2D and 3D Bulk Materials for Environmental Remediation: Air Filtration and Oil/Water Separation
Abstract
:1. Introduction
2. Air Filtration (Part 1)
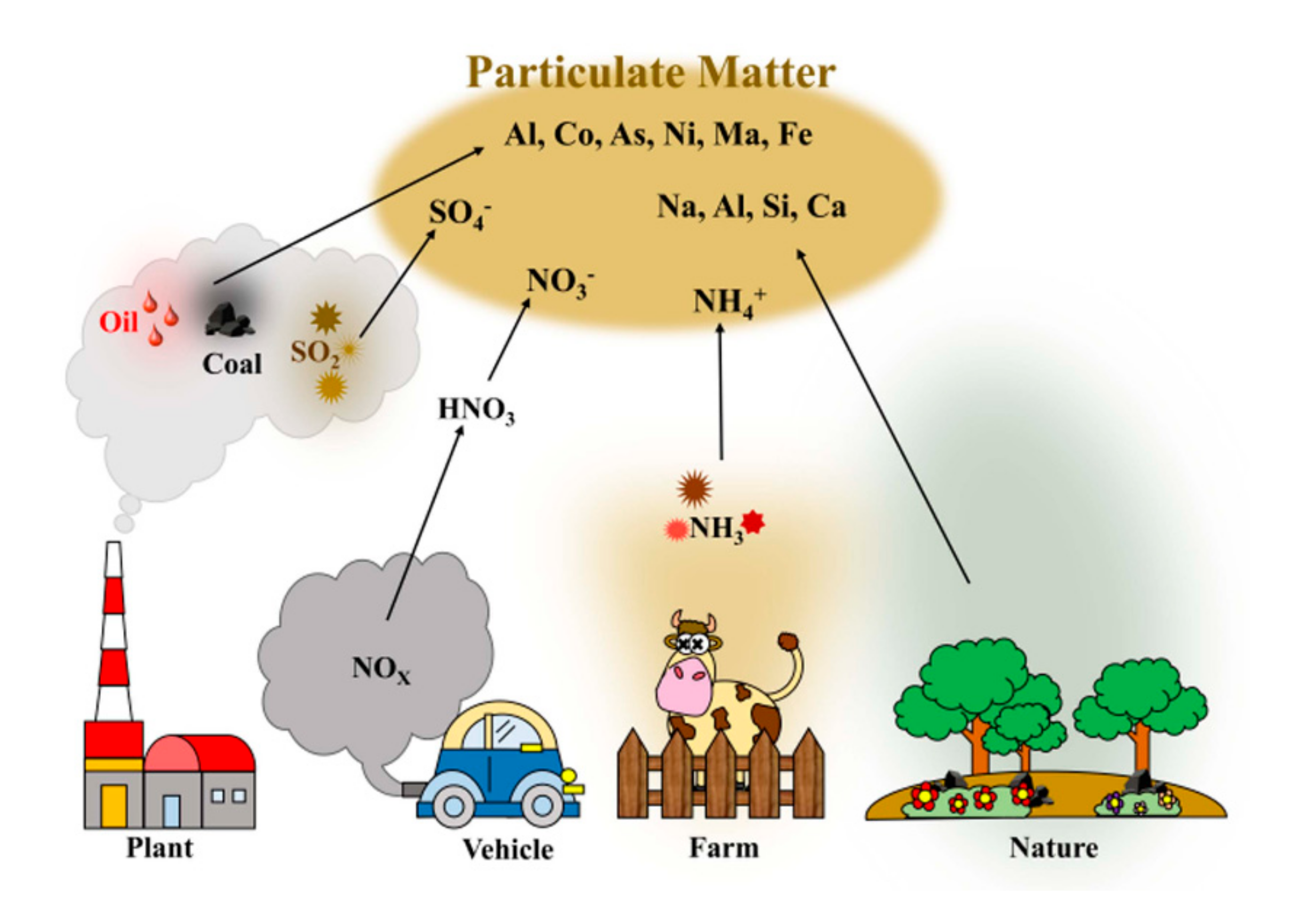
2.1. Basic Concept of PM
2.2. Classification of Air Filters
2.3. Particulate Capturing Mechanisms
2.4. Characterization of PM Filtration
2.4.1. Removal Efficiency and Pressure Drop
2.4.2. Quality Factor (QF)
2.5. Air Filter Materials
2.5.1. Synthetic Polymer-Based Materials
2.5.2. Natural Polymer-Based Materials
Cellulose
Chitosan
2.5.3. Carbon-Based Materials
2.5.4. Inorganic-Based Materials
2.5.5. Organic/Inorganic Hybrid-Based Materials
3. Oil/Water Separation (Part 2)
3.1. Basic Concept of Oil/Water Separation
3.1.1. Oil Penetration or Absorption
3.1.2. Water Penetration or Absorption
3.2. Mechanisms of Oil/Water Separation
3.3. Materials for Oil/Water Separation
3.3.1. Filtration-Based Materials
Mesh-Based Materials
Fabric-Based Materials
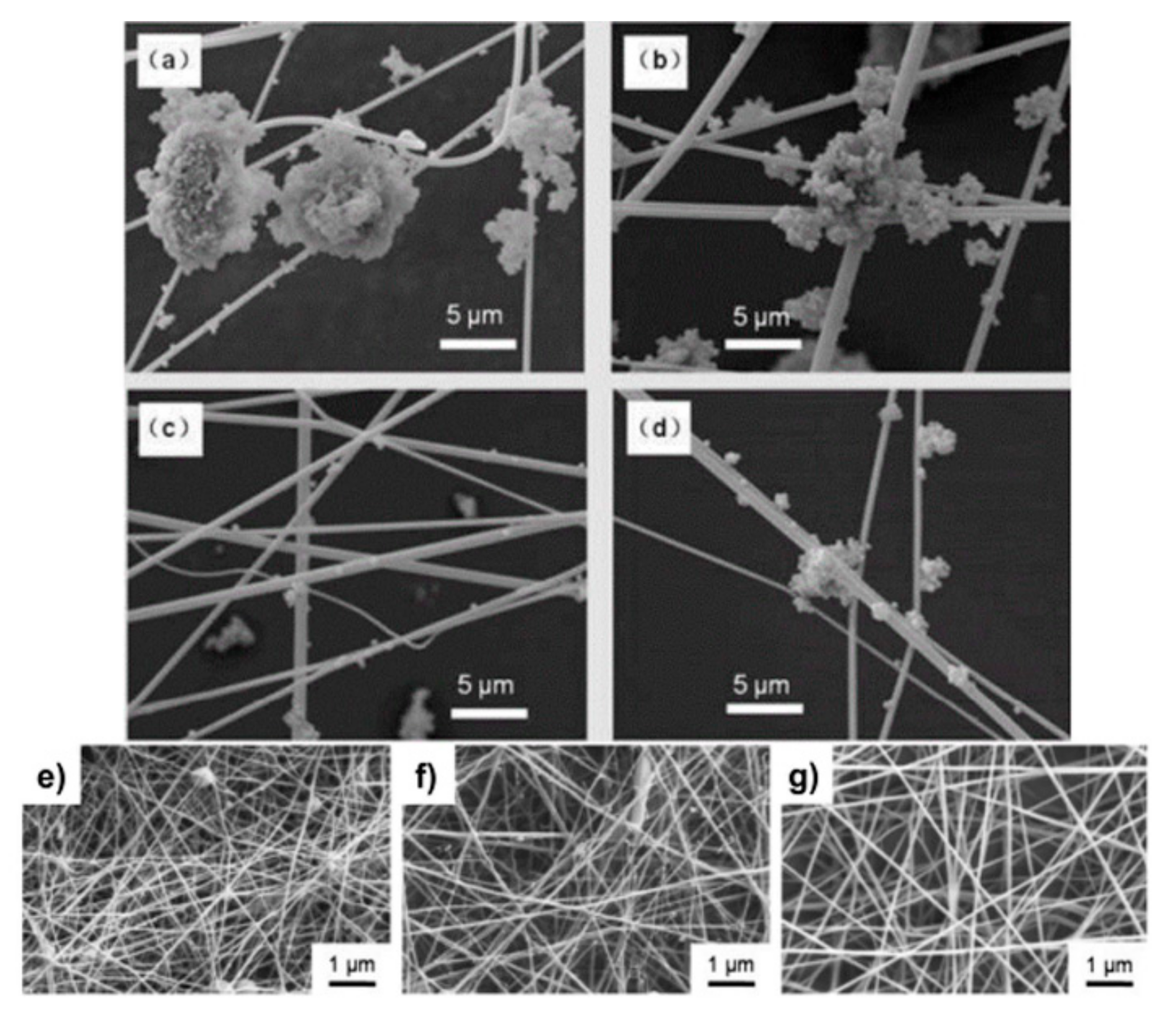
Fiber-Based Materials
3.3.2. Absorption-Based Materials
General Absorbents
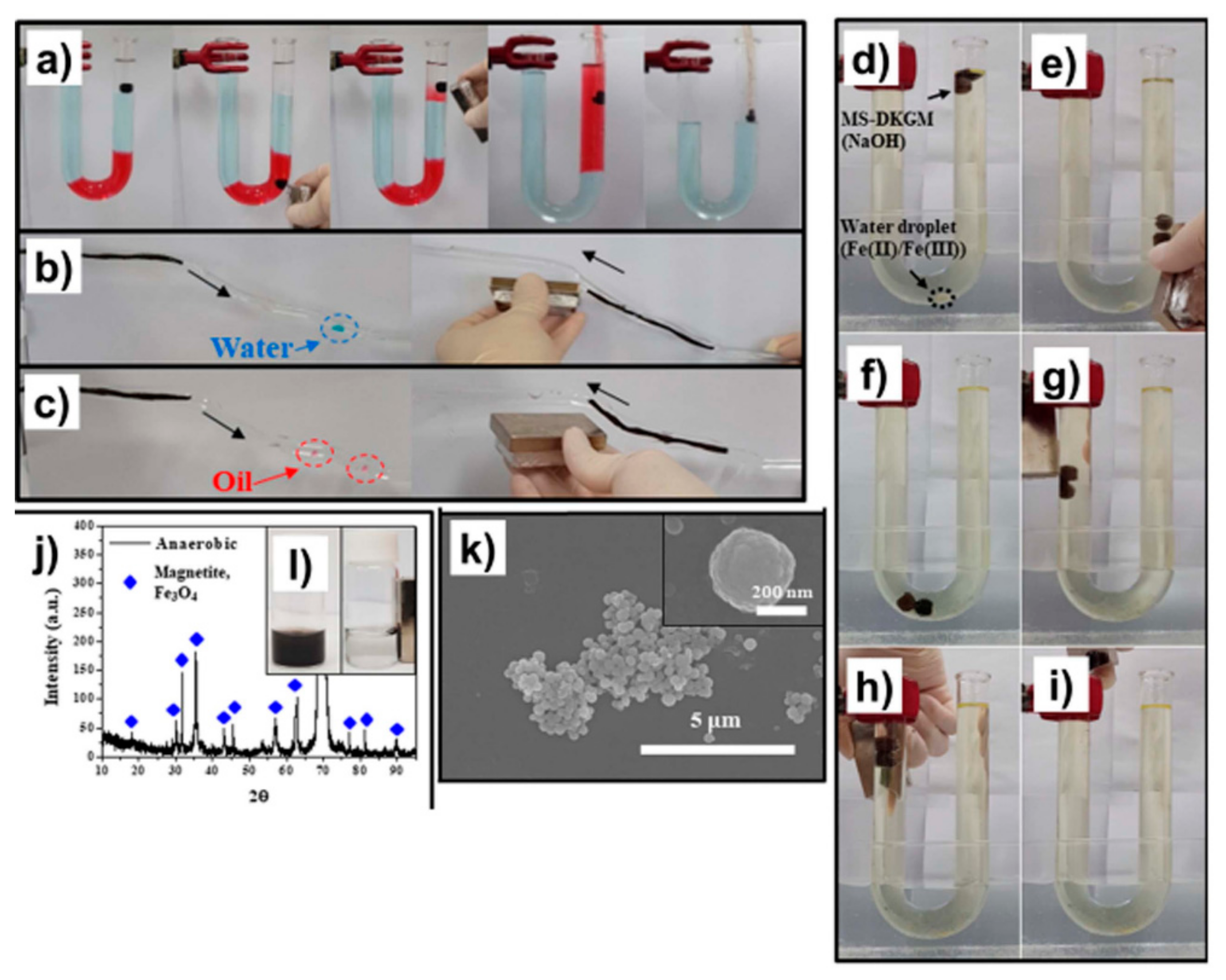
Multifunctional Absorbents
4. Summary and Perspective
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nel, A. Air Pollution-Related Illness: Effects of Particles. Science 2005, 308, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.W.; Pope, C.A.; Dockery, D.W.; Wang, Y.; Ezzati, M.; Dominici, F. The effect of air pollution control on life expectancy in the United States: An analysis of 545 US counties for the period 2000 to 2007. Epidemiology 2013, 24, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, R.W.; Mills, I.C.; Walton, H.A.; Anderson, H.R. Fine particle components and health—A systematic review and meta-analysis of epidemiological time series studies of daily mortality and hospital admissions. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 208–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betha, R.; Behera, S.N.; Balasubramanian, R. 2013 Southeast Asian smoke haze: Fractionation of particulate-bound elements and associated health risk. Environ. Sci. Technol. 2014, 48, 4327–4335. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Air Pollution Levels Rising in Many of the World’s Poorest Cities; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Senaratne, I.; Shooter, D. Elemental composition in source identification of brown haze in Auckland, New Zealand. Atmos. Environ. 2004, 38, 3049–3059. [Google Scholar] [CrossRef]
- Wang, Q.; Zhuang, G.; Huang, K.; Liu, T.; Deng, C.; Xu, J.; Lin, Y.; Guo, Z.; Chen, Y.; Fu, Q.; et al. Probing the severe haze pollution in three typical regions of China: Characteristics, sources and regional impacts. Atmos. Environ. 2015, 120, 76–88. [Google Scholar] [CrossRef]
- Liu, G.; Xiao, M.; Zhang, X.; Gal, C.; Chen, X.; Liu, L.; Pan, S.; Wu, J.; Tang, L.; Clements-Croome, D. A review of air filtration technologies for sustainable and healthy building ventilation. Sustain. Cities Soc. 2017, 32, 375–396. [Google Scholar] [CrossRef]
- Liu, H.; Cao, C.; Huang, J.; Chen, Z.; Chene, G.; Lai, Y. Progress on particulate matter filtration technology: Basic concepts, advanced materials, and performances. Nanoscale 2020, 12, 437–453. [Google Scholar] [CrossRef]
- Souzandeh, H.; Wang, Y.; Netravali, A.N.; Zhong, W.H. towards Sustainable and Multifunctional Air Filters: A Review on Biopolymer-Based Filtration Materials. Polym. Rev. 2019, 59, 651–686. [Google Scholar] [CrossRef]
- Graham, W.M.; Condon, R.H.; Carmichael, R.H.; D’Ambra, I.; Patterson, H.K.; Linn, L.J.; Hernandez, F.J., Jr. Oil carbon entered the coastal planktonic food web during the Deepwater Horizon oil spill. Environ. Res. Lett. 2010, 5, 045301. [Google Scholar] [CrossRef]
