. Sci. 2020, 174, 108841. [Google Scholar] [CrossRef]
- Maurice, V.; Marcus, P. Progress in corrosion science at atomic and nanometric scales. Prog. Mater. Sci. 2018, 95, 132–171. [Google Scholar] [CrossRef]
- Yongqiang, W.; Hao, S.; Na, L.; Yanhao, X.; Hemin, J. Effect of sigma phase precipitation on the pitting corrosion mechanism of duplex stainless steels. Int. J. Electrochem. Sci. 2018, 13, 9868–9887. [Google Scholar] [CrossRef]
- Sieurin, H.; Sandström, R. Sigma phase precipitation in duplex stainless steel 2205. Mater. Sci. Eng. A 2007, 444, 271–276. [Google Scholar] [CrossRef]
- Llorca-Isern, N.; López-Luque, H.; López-Jiménez, I.; Biezma, M.V. Identification of sigma and chi phases in duplex stainless steels. Mater. Charact. 2016, 112, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Pohl, M.; Storz, O.; Glogowski, T. Effect of intermetallic precipitations on the properties of duplex stainless steel. Mater. Charact. 2007, 58, 65–71. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Jiang, Y.; Deng, B.; Sun, D.; Li, J. Influence of annealing treatment on the corrosion resistance of lean duplex stainless steel 2101. Electrochim. Acta 2009, 54, 5387–5392. [Google Scholar] [CrossRef]
- Kumar, S.; Krisam, S.; Jacob, A.; Kiraly, F.; Keplinger, A.; Abart, R.; Povoden-Karadeniz, E. Microstructures and element distributions in an aged hyper duplex stainless steel and corresponding hardness variation. Mater. Des. 2020, 194, 108951. [Google Scholar] [CrossRef]
- Gennari, C.; Pezzato, L.; Piva, E.; Gobbo, R.; Calliari, I. Influence of small amount and different morphology of secondary phases on impact toughness of UNS S32205 Duplex Stainless Steel. Mater. Sci. Eng. A 2018, 729, 149–156. [Google Scholar] [CrossRef]
- Biezmaa, M.V.; Berlangab, C.; Argandonac, G. Relationship between Microstructure and Fracture Types in a UNS S32205 Duplex Stainless Steel. Mater. Res. 2013, 16, 965–969. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.D.S.; Sobrinho, J.M.B.; Souto, C.R.; Gomes, R.M. Application of electromechanical impedance technique in the monitoring of sigma phase embrittlement in duplex stainless steel. Mater. Sci. Eng. A 2020, 139457. [Google Scholar] [CrossRef]
- Silva, D.D.S.; Raimundo, R.A.; Torquato, R.A.; Faria, G.L.; Morales, M.A.; Simões, T.A.; Gomes, R.M. Low-Field Magnetic Analysis for Sigma Phase Embrittlement Monitoring in Thermally Aged 22Cr Duplex Stainless Steel. J. Magn. Magn. Mater. 2020, 167072. [Google Scholar] [CrossRef]
- Haghdadi, N.; Laleh, M.; Kosari, A.; Moayed, M.H.; Cizek, P.; Hodgson, P.D.; Beladi, H. The effect of phase transformation route on the intergranular corrosion susceptibility of 2205 duplex stainless steel. Mater. Lett. 2019, 238, 26–30. [Google Scholar] [CrossRef]
- Zanotto, F.; Grassi, V.; Balbo, A.; Monticelli, C.; Melandri, C.; Zucchi, F. Effect of brief thermal aging on stress corrosion cracking susceptibility of LDSS 2101 in the presence of chloride and thiosulphate ions. Corros. Sci. 2018, 130, 22–30. [Google Scholar] [CrossRef]
- Zhang, Z.; Jing, H.; Xu, L.; Han, Y.; Zhao, L.; Lv, X. Effect of post-weld heat treatment on microstructure evolution and pitting corrosion resistance of electron beam-welded duplex stainless steel. Corros. Sci. 2018, 141, 30–45. [Google Scholar] [CrossRef]
- ASTM A 240/A 240M-20: Standard Specification for Chromium and Chromium-Nickel Stainless Steel Plate, Sheet, and Strip for Pressure Vessels and for General Applications; ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM A923-14: Standard Test Methods for Detecting Detrimental Intermetallic Phase in Duplex Austenitic/Ferritic Stainless Steels; ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM A370-20: Standard Test Methods and Definitions for Mechanical Testing of Steel Products; ASTM International: West Conshohocken, PA, USA, 2020.
