Anisotropic Response of CoCrFeMnNi High-Entropy Alloy Fabricated by Selective Laser Melting
Abstract
:1. Introduction
2. Materials and Methods
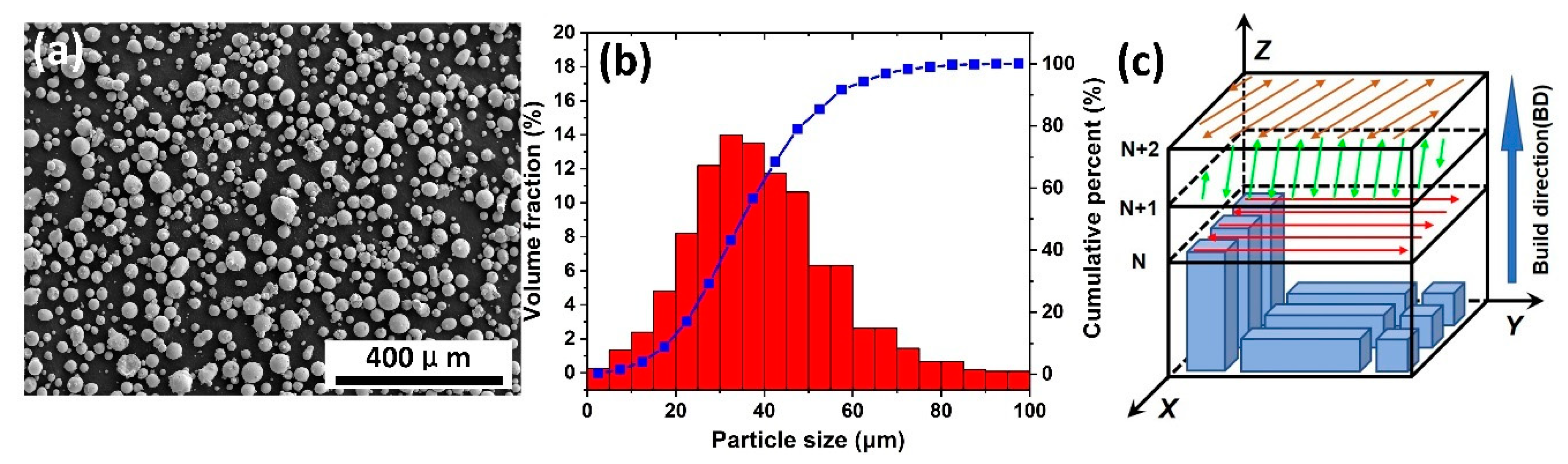
2.1. Sample Preparation
2.2. Microstructural Characterization
2.3. Tensile Tests
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Phase Identification
