The Impact of Graphene and Diatomite Admixtures on the Performance and Properties of High-Performance Magnesium Oxychloride Cement Composites
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- the identified crystalline phases in both composites were well stable and durable phase 5 (Mg3(OH)5Cl·4H2O) and unreacted MgO residue;
- the highly compacted structure of graphene-doped composite was identified, where possible defects were inter-grown by phase 5 needle like crystals;
- diatomite and graphene were well distributed and fixed in MOC matrix;
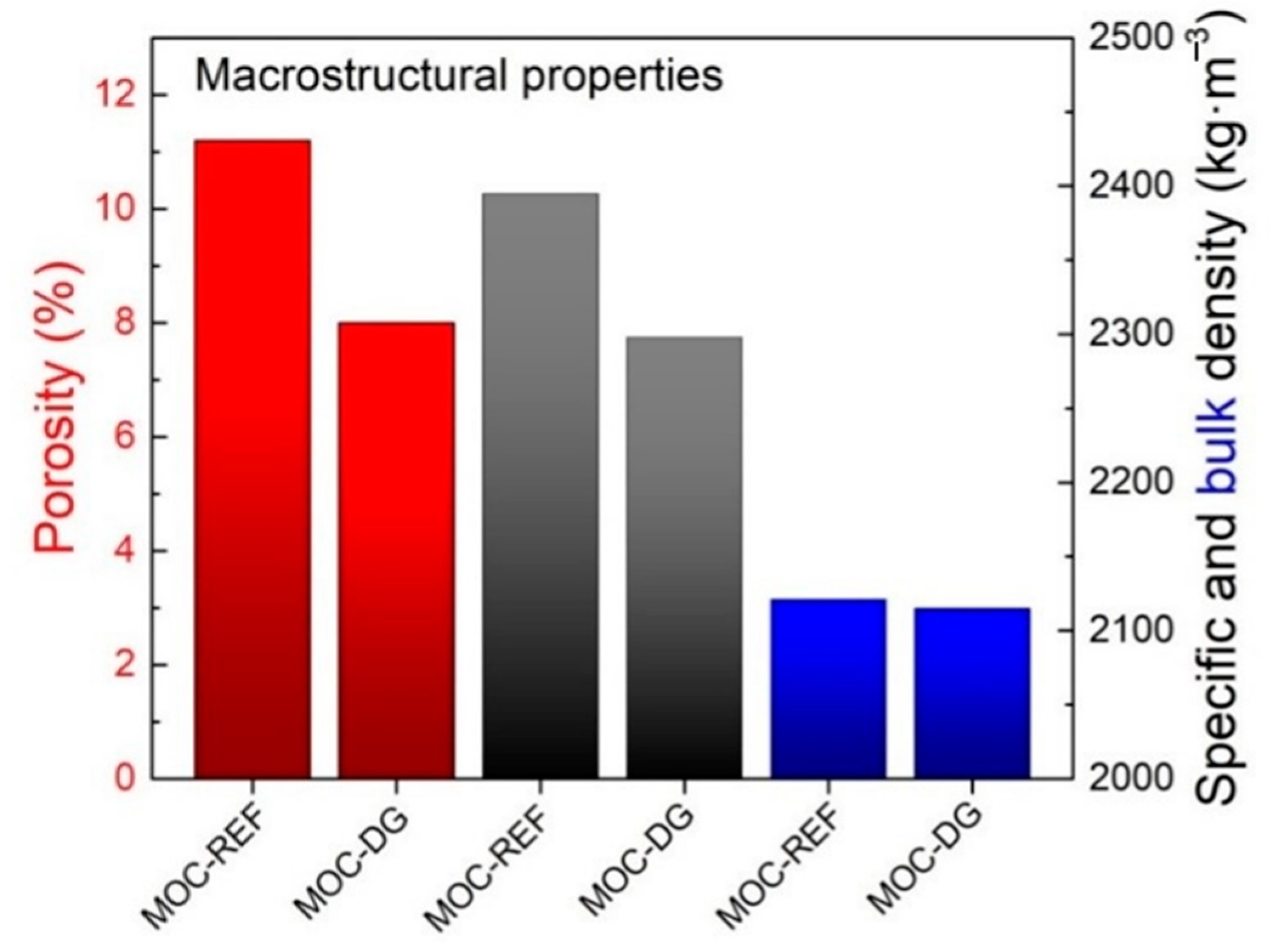
- the porosity, bulk density, and specific density were reduced by the use of graphene nanoplatelets and diatomite;