- Örnek, C.; Engelberg, D.L. Correlative EBSD and SKPFM characterisation of microstructure development to assist determination of corrosion propensity in grade 2205 duplex stainless steel. J. Mater. Sci. 2016, 51, 1931–1948. [Google Scholar] [CrossRef]
- Warren, A.D.; Harniman, R.L.; Guo, Z.; Younes, C.M.; Flewitt, P.E.J.; Scott, T.B. Quantification of sigma-phase evolution in thermally aged 2205 duplex stainless steel. J. Mater. Sci. 2016, 51, 694–707. [Google Scholar] [CrossRef] [Green Version]
- Michalska, J.; Sozańska, M. Qualitative and quantitative analysis of σ and χ phases in 2205 duplex stainless steel. Mater. Charact. 2006, 56, 355–362. [Google Scholar] [CrossRef]
- Breda, M.; Calliari, I.; Ramous, E.; Pizzo, M.; Corain, L.; Straffelini, G. Ductile-to-brittle transition in a Zeron®100 SDSS in wrought and aged conditions. Mater. Sci. Eng. A 2013, 585, 57–65. [Google Scholar] [CrossRef]
- Hong, T.; Nagumo, M. Effect of surface roughness on early stages of pitting corrosion of Type 301 stainless steel. Corros. Sci. 1997, 39, 1665–1672. [Google Scholar] [CrossRef]
- Abosrra, L.; Ashour, A.F.; Mitchell, S.C.; Youseffi, M. Corrosion of Mild Steel and 316L Austenitic Stainless Steel with Different Surface Roughness in Sodium Chloride Saline Solutions. WIT Trans. Eng. Sci. 2009, 65, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, N.; Momeni, M.; Kosari, A.; Zakeri, M.; Moayed, M.H. A comparative study of critical pitting temperature (CPT) of stainless steels by electrochemical impedance spectroscopy (EIS), potentiodynamic and potentiostatic techniques. Corros. Sci. 2012, 59, 96–102. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance Spectroscopy: Emphasizing Solid Materials and Systems; Wiley: New York, NY, USA, 1987; ISBN 0471831220. [Google Scholar]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, S.; Li, W.; Yin, L.; He, J.; Wu, J. Investigation of 1-butyl-3-methyl-1H-benzimidazolium iodide as inhibitor for mild steel in sulfuric acid solution. Corros. Sci. 2014, 80, 383–392. [Google Scholar] [CrossRef]
- Frankel, G.S. Pitting Corrosion of Metals. J. Electrochem. Soc. 1998, 145, 2186. [Google Scholar] [CrossRef]
- Ayati, N.S.; Khandandel, S.; Momeni, M.; Moayed, M.H.; Davoodi, A.; Rahimizadeh, M. Inhibitive effect of synthesized 2-(3-pyridyl)-3,4-dihydro-4-quinazolinone as a corrosion inhibitor for mild steel in hydrochloric acid. Mater. Chem. Phys. 2011, 126, 873–879. [Google Scholar] [CrossRef]
- He, L.; Wirian, L.; Singh, P.M. Effects of Isothermal Aging on the Microstructure Evolution and Pitting Corrosion Resistance of Lean Duplex Stainless Steel UNS S32003. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2019. [Google Scholar] [CrossRef]