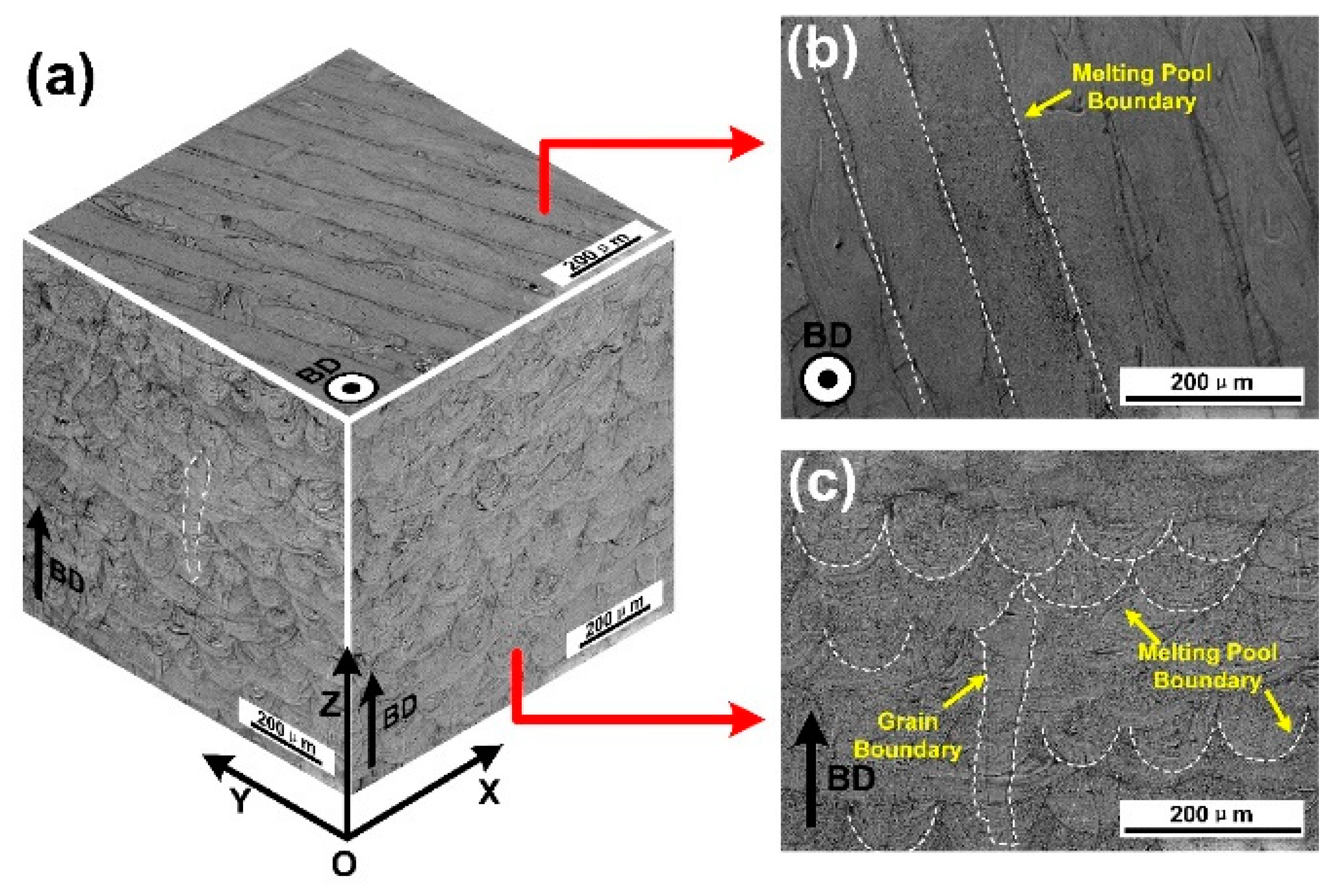
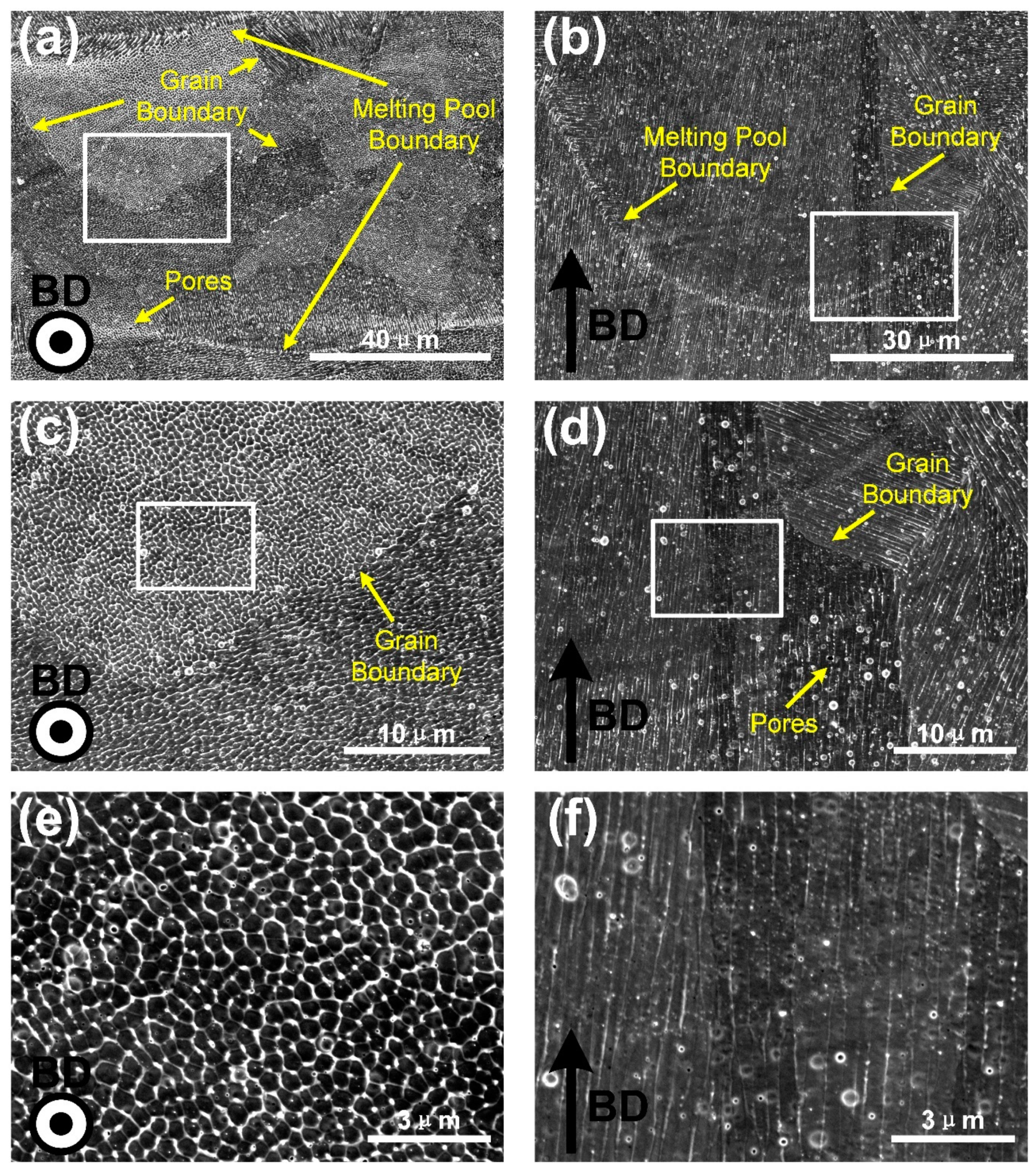
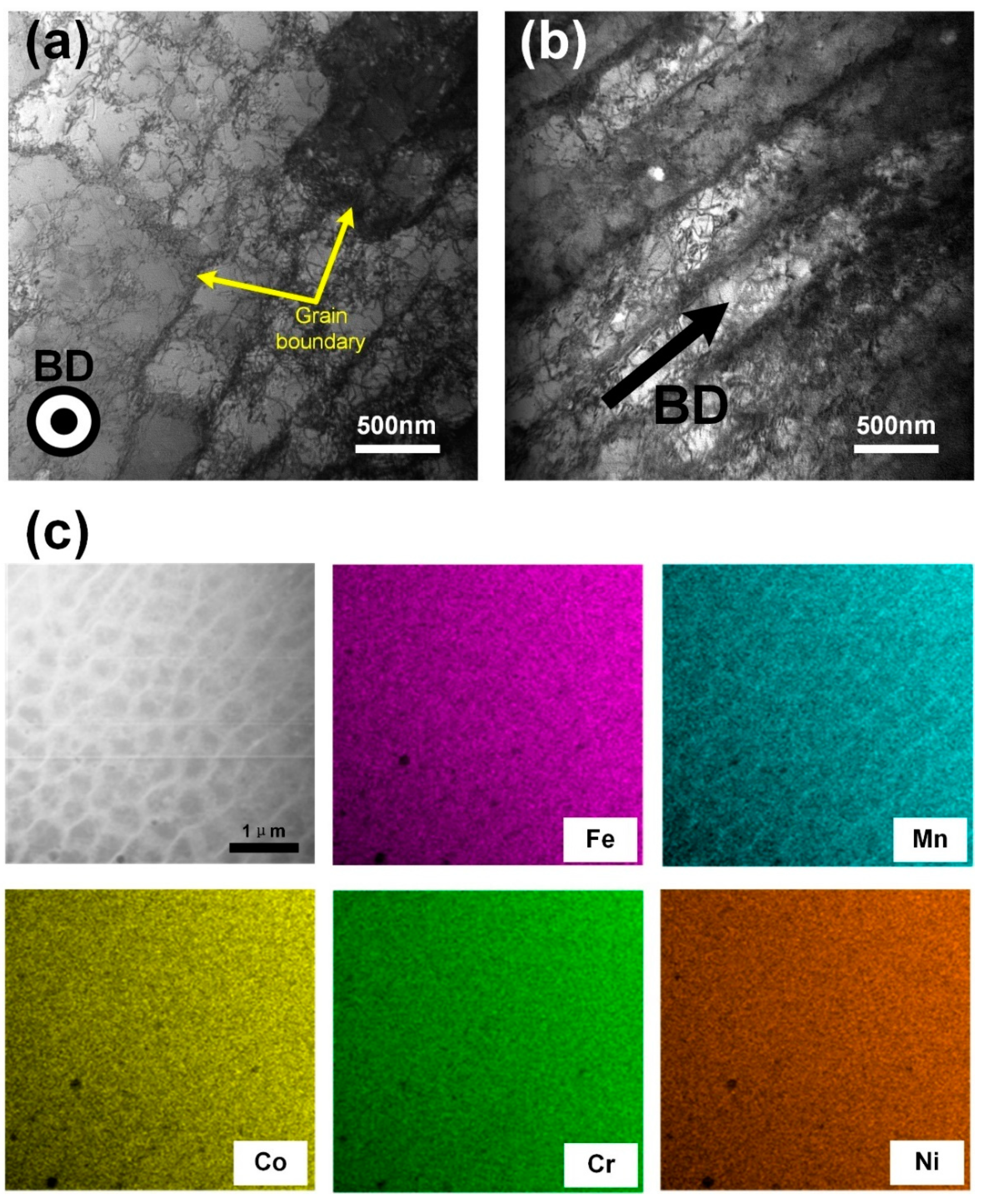
3.2. Microstructures Characterization
3.3. Mechanical Property Evaluation
3.4. Corrosion Property Assessment
4. Conclusions
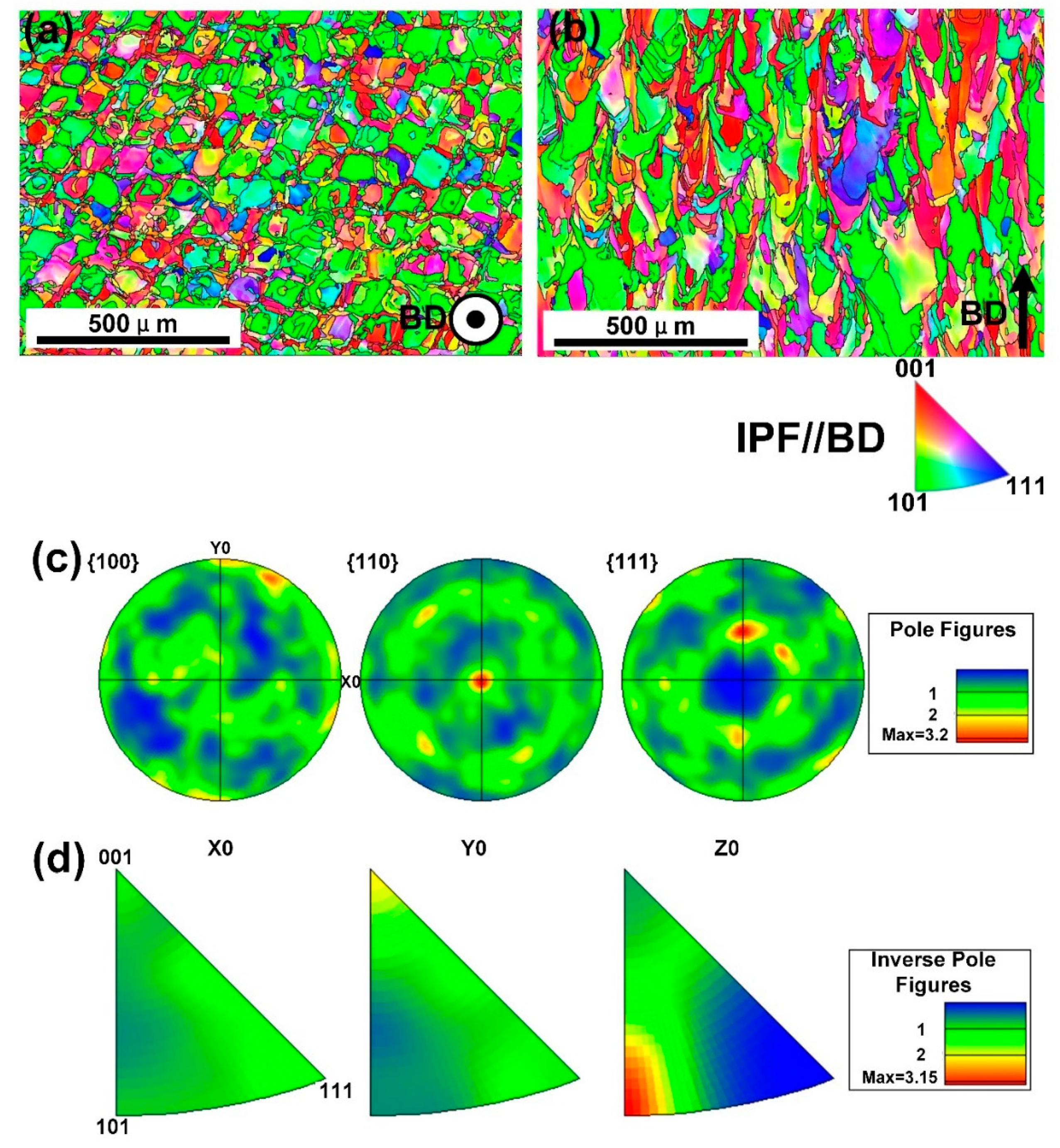
- The columnar crystal structure with the long axis direction consistent with the BD direction was formed under the extremely high thermal gradient during the SLM process. Within the columnar crystal, cellular-dendritic sub-structures with a size of about 500 nm were found, and their long axis direction were consistent with that of the columnar crystal. The walls of the sub-structures, decorated with high density dislocations, had Mn segregations with the thickness of about 500 nm.
- The SLMed samples showed fiber textures with dominant {110} directions mixed with some {100} directions perpendicular to the building direction, under the current building strategy.