- Reddy, C.M.; Arey, J.S.; Seewald, J.S.; Sylva, S.P.; Lemkau, K.L.; Nelson, R.K.; Carmichael, C.A.; McIntyre, C.P.; Fenwick, J.; Ventura, G.T.; et al. Composition and fate of gas and oil released to the water column during the Deepwater Horizon oil spill. Proc. Natl. Acad. Sci. USA 2012, 109, 20229–20234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redmond, M.C.; Valentine, D.L. Natural gas and temperature structured a microbial community response to the Deepwater Horizon oil spill. Proc. Natl. Acad. Sci. USA 2012, 109, 20292–20297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, O.U.; Hazen, T.C.; Borglin, S.; Chain, P.S.G.; Dubinsky, E.A.; Fortney, J.L.; Han, J.; Holman, H.Y.; Hultman, J.; Lamendella, R.; et al. Metagenome, metatranscriptome and single-cell sequencing reveal microbial response to Deepwater Horizon oil spill. ISME J. 2012, 6, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Kujawinski, E.B.; KidoSoule, M.C.; Valentine, D.L.; Boysen, A.K.; Longnecker, K.; Redmond, M.C. Fate of dispersants associated with the Deepwater Horizon Oil Spill. Environ. Sci. Technol. 2011, 45, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Albaiges, J. The basics of oil spill cleanup. Int. J. Environ. Anal. Chem. 2014, 94, 1512–1514. [Google Scholar] [CrossRef]
- Li, Q.; Kang, C.; Zhang, C. Wastewater produced from an oilfield and continuous treatment with an oil-degrading bacterium. Process Biochem. 2005, 40, 873–877. [Google Scholar] [CrossRef]
- Yuliwati, E.; Ismail, A.F. Effect of additives concentration on the surface properties and performance of PVDF ultrafiltration membranes for refinery produced wastewater treatment. Desalination 2011, 273, 226–234. [Google Scholar] [CrossRef]
- Pendashteh, A.R.; Fakhru’l-Razi, A.; Madaeni, S.S.; Abdullah, L.C.; Abidin, Z.Z.; Biak, D.R.A. Membrane foulants characterization in a membrane bioreactor (MBR) treating hypersaline oily wastewater. Chem. Eng. J. 2011, 168, 140–150. [Google Scholar] [CrossRef]