- the compressive strength of MOC-DG composite was greatly improved due to the high hardness and mechanical strength of graphene, lowered porosity, and activation of two-dimensional graphene-based reinforcement;
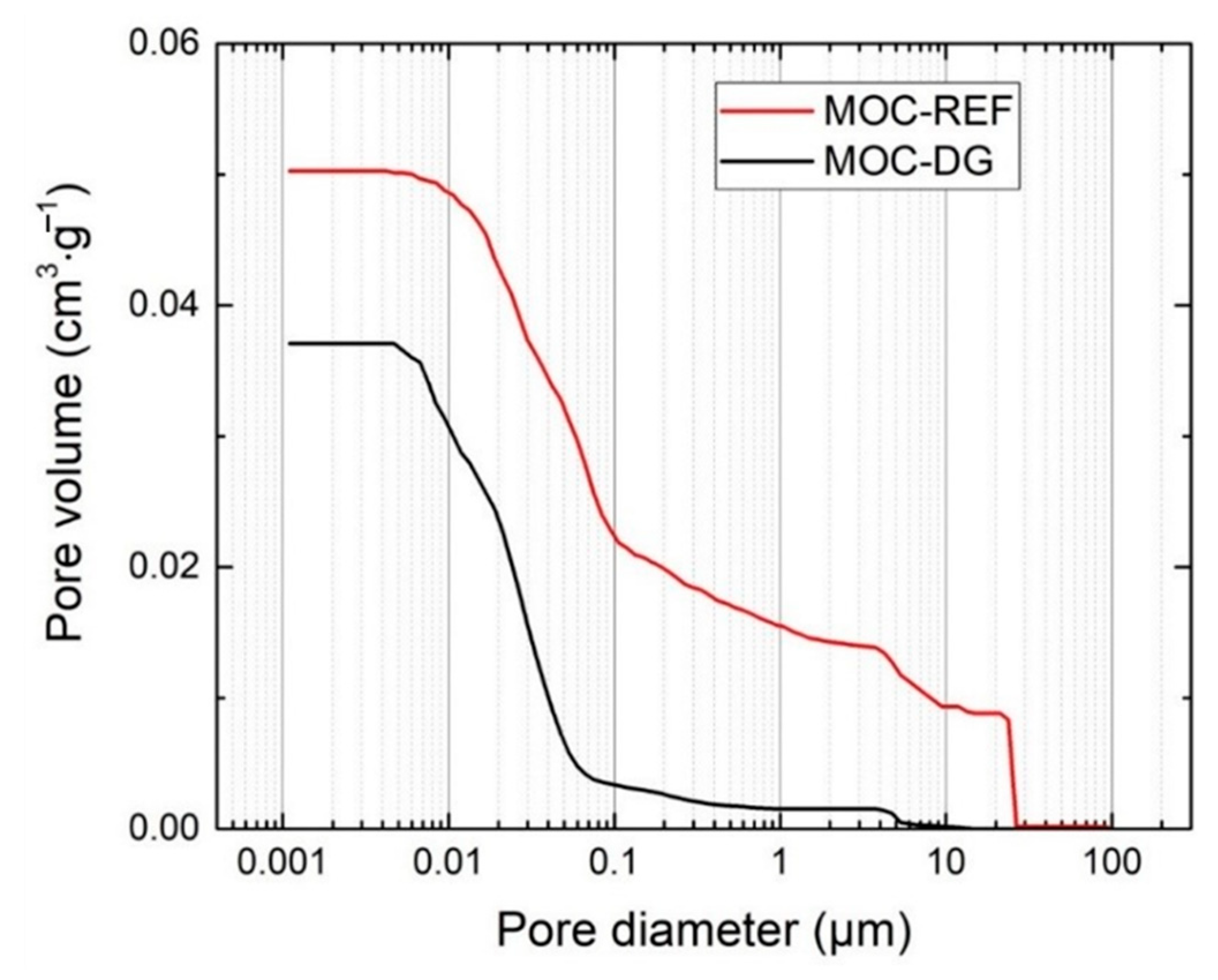
- as the average pore size was significantly reduced by the mutual action of diatomite and graphene in composite mixture, the water transport and accumulation was highly limited in MOC-DG materials which is very promising finding for its improved durability in the sense of moisture damage;
- the use of diatomite slightly reduced the thermal conductivity of the newly developed composite, but its heat transport properties remained high.