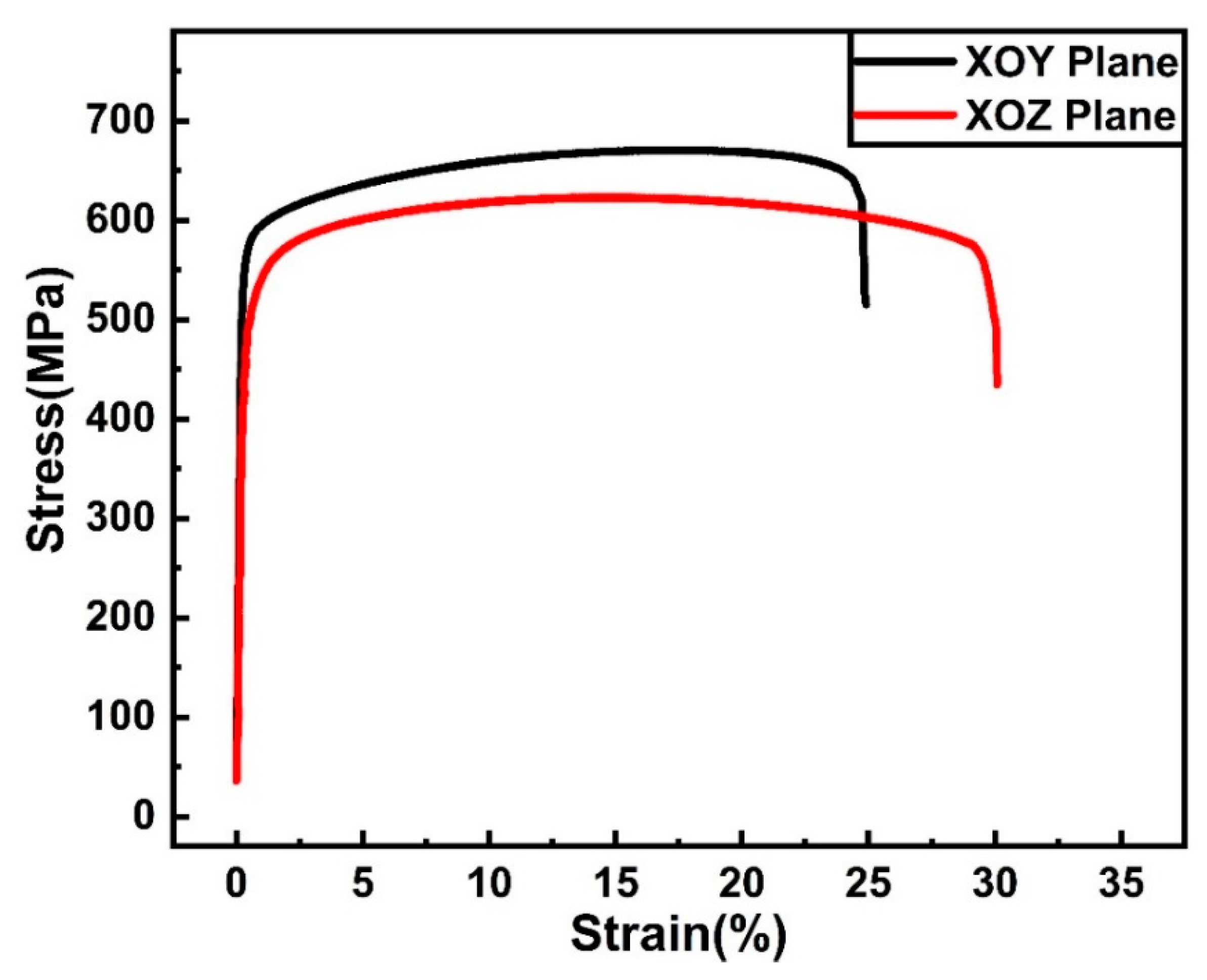
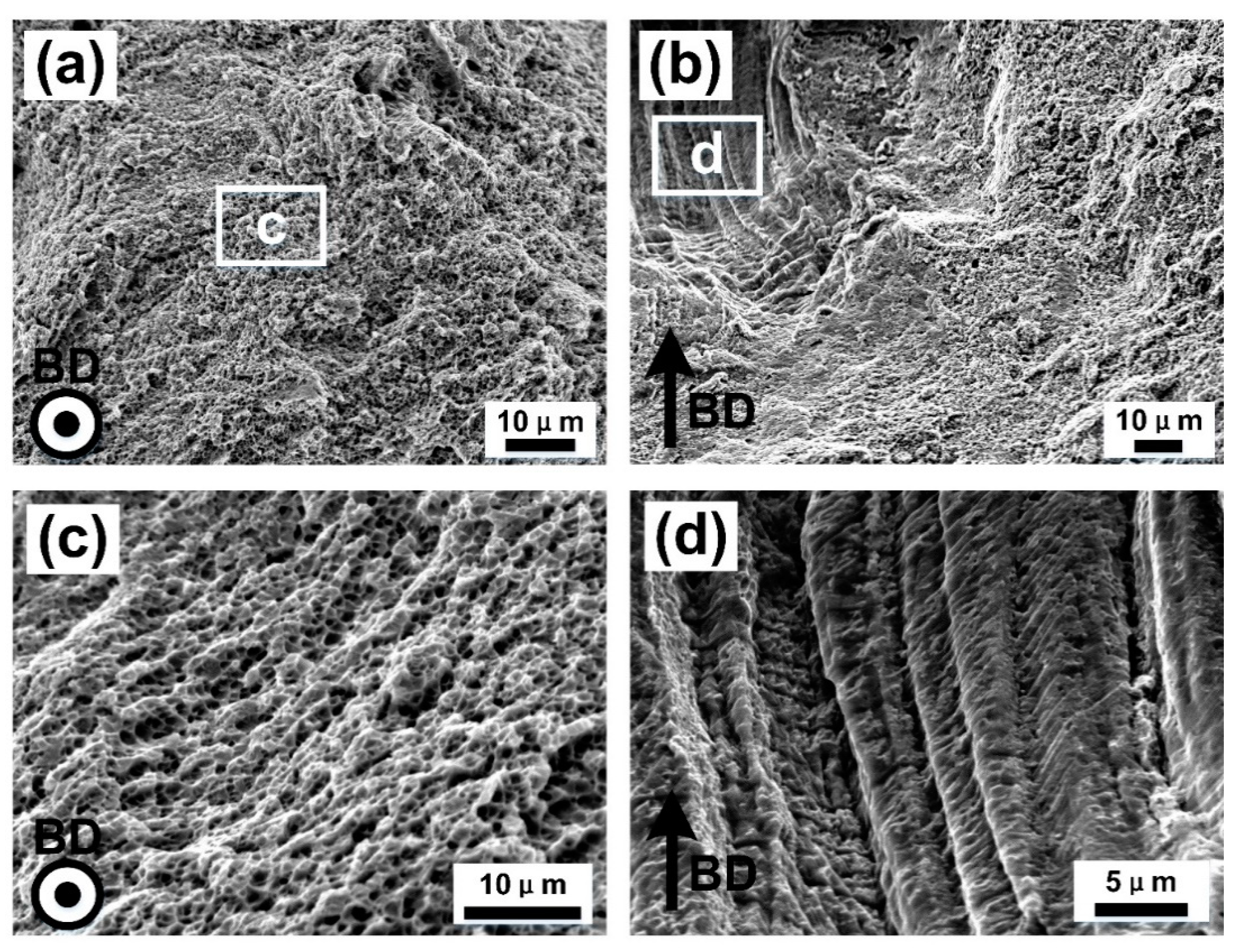
- The XOY plane sample (perpendicular to building direction) exhibited higher mechanical properties of ultimate tensile strength and yield strength (σ0.2) (UTS of 679.8 ± 12.9 MPa, and σ0.2 of 582.9 ± 10.8 MPa), but lower elongation to failure (εf of 23.8 ± 1.4%), compared to the XOZ plane (parallel to building direction) (UTS of 635.9 ± 13.4 MPa, σ0.2 of 503.8 ± 10.6 MPa and εf of 31.8 ± 1.5%), which was due to the anisotropy of microstructure.
- In the 3.5 wt % NaCl solution, the XOZ plane exhibited higher , and polarization resistance, indicating that the XOZ plane had better corrosion resistance in comparison with the XOY plane. The difference of the corrosion properties between the XOY and XOZ planes were caused by the microstructural features exposed by the tested plane.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chlebus, E.; Kuźnicka, B.; Kurzynowski, T.; Dybała, B. Microstructure and mechanical behaviour of Ti―6Al―7Nb alloy produced by selective laser melting. Mater. Charact. 2011, 62, 488–495. [Google Scholar] [CrossRef]
- Tang, M.; Pistorius, P.C. Oxides, porosity and fatigue performance of AlSi10Mg parts produced by selective laser melting. Int. J. Fatigue 2017, 94, 192–201. [Google Scholar] [CrossRef]
- Shlyarov, V.; Zagulyaev, D.; Chen, X.; Gromov, V. Hardness of low-alloy steel obtained by cold metal transfer in the magnetic field. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 866, p. 012048. [Google Scholar]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.; Knight, P.; Vincent, A. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Otto, F.; Dlouhý, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef] [Green Version]
- Piglione, A.; Dovgyy, B.; Liu, C.; Gourlay, C.; Hooper, P.; Pham, M. Printability and microstructure of the CoCrFeMnNi high-entropy alloy fabricated by laser powder bed fusion. Mater. Lett. 2018, 224, 22–25. [Google Scholar] [CrossRef]
- Li, R.; Niu, P.; Yuan, T.; Cao, P.; Chen, C.; Zhou, K. Selective laser melting of an equiatomic CoCrFeMnNi high-entropy alloy: Processability, non-equilibrium microstructure and mechanical property. J. Alloys Compd. 2018, 746, 125–134. [Google Scholar] [CrossRef]