- Zeng, H.; Yang, Y.; Xu, R.; Xin, S.; Li, M. Pitting corrosion resistance of sensitized type 2205 duplex stainless steel in hot concentrated seawater. J. Solid State Electrochem. 2019, 23, 2793–2801. [Google Scholar] [CrossRef]
- Silva, D.D.S.; Simões, T.A.; Macedo, D.A.; Bueno, A.H.S.; Torres, S.M.; Gomes, R.M. Microstructural influence of sigma phase on pitting corrosion behavior of duplex stainless steel/NaCl electrolyte couple. Mater. Chem. Phys. 2020, 124056. [Google Scholar] [CrossRef]
- Ramirez, A.J.; Lippold, J.C.; Brandi, S.D. The relationship between chromium nitride and secondary austenite precipitation in duplex stainless steels. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2003, 34, 1575–1597. [Google Scholar] [CrossRef]
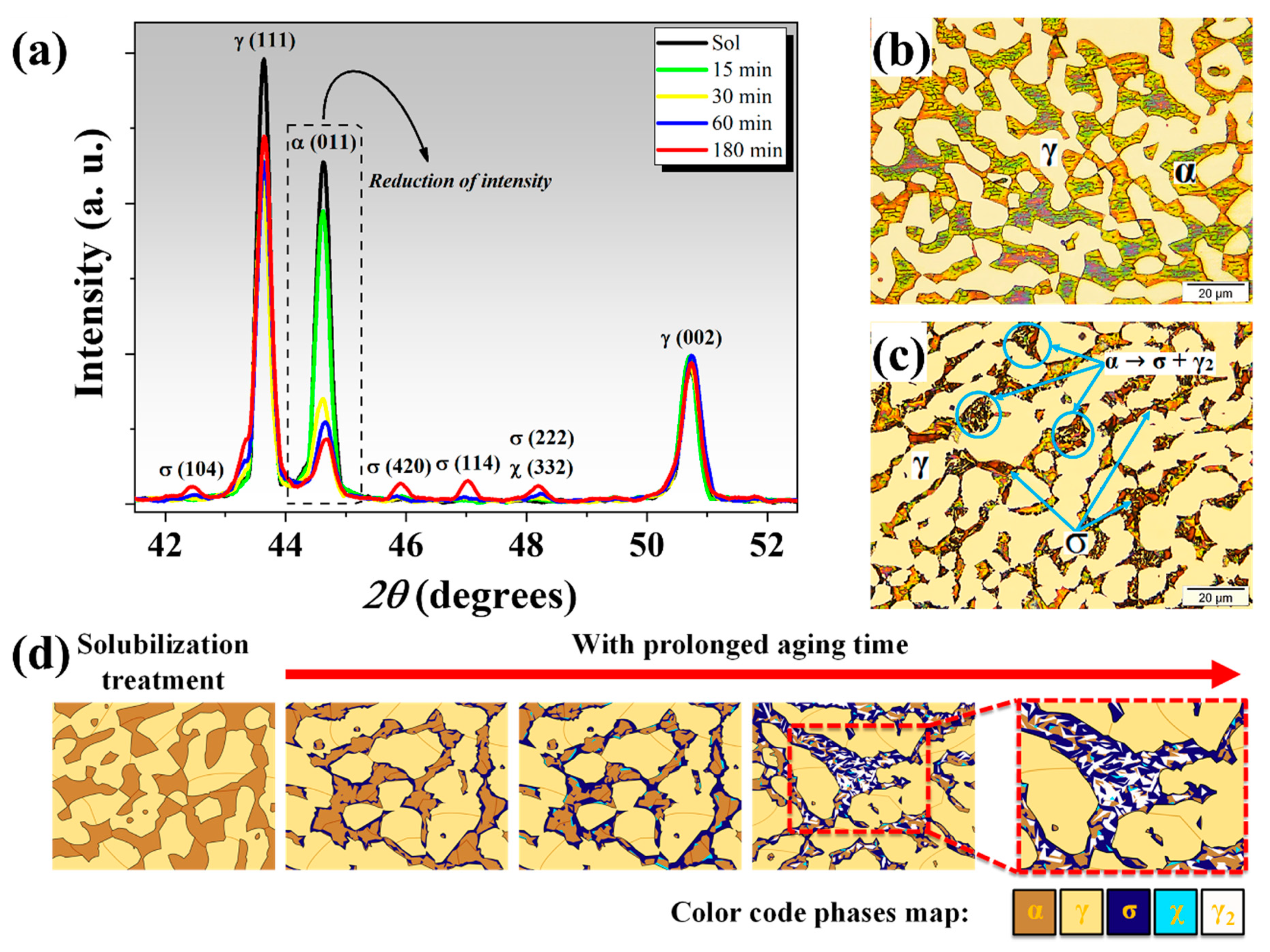
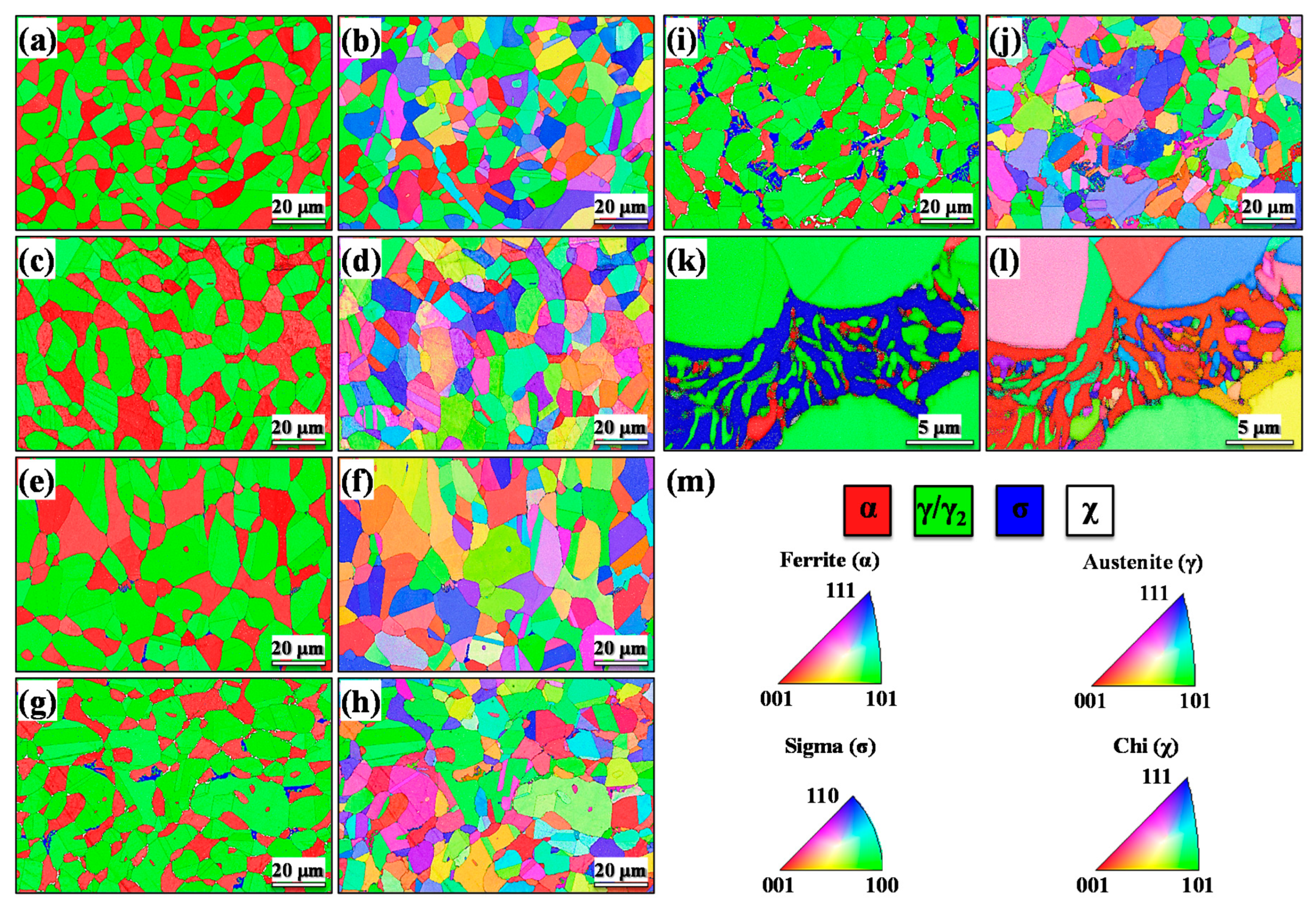
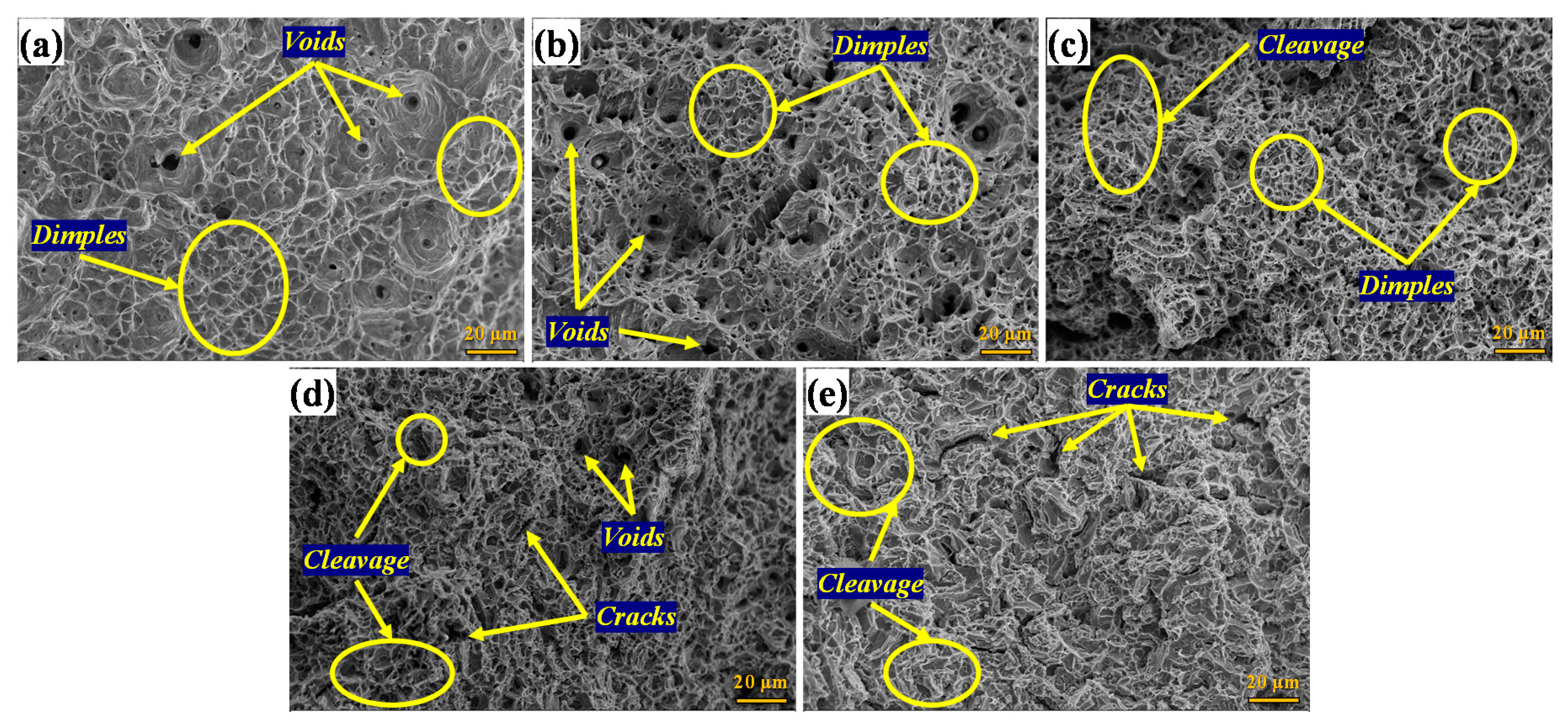
| Phase | Solubilized | 15 min | 30 min | 60 min | 180 min |
|---|---|---|---|---|---|
| % α | 37.5 ± 0.7 | 35.6 ± 2.2 | 31.4 ± 1.3 | 27.8 ± 0.6 | 17.2 ± 0.6 |
| % γ | 62.5 ± 0.7 | 64.0 (*) ± 2.2 | 67.7 (*) ± 1.1 | 69.3 (*) ± 0.9 | 73.7 (*) ± 0.8 |
| % σ | n/a | 0.4 ± 0.1 | 0.9 ± 0.2 | 1.7 ± 0.1 | 6.8 ± 0.3 |
| % χ | n/a | n/a | n/a | 1.2 ± 0.1 | 2.3 ± 0.3 |
| Specimen | (HV0.2) | σu (MPa) | σy (MPa) | ε (%) | (%RA) |
|---|---|---|---|---|---|