Author Contributions
Funding
Conflicts of Interest
References
- Friedlingstein, P.; Houghton, R.A.; Marland, G.; Hackler, J.; Boden, T.A.; Conway, T.J.; Canadell, J.G.; Raupach, M.R.; Ciais, P.; Le Quéré, C. Update on CO2 emissions. Nat. Geosci. 2010, 3, 811–812. [Google Scholar] [CrossRef]
- Allwood, J.M.; Cullen, J.M.; Milford, R.L. Options for Achieving a 50% Cut in Industrial Carbon Emissions by 2050. Environ. Sci. Technol. 2010, 44, 1888–1894. [Google Scholar] [CrossRef] [PubMed]
- Arıoğlu Akan, M.Ö.; Dhavale, D.G.; Sarkis, J. Greenhouse gas emissions in the construction industry: An analysis and evaluation of a concrete supply chain. J. Clean. Prod. 2017, 167, 1195–1207. [Google Scholar] [CrossRef]
- Flower, D.J.M.; Sanjayan, J.G. Green house gas emissions due to concrete manufacture. Int. J. Life Cycle Assess. 2007, 12, 282. [Google Scholar] [CrossRef]
- Ay, N.; Ünal, M. The use of waste ceramic tile in cement production. Cem. Concr. Res. 2000, 30, 497–499. [Google Scholar] [CrossRef]
- Jang, H.-S.; So, S.-Y. The properties of cement-based mortar using different particle size of grinding waste insulator powder. J. Build. Eng. 2015, 3, 48–57. [Google Scholar] [CrossRef]
- Segre, N.; Joekes, I. Use of tire rubber particles as addition to cement paste. Cem. Concr. Res. 2000, 30, 1421–1425. [Google Scholar] [CrossRef]
- Nochaiya, T.; Wongkeo, W.; Chaipanich, A. Utilization of fly ash with silica fume and properties of Portland cement–fly ash–silica fume concrete. Fuel 2010, 89, 768–774. [Google Scholar] [CrossRef]
- Sorel, S. On a new magnesium cement. CR Acad. Sci. 1867, 65, 102–104. [Google Scholar]
- Kiyanets, A.V. Prospects for application of magnesium binder in construction. IOP Conf. Ser. Mater. Sci. Eng. 2018, 451, 012074. [Google Scholar] [CrossRef]
- Bilinski, H.; Matkovic, B.; Mazuranic, C.; Zunic, T. The Formation of Magnesium Oxychloride Phases in the Systems MgO-MgCl2-H2O and NaOH-MgCl2-H2O. J. Am. Ceram. Soc. 1984, 67, 266–269. [Google Scholar] [CrossRef]
- Lojka, M.; Jankovský, O.; Jiříčková, A.; Lauermannová, A.-M.; Antončík, F.; Sedmidubský, D.; Pavlík, Z. Thermal Stability and Kinetics of Formation of Magnesium Oxychloride Phase 3Mg(OH)2·MgCl2·8H2O. Materials 2020, 13, 767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiříčková, A.; Lojka, M.; Lauermannová, A.-M.; Antončík, F.; Sedmidubský, D.; Pavlíková, M.; Záleská, M.; Pavlík, Z.; Jankovský, O. Synthesis, Structure, and Thermal Stability of Magnesium Oxychloride 5Mg(OH) 2MgCl2 8H2O. Appl. Sci. 2020, 10, 1683. [Google Scholar] [CrossRef] [Green Version]
- Dinnebier, R.E.; Freyer, D.; Bette, S.; Oestreich, M. 9Mg(OH)2·MgCl2·4H2O, a High Temperature Phase of the Magnesia Binder System. Inorg. Chem. 2010, 49, 9770–9776. [Google Scholar] [CrossRef] [PubMed]
- Dinnebier, R.E.; Oestreich, M.; Bette, S.; Freyer, D. 2Mg(OH)2·MgCl2·2H2O and 2Mg(OH)2·MgCl2·4H2O, Two High Temperature Phases of the Magnesia Cement System. Z. Anorg. Allg. Chem. 2012, 638, 628–633. [Google Scholar] [CrossRef]
- Matkovic, B.; Young, J. Microstructure of magnesium oxychloride cements. Nat. Phys. Sci. 1973, 246, 79. [Google Scholar] [CrossRef]
- Misra, A.; Mathur, R. Magnesium oxychloride cement concrete. Bull. Mater. Sci. 2007, 30, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Montle, J.; Mayhan, K. The role of magnesium oxychloride as a fire-resistive material. Fire Technol. 1974, 10, 201–210. [Google Scholar] [CrossRef]