- Chen, P.; Li, S.; Zhou, Y.; Yan, M.; Attallah, M.M. Fabricating CoCrFeMnNi high entropy alloy via selective laser melting in-situ alloying. J. Mater. Sci. Technol. 2020, 43, 40–43. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Choe, J.; Lee, K. Selective laser melted equiatomic CoCrFeMnNi high-entropy alloy: Microstructure, anisotropic mechanical response, and multiple strengthening mechanism. J. Alloys Compd. 2019, 805, 680–691. [Google Scholar] [CrossRef]
- Chew, Y.; Bi, G.; Zhu, Z.; Ng, F.; Weng, F.; Liu, S.; Nai, S.; Lee, B. Microstructure and enhanced strength of laser aided additive manufactured CoCrFeNiMn high entropy alloy. Mater. Sci. Eng. A 2019, 744, 137–144. [Google Scholar] [CrossRef]
- Ren, J.; Mahajan, C.; Liu, L.; Follette, D.; Chen, W.; Mukherjee, S. Corrosion Behavior of Selectively Laser Melted CoCrFeMnNi High Entropy Alloy. Metals 2019, 9, 1029. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Zhang, H.; Du, X.; He, Y.; Luo, H.; Song, G.; Mao, L.; Zhou, T.; Wang, L. Corrosion resistance enhancement of CoCrFeMnNi high-entropy alloy fabricated by additive manufacturing. Corros. Sci. 2020, 177, 108954. [Google Scholar] [CrossRef]
- Wysocki, B.; Maj, P.; Krawczyńska, A.; Rożniatowski, K.; Zdunek, J.; Kurzydłowski, K.J.; Święszkowski, W. Microstructure and mechanical properties investigation of CP titanium processed by selective laser melting (SLM). J. Mater. Process. Technol. 2017, 241, 13–23. [Google Scholar]
- Olakanmi, E.; Cochrane, R.; Dalgarno, K. A review on selective laser sintering/melting (SLS/SLM) of aluminium alloy powders: Processing, microstructure, and properties. Prog. Mater. Sci. 2015, 74, 401–477. [Google Scholar] [CrossRef]
- Wang, Y.M.; Voisin, T.; McKeown, J.T.; Ye, J.; Calta, N.P.; Li, Z.; Zeng, Z.; Zhang, Y.; Chen, W.; Roehling, T.T.; et al. Additively manufactured hierarchical stainless steels with high strength and ductility. Nat. Mater. 2018, 17, 63–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prashanth, K.; Eckert, J. Formation of metastable cellular microstructures in selective laser melted alloys. J. Alloys Compd. 2017, 707, 27–34. [Google Scholar] [CrossRef]
- Liu, L.; Ding, Q.; Zhong, Y.; Zou, J.; Wu, J.; Chiu, Y.-L.; Li, J.; Zhang, Z.; Yu, Q.; Shen, Z. Dislocation network in additive manufactured steel breaks strength–ductility trade-off. Mater. Today 2018, 21, 354–361. [Google Scholar] [CrossRef] [Green Version]
- Kong, D.; Ni, X.; Dong, C.; Zhang, L.; Yao, J.; Man, C.; Wang, L.; Xiao, K.; Li, X. Anisotropic response in mechanical and corrosion properties of hastelloy X fabricated by selective laser melting. Constr. Build. Mater. 2019, 221, 720–729. [Google Scholar] [CrossRef]