| Solubilized | 257 ± 0.5 | 993 ± 2.8 | 507 ± 1.5 | 34 ± 1.2 | 57 ± 1.3 |
| 15 min | 266 ± 3.1 | 981 ± 3.1 | 507 ± 2.9 | 34 ± 1.7 | 49 ± 0.7 |
| 30 min | 271 ± 2.6 | 1001 ± 2.5 | 558 ± 1.3 | 32 ± 0.8 | 37 ± 1.2 |
| 60 min | 287 ± 0.7 | 977 ± 2.7 | 561 ± 2.2 | 29 ± 1.5 | 25 ± 0.6 |
| 180 min | 305 ± 2.7 | 946 ± 3.3 | 561 ± 1.6 | 22 ± 1.4 | 15 ± 0.7 |
| Test Results at 25 °C | ||||
| Specimen | Parameters | |||
| (Ω cm2) | (µF cm−2) | (kΩ cm2) | ||
| Solubilized | 3.14 | 4.31 | 0.85 | 21.11 |
| 15 min | 2.35 | 6.84 | 0.75 | 12.88 |
| 30 min | 1.92 | 7.91 | 0.77 | 9.74 |
| 60 min | 1.06 | 8.77 | 0.78 | 0.84 |
| 180 min | 0.98 | 10.84 | 0.77 | 0.84 |
| Test Results at 40 °C | ||||
| Specimen | Parameters | |||
| (Ω cm2) | (µF cm−2) | (kΩ cm2) | ||
| Solubilized | 4.36 | 8.24 | 0.86 | 3.50 |
| 15 min | 2.86 | 12.84 | 0.73 | 3.49 |
| 30 min | 0.68 | 11.21 | 0.78 | 0.73 |
| 60 min | 0.54 | 12.23 | 0.82 | 0.40 |
| 180 min | 0.48 | 20.75 | 0.72 | 0.26 |
| Test Results at 65 °C | ||||
| Specimen | Parameters | |||
| (Ω cm2) | (µF cm−2) | (kΩ cm2) | ||
| Solubilized | 2.37 | 10.97 | 0.81 | 3.08 |
| 15 min | 1.84 | 12.71 | 0.71 | 2.27 |
| 30 min | 0.53 | 16.75 | 0.79 | 1.07 |
| 60 min | 0.41 | 17.45 | 0.77 | 0.32 |