- Plekhanova, T.; Keriene, J.; Gailius, A.; Yakovlev, G. Structural, physical and mechanical properties of modified wood–magnesia composite. Constr. Build. Mater. 2007, 21, 1833–1838. [Google Scholar] [CrossRef]
- Qiao, H.X.; Zhu, B.R.; Shi, Y.Y.; Dong, J.M.; Elizabeth Wanjiru, M. Strength development and micro-mechanism of magnesium oxychloride cement concrete. Mater. Res. Innov. 2015, 19, S1–S185, S181–S190. [Google Scholar] [CrossRef]
- Sorre, C.A.; Armstrong, C.R. Reactions and Equi ibria in Magnesium Oxych oride Cements. J. Am. Ceram. Soc. 1976, 59, 51–54. [Google Scholar] [CrossRef]
- Thompson, H.C. Fireproof Product Using Magnesium Oxychloride Cement. U.S. Patent 3,963,849, 15 June 1976. [Google Scholar]
- Sofer, Z.; Šimek, P.; Jankovský, O.; Sedmidubský, D.; Beran, P.; Pumera, M. Neutron diffraction as a precise and reliable method for obtaining structural properties of bulk quantities of graphene. Nanoscale 2014, 6, 13082–13089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouša, D.; Luxa, J.; Mazanek, V.; Jankovský, O.; Sedmidubský, D.; Klimova, K.; Pumera, M.; Sofer, Z. Toward graphene chloride: Chlorination of graphene and graphene oxide. RSC Adv. 2016, 6, 66884–66892. [Google Scholar] [CrossRef] [Green Version]
- Jankovsky, O.; Novacek, M.; Luxa, J.; Sedmidubsky, D.; Fila, V.; Pumera, M.; Sofer, Z. A New Member of the Graphene Family: Graphene Acid. Chem. Eur. J. 2016, 22, 17416–17424. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Morozov, S.V.; Novoselov, K.S.; Katsnelson, M.I.; Schedin, F.; Elias, D.C.; Jaszczak, J.A.; Geim, A.K. Giant Intrinsic Carrier Mobilities in Graphene and Its Bilayer. Phys. Rev. Lett. 2008, 100, 016602. [Google Scholar] [CrossRef] [Green Version]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhi, L.; Müllen, K. Transparent, Conductive Graphene Electrodes for Dye-Sensitized Solar Cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef]
- Blake, P.; Brimicombe, P.D.; Nair, R.R.; Booth, T.J.; Jiang, D.; Schedin, F.; Ponomarenko, L.A.; Morozov, S.V.; Gleeson, H.F.; Hill, E.W.; et al. Graphene-Based Liquid Crystal Device. Nano Lett. 2008, 8, 1704–1708. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Zhang, D.; Gong, X.-G. Thermal conductivity of graphene nanoribbons. Appl. Phys. Lett. 2009, 95, 163103. [Google Scholar] [CrossRef] [Green Version]
- Calizo, I.; Balandin, A.A.; Bao, W.; Miao, F.; Lau, C.N. Temperature Dependence of the Raman Spectra of Graphene and Graphene Multilayers. Nano Lett. 2007, 7, 2645–2649. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Shi, L.; Yao, Z.; Li, D.; Majumdar, A. Thermal Conductance and Thermopower of an Individual Single-Wall Carbon Nanotube. Nano Lett. 2005, 5, 1842–1846. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385. [Google Scholar] [CrossRef]
- Jiang, J.-W.; Wang, J.-S.; Li, B. Young’s modulus of graphene: A molecular dynamics study. Phys. Rev. B 2009, 80, 113405. [Google Scholar] [CrossRef] [Green Version]
- Frank, I.W.; Tanenbaum, D.M.; van der Zande, A.M.; McEuen, P.L. Mechanical properties of suspended graphene sheets. J. Vac. Sci. Technol. B 2007, 25, 2558–2561. [Google Scholar] [CrossRef] [Green Version]
- Jankovský, O.; Lojka, M.; Lauermannová, A.-M.; Antončík, F.; Pavlíková, M.; Záleská, M.; Pavlík, Z.; Pivák, A.; Sedmidubský, D. Towards novel building materials: High-strength nanocomposites based on graphene, graphite oxide and magnesium oxychloride. Appl. Mater. Today 2020, 20, 100766. [Google Scholar] [CrossRef]