- Rubio, J.; Souza, M.L.; Smith, R.W. Overview of flotation as a wastewater treatment technique. Miner. Eng. 2002, 15, 139–155. [Google Scholar] [CrossRef]
- Zhong, J.; Sun, X.; Wang, C. Treatment of oily wastewater produced from refinery processes using flocculation and ceramic membrane filtration. Sep. Purif. Technol. 2003, 32, 93–98. [Google Scholar] [CrossRef]
- Gupta, V.K.; Rastogi, A.; Nayak, A. Adsorption studies on the removal of hexavalent chromium from aqueous solution using a low cost fertilizer industry waste material. J. Colloid Interface Sci. 2010, 342, 135–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, M.H.; Gao, P.; Tan, B.Y.L.; Sun, D.D.; Leckie, J.O. Highly Efficient and Flexible Electrospun Carbon–Silica Nanofibrous Membrane for Ultrafast Gravity–Driven Oil–Water Separation. ACS Appl. Mater. Interfaces 2014, 6, 9393–9401. [Google Scholar] [CrossRef] [PubMed]
- Yeber, M.; Paul, E.; Soto, C. Chemical and biological treatments to clean oily wastewater: Optimization of the photocatalytic process using experimental design. Desalin. Water Treat. 2012, 47, 295–299. [Google Scholar] [CrossRef]
- Huang, Q.; Mao, F.; Han, X.; Yan, J.; Chi, Y. Migration of emulsified water droplets in petroleum sludge during centrifugation. Energy Fuels 2014, 28, 4918–4924. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, X. Treatment of refectory oily wastewater by electro-coagulation process. Chemosphere 2004, 56, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/Water Separation Techniques: A Review of Recent Progresses and Future Directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Islam, M.S.; Choi, W.S.; Kim, S.H.; Han, O.H.; Lee, H.J. Inorganic Micelles (Hydrophilic Core@Amphiprotic Shell) for Multiple Applications. Adv. Funct. Mater. 2015, 25, 6061–6070. [Google Scholar] [CrossRef]