| 180 min | 0.31 | 41.78 | 0.78 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, D.D.S.; Lima, L.S.D.C.; Araújo, A.J.M.; Silva, V.D.; Raimundo, R.A.; Damasceno, I.Z.; Simões, T.A.; Gomes, R.M. The Effect of Microstructural Changes on Mechanical and Electrochemical Corrosion Properties of Duplex Stainless Steel Aged for Short Periods. Materials 2020, 13, 5511. https://doi.org/10.3390/ma13235511
Silva DDS, Lima LSDC, Araújo AJM, Silva VD, Raimundo RA, Damasceno IZ, Simões TA, Gomes RM. The Effect of Microstructural Changes on Mechanical and Electrochemical Corrosion Properties of Duplex Stainless Steel Aged for Short Periods. Materials. 2020; 13(23):5511. https://doi.org/10.3390/ma13235511
Chicago/Turabian StyleSilva, David D. S., Lindolpho S. D. C. Lima, Allan J. M. Araújo, Vinícius D. Silva, Rafael A. Raimundo, Igor Z. Damasceno, Thiago A. Simões, and Rodinei M. Gomes. 2020. "The Effect of Microstructural Changes on Mechanical and Electrochemical Corrosion Properties of Duplex Stainless Steel Aged for Short Periods" Materials 13, no. 23: 5511. https://doi.org/10.3390/ma13235511
APA StyleSilva, D. D. S., Lima, L. S. D. C., Araújo, A. J. M., Silva, V. D., Raimundo, R. A., Damasceno, I. Z., Simões, T. A., & Gomes, R. M. (2020). The Effect of Microstructural Changes on Mechanical and Electrochemical Corrosion Properties of Duplex Stainless Steel Aged for Short Periods. Materials, 13(23), 5511. https://doi.org/10.3390/ma13235511