- Dimov, D.; Amit, I.; Gorrie, O.; Barnes, M.D.; Townsend, N.J.; Neves, A.I.S.; Withers, F.; Russo, S.; Craciun, M.F. Ultrahigh Performance Nanoengineered Graphene–Concrete Composites for Multifunctional Applications. Adv. Funct. Mater. 2018, 28, 1705183. [Google Scholar] [CrossRef]
- Shamsaei, E.; de Souza, F.B.; Yao, X.; Benhelal, E.; Akbari, A.; Duan, W. Graphene-based nanosheets for stronger and more durable concrete: A review. Constr. Build. Mater. 2018, 183, 642–660. [Google Scholar] [CrossRef]
- Brichni, A.; Hammi, H.; Aggoun, S.; Mnif, A. Optimisation of magnesium oxychloride cement properties by silica glass. Adv. Cem. Res. 2016, 28, 654–663. [Google Scholar] [CrossRef]
- He, P.; Poon, C.S.; Tsang, D.C. Comparison of glass powder and pulverized fuel ash for improving the water resistance of magnesium oxychloride cement. Cem. Concr. Compos. 2018, 86, 98–109. [Google Scholar] [CrossRef]
- Li, C.D.; Yu, H.F. Study on Recycle of Sawdust Sorel’s cement concrete waste. In Advanced Materials Research; Trans Tech Publications Ltd.: Baech, Switzerland, 2010; pp. 382–385. [Google Scholar]
- Chau, C.; Chan, J.; Li, Z. Influences of fly ash on magnesium oxychloride mortar. Cem. Concr. Compos. 2009, 31, 250–254. [Google Scholar] [CrossRef]
- Paschen, S. Kieselgur—Mining, Processing and Use; Industriebetriebe Heinrich Meyer—Werke Breloh G.m.b.H. und Co. K.G; Vereinigte Deutsche Kieselgurwerke: Munster, Germany, 1986; pp. 158–162. [Google Scholar]
- Fuya, W.; Huifen, Z.; Huang, F.; Guoxi, C.; Deqiang, W.; Hongping, H. A mineralogical study of diatomite in Leizhou Peninsula. Chin. J. Geochem. 1995, 14, 140–151. [Google Scholar] [CrossRef]
- Duraia, E.-S.M.; Burkitbaev, M.; Mohamedbakr, H.; Mansurov, Z.; Tokmolden, S.; Beall, G.W. Growth of carbon nanotubes on diatomite. Vacuum 2009, 84, 464–468. [Google Scholar] [CrossRef]
- Irani, M.; Mousavian, M.; Keshtkar, A. Adsorption of Lead from Aqueous Solutions Using Natural Diatomite. In Proceedings of the 7th International Chemical Engineering Congress & Exhibition, Kish Island, Hormozgan, Iran, 21–24 November 2011. [Google Scholar]
- Beheshti, H.; Irani, M. Removal of lead(II) ions from aqueous solutions using diatomite nanoparticles. Desalin. Water Treat. 2016, 57, 18799–18805. [Google Scholar] [CrossRef]
- Aytaş, Ş.; Akyil, S.; Aslani, M.A.A.; Aytekin, U. Removal of uranium from aqueous solutions by diatomite (Kieselguhr). J. Radioanal. Nucl. Chem. 1999, 240, 973–976. [Google Scholar] [CrossRef]
- Yılmaz, B.; Ediz, N. The use of raw and calcined diatomite in cement production. Cem. Concr. Compos. 2008, 30, 202–211. [Google Scholar] [CrossRef]
- Degirmenci, N.; Yilmaz, A. Use of diatomite as partial replacement for Portland cement in cement mortars. Constr. Build. Mater. 2009, 23, 284–288. [Google Scholar] [CrossRef]
- Ergün, A. Effects of the usage of diatomite and waste marble powder as partial replacement of cement on the mechanical properties of concrete. Constr. Build. Mater. 2011, 25, 806–812. [Google Scholar] [CrossRef]
- Kastis, D.; Kakali, G.; Tsivilis, S.; Stamatakis, M.G. Properties and hydration of blended cements with calcareous diatomite. Cem. Concr. Res. 2006, 36, 1821–1826. [Google Scholar] [CrossRef]
- EN 933-1, Test for Geometrical Properties of Aggregates—Part 1: Determination of Particle Size Distribution—Sieving Method; European Committee for Standardization: Brussels, Belgium, 2012.