- Sun, Z.; Tan, X.; Tor, S.B.; Chua, C.K. Simultaneously enhanced strength and ductility for 3D-printed stainless steel 316L by selective laser melting. NPG Asia Mater. 2018, 10, 127–136. [Google Scholar] [CrossRef]
- Sun, S.-H.; Hagihara, K.; Nakano, T. Effect of scanning strategy on texture formation in Ni-25 at.%Mo alloys fabricated by selective laser melting. Mater. Des. 2018, 140, 307–316. [Google Scholar] [CrossRef]
- Zhou, X.; Li, K.; Zhang, D.; Liu, X.; Ma, J.; Liu, W.; Shen, Z. Textures formed in a CoCrMo alloy by selective laser melting. J. Alloys Compd. 2015, 631, 153–164. [Google Scholar] [CrossRef]
- Chen, J.; Yao, Z.; Wang, X.; Lu, Y.; Wang, X.; Liu, Y.; Fan, X. Effect of C content on microstructure and tensile properties of as-cast CoCrFeMnNi high entropy alloy. Mater. Chem. Phys. 2018, 210, 136–145. [Google Scholar] [CrossRef]
- Hosford, W.F. Mechanical Behavior of Materials; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Zhu, Z.; Nguyen, Q.; Ng, F.; An, X.; Liao, X.; Liaw, P.; Nai, M.L.S.; Wei, J. Hierarchical microstructure and strengthening mechanisms of a CoCrFeNiMn high entropy alloy additively manufactured by selective laser melting. Scr. Mater. 2018, 154, 20–24. [Google Scholar] [CrossRef]
- Hou, J.; Chen, W.; Chen, Z.; Zhang, K.; Huang, A. Microstructure, tensile properties and mechanical anisotropy of selective laser melted 304L stainless steel. J. Mater. Sci. Technol. 2020, 48, 63–71. [Google Scholar] [CrossRef]
- Jeon, J.M.; Park, J.M.; Yu, J.-H.; Kim, J.G.; Seong, Y.; Park, S.H.; Kim, H.S. Effects of microstructure and internal defects on mechanical anisotropy and asymmetry of selective laser-melted 316L austenitic stainless steel. Mater. Sci. Eng. A 2019, 763, 138152. [Google Scholar] [CrossRef]
- Yu, H.; Yang, J.; Yin, J.; Wang, Z.; Zeng, X. Comparison on mechanical anisotropies of selective laser melted Ti-6Al-4V alloy and 304 stainless steel. Mater. Sci. Eng. A 2017, 695, 92–100. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, S.; Wei, Z.; Zhang, L.; Wei, P.; Lu, B.; Zhang, S.; Xiang, Y. Anisotropy of nickel-based superalloy K418 fabricated by selective laser melting. Prog. Nat. Sci. 2018, 28, 496–504. [Google Scholar] [CrossRef]
- Brito, C.; Vida, T.; Freitas, E.S.; Cheung, N.; Spinelli, J.E.; Garcia, A. Cellular/dendritic arrays and intermetallic phases affecting corrosion and mechanical resistances of an Al–Mg–Si alloy. J. Alloys Compd. 2016, 673, 220–230. [Google Scholar] [CrossRef]