- EN 1015-10, Methods of Test for Mortar for Masonry—Part 10: Determination of Dry Bulk Density of Hardened Mortar; European Committee for Standardization: Brussels, Belgium, 1999.
- Pavlíková, M.; Zemanová, L.; Pokorný, J.; Záleská, M.; Jankovský, O.; Lojka, M.; Sedmidubský, D.; Pavlík, Z. Valorization of wood chips ash as an eco-friendly mineral admixture in mortar mix design. Waste Manag. 2018, 80, 89–100. [Google Scholar] [CrossRef] [PubMed]
- EN 1015-11, Methods of Test for Mortar for Masonry—Part 10: Determination of Flexural and Compressive Strength of Hardened Mortar; European Committee for Standardization: Brussels, Belgium, 1999.
- He, P.; Poon, C.S.; Richardson, I.G.; Tsang, D.C.W. The mechanism of supplementary cementitious materials enhancing the water resistance of magnesium oxychloride cement (MOC): A comparison between pulverized fuel ash and incinerated sewage sludge ash. Cem. Concr. Compos. 2020, 109, 103562. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Bi, W.; Cheeseman, C. Effect of tartaric acid and phosphoric acid on the water resistance of magnesium oxychloride (MOC) cement. Constr. Build. Mater. 2019, 213, 528–536. [Google Scholar] [CrossRef]
- EN 13755, Natural Stone Test Methods: Determination of Water Absorption at Atmospheric Pressure; European Committee for Standardization: Brussels, Belgium, 2008.
- EN 1015–18, Methods of Test for Mortar for Masonry—Part 18: Determination of Water Absorption Coefficient Due to Capillary Action of Hardened Mortar; European Committee for Standardization: Brussels, Belgium, 2002.
- Feng, C.; Guimarães, A.S.; Ramos, N.; Sun, L.; Gawin, D.; Konca, P.; Hall, C.; Zhao, J.; Hirsch, H.; Grunewald, J.; et al. Hygric properties of porous building materials (VI): A round robin campaign. Build. Environ. 2020, 185, 107242. [Google Scholar] [CrossRef]
- Gustafsson, S.E. Transient plane source techniques for thermal conductivity and thermal diffusivity measurements of solid materials. Rev. Sci. Instrum. 1991, 62, 797–804. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Konishi, H.; Fehrenbacher, A.; Ma, C.; Xu, J.-Q.; Choi, H.; Xu, H.-F.; Pfefferkorn, F.E.; Li, X.-C. Novel nanoprocessing route for bulk graphene nanoplatelets reinforced metal matrix nanocomposites. Scr. Mater. 2012, 67, 29–32. [Google Scholar] [CrossRef]
- Güler, Ö.; Bağcı, N. A short review on mechanical properties of graphene reinforced metal matrix composites. J. Mater. Res. Technol. 2020, 9, 6808–6833. [Google Scholar] [CrossRef]
- Chu, H.; Wang, Z.; Zhang, Y.; Wang, F.; Ju, S.; Wang, L.; Wang, D.J.M. Using Graphene Sulfonate Nanosheets to Improve the Properties of Siliceous Sacrificial Materials: An Experimental and Molecular Dynamics Study. Materials 2020, 13, 4824. [Google Scholar] [CrossRef]
- Veiga, M.R.; Magalhães, A.; Bokan-Bosilikov, V. Capillarity tests on historic mortar samples extracted from site. Methodology and compared results. In Proceedings of the 13th International Masonry Conference, Amsterdam, The Netherlands, 4–7 July 2004. [Google Scholar]
- Vyšvařil, M.; Pavlíková, M.; Záleská, M.; Pivák, A.; Žižlavský, T.; Rovnaníková, P.; Bayer, P.; Pavlík, Z. Non-hydrophobized perlite renders for repair and thermal insulation purposes: Influence of different binders on their properties and durability. Constr. Build. Mater. 2020, 263, 120617. [Google Scholar] [CrossRef]
- EN 998-1, Specification for Mortar for Masonry—Part 1: Rendering and Plastering Mortar; European Committee for Standardization: Brussels, Belgium, 2010.
- Lanzón, M.; García-Ruiz, P.A. Evaluation of capillary water absorption in rendering mortars made with powdered waterproofing additives. Constr. Build. Mater. 2009, 23, 3287–3291. [Google Scholar] [CrossRef]
- Xu, B.; Ma, H.; Hu, C.; Li, Y. Influence of cenospheres on properties of magnesium oxychloride cement-based composites. Mater. Struct. 2016, 49, 1319–1326. [Google Scholar] [CrossRef]





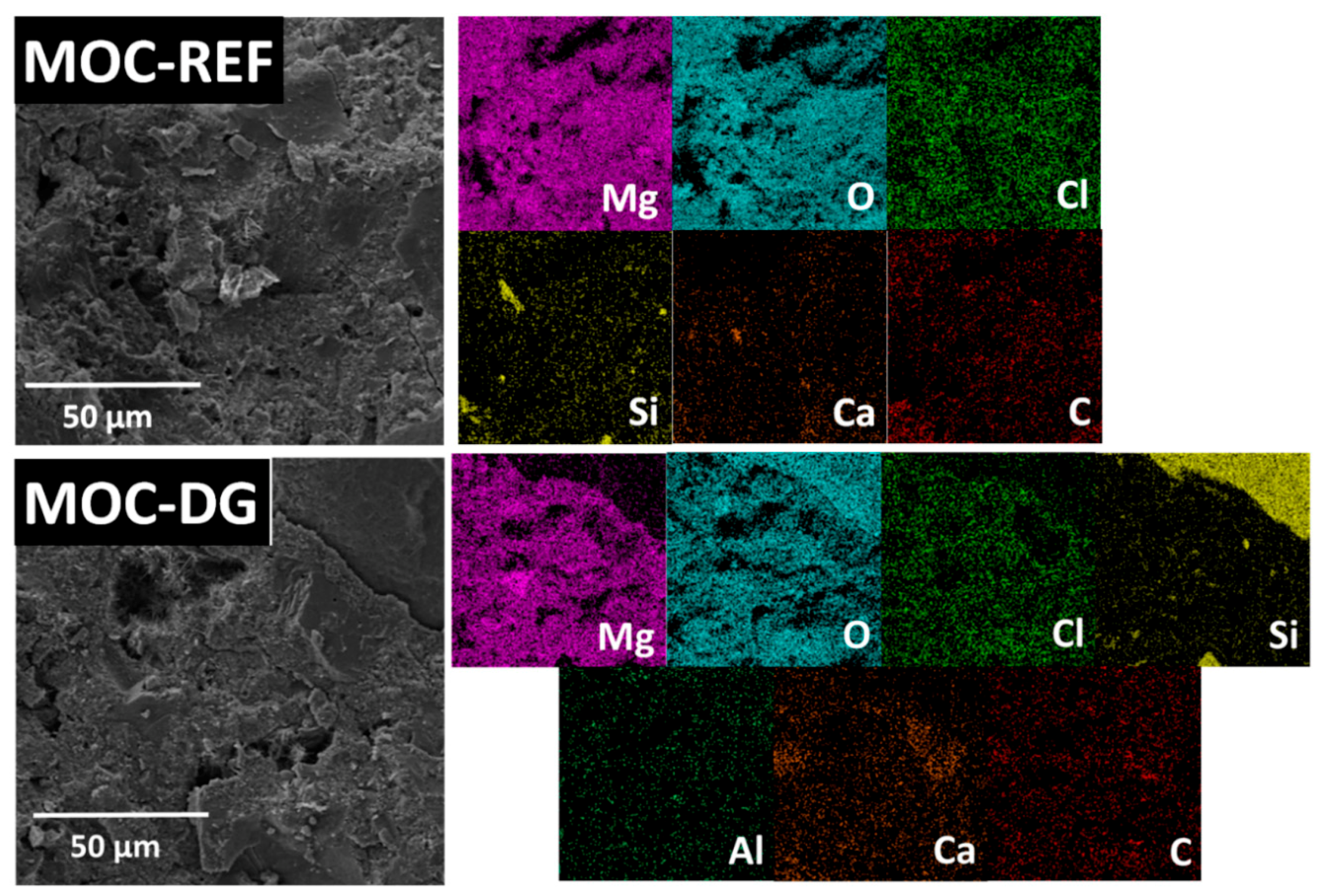


| Composite | MgO | MgCl2·6H2O | Water | Silica Sand | Diatomite | Graphene |
|---|---|---|---|---|---|---|
| MOC-REF | 584.4 | 258.9 | 215 | 3 × 497.7 | - | - |
| MOC-DG | 584.4 | 258.9 | 215 | 3 × 382.8 | 26.6 | 4.2 |
| Element | MOC-REF | MOC-DG |
|---|---|---|
| Mg | 32.7 | 21.8 |
| O | 46.1 | 45.7 |
| C | 11.3 | 12.0 |
| Cl | 8.0 | 6.0 |
| Ca | 1.2 | 5.0 |
| Si | 0.7 | 9.2 |
| Al | 0.0 | 0.3 |
| Material | ρs (kg·m−3) | ρb (kg·m−3) | P (%) | PHg (%) | ff (MPa) | fc (MPa) | Ed (GPa) |
|---|---|---|---|---|---|---|---|
| MOC-REF | 2395 ± 29 | 2121 ± 30 | 11.2 ± 0.2 | 10.8 | 23.1± 0.3 | 67.3 ± 0.9 | 33.8 ± 0.8 |
| MOC-DG | 2298 ± 28 | 2115 ± 30 | 8.0 ± 0.2 | 8.11 | 25.6 ± 0.3 | 87.7 ± 1.2 | 37.5 ± 0.9 |
| Parameter | MOC-REF | MOC-DG |
|---|---|---|
| Wa (%) | 4.28 | 1.94 |
| Wa24 (%) | 2.85 | 0.94 |
| Aw (kg·m−2·s−1/2) | 0.0061 | 0.0023 |
| κapp × 10−11 (m2·s−1) | 4.90 | 3.47 |
| λd (W·m−1·K−1) | 3.270 | 3.151 |
| ad × 10−6 (m2·s−1) | 2.112 | 2.154 |