- Pech-Canul, M.; Bartolo-Pérez, P.; Echeverría, M. The role of silicon alloying addition on the pitting corrosion resistance of an Al-12wt.%Si alloy. Electrochimica Acta 2014, 140, 258–265. [Google Scholar] [CrossRef]
- Melia, M.A.; Carroll, J.D.; Whetten, S.R.; Esmaeely, S.N.; Locke, J.; White, E.; Anderson, I.; Chandross, M.; Michael, J.R.; Argibay, N.; et al. Mechanical and Corrosion Properties of Additively Manufactured CoCrFeMnNi High Entropy Alloy. Addit. Manuf. 2019, 29, 100833. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Gu, X.; Dai, N.; Qin, P.; Zhang, L. Distinction of corrosion resistance of selective laser melted Al-12Si alloy on different planes. J. Alloys Compd. 2018, 747, 648–658. [Google Scholar] [CrossRef]




| Elements (wt %) | Co | Cr | Fe | Mn | Ni |
|---|---|---|---|---|---|
| Powder | 20.1 ± 0.25 | 19.5 ± 0.45 | 20.7 ± 0.5 | 19.9 ± 0.35 | 20.8 ± 0.35 |
| As-built | 20.6 ± 0.35 | 18.7 ± 0.8 | 21.0 ± 0.6 | 18.3 ± 0.25 | 21.3 ± 0.35 |
| Intensity Ratio | Original Powder | XOY Plane | XOZ Plane |
|---|---|---|---|
| 0.37 | 1.03 | 0.15 | |
| 0.27 | 1.98 | 0.11 |
| Processing | σ0.2 (MPa) | UTS (MPa) | εf (%) | Ref. |
|---|---|---|---|---|
| SLM produced, XOY plane | 582.9 ± 10.8 | 679.8 ± 12.9 | 23.8 ± 1.4 | This work |
| SLM produced, XOZ plane | 503.8 ± 10.6 | 635.9 ± 13.4 | 31.8 ± 1.5 | This work |
| Cast | 275 | 475 | 51 | [24] |
| Corrosion Parameter | XOY Plane | XOZ Plane | As-Cast |
|---|---|---|---|
| −86.83 | −38.04 | −457 | |
| 0.19 | 0.1 | 1.123 | |
| 70.18 | 148.56 | - | |
| 157.01 | 186.60 | - | |
| Polarization Resistance, | 65,903 | 71,466 | 12,700 |
| Solution Resistance, | 5.982 | 5.988 | 5.2 |
| Double Layer Capacitance, | 35.29 | 32.47 | 19.37 |
| This work | This work | [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Sun, M.; Li, B.; Zhang, L.; Lu, B. Anisotropic Response of CoCrFeMnNi High-Entropy Alloy Fabricated by Selective Laser Melting. Materials 2020, 13, 5687. https://doi.org/10.3390/ma13245687
Wang B, Sun M, Li B, Zhang L, Lu B. Anisotropic Response of CoCrFeMnNi High-Entropy Alloy Fabricated by Selective Laser Melting. Materials. 2020; 13(24):5687. https://doi.org/10.3390/ma13245687
Chicago/Turabian StyleWang, Bowen, Miao Sun, Bobo Li, Lijuan Zhang, and Bingheng Lu. 2020. "Anisotropic Response of CoCrFeMnNi High-Entropy Alloy Fabricated by Selective Laser Melting" Materials 13, no. 24: 5687. https://doi.org/10.3390/ma13245687