| Cvd × 106 (J·m−3·K−1) | 1.548 | 1.463 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauermannová, A.-M.; Antončík, F.; Lojka, M.; Jankovský, O.; Pavlíková, M.; Pivák, A.; Záleská, M.; Pavlík, Z. The Impact of Graphene and Diatomite Admixtures on the Performance and Properties of High-Performance Magnesium Oxychloride Cement Composites. Materials 2020, 13, 5708. https://doi.org/10.3390/ma13245708
Lauermannová A-M, Antončík F, Lojka M, Jankovský O, Pavlíková M, Pivák A, Záleská M, Pavlík Z. The Impact of Graphene and Diatomite Admixtures on the Performance and Properties of High-Performance Magnesium Oxychloride Cement Composites. Materials. 2020; 13(24):5708. https://doi.org/10.3390/ma13245708
Chicago/Turabian StyleLauermannová, Anna-Marie, Filip Antončík, Michal Lojka, Ondřej Jankovský, Milena Pavlíková, Adam Pivák, Martina Záleská, and Zbyšek Pavlík. 2020. "The Impact of Graphene and Diatomite Admixtures on the Performance and Properties of High-Performance Magnesium Oxychloride Cement Composites" Materials 13, no. 24: 5708. https://doi.org/10.3390/ma13245708
APA StyleLauermannová, A.-M., Antončík, F., Lojka, M., Jankovský, O., Pavlíková, M., Pivák, A., Záleská, M., & Pavlík, Z. (2020). The Impact of Graphene and Diatomite Admixtures on the Performance and Properties of High-Performance Magnesium Oxychloride Cement Composites. Materials, 13(24), 5708. https://doi.org/10.3390/ma13245708