- Deng, Z.Y.; Wang, W.; Mao, L.H.; Wang, C.F.; Chen, S. Versatile Superhydrophobic and Photocatalytic Films Generated from TiO2−SiO2@PDMS and Their Applications on Fabrics. J. Mater. Chem. A 2014, 2, 4178–4184. [Google Scholar] [CrossRef]
- Chen, Y.; Li, F.; Cao, W.; Li, T. Preparation of Recyclable CdS Photocatalytic and Superhydrophobic Films with Photostability by Using a Screen-Printing Technique. J. Mater. Chem. A 2015, 3, 16934–16940. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, Y.; Wang, H.; Wang, X.; Lin, T. A Superamphiphobic Coating with an Ammonia-Triggered Transition to Superhydrophilic and Superoleophobic for Oil−Water Separation. Angew. Chem. Int. Ed. 2015, 54, 4527–4530. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Pan, L.; Yang, M.; Peng, L.; Zong, S.; Shi, Y.; Yu, G. Multifunctional Superhydrophobic Surfaces Templated from Innately Microstructured Hydrogel Matrix. Nano Lett. 2014, 14, 4803–4809. [Google Scholar] [CrossRef] [PubMed]
- Crick, C.R.; Gibbins, J.A.; Parkin, I.P. Superhydrophobic Polymer-Coated Copper-Mesh; Membranes for Highly Efficient Oil−Water Separation. J. Mater. Chem. A 2013, 1, 5943–5948. [Google Scholar] [CrossRef]
- Liu, X.; Ge, L.; Li, W.; Wang, X.; Li, F. Layered Double Hydroxide Functionalized Textile for Effective Oil/water Separation and Selective Oil Adsorption. ACS Appl. Mater. Interfaces 2015, 7, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yao, T.; Wu, J.; Ma, C.; Gan, Z.; Wang, Z.; Cheng, Y. Facile Approach in Fabricating Superhydrophobic and Superoleophilic Surface for Water and Oil Mixture Separation. ACS Appl. Mater. Interfaces 2009, 1, 2613–2617. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.; Liu, X.; Wang, D.; Li, J.; Jiang, L.; Jin, J. Salt-Induced Fabrication of Superhydrophilic and Underwater Superoleophobic PAA-g-PVDF Membranes for Effective Separation of Oil-in-Water Emulsions. Angew. Chem. Int. Ed. 2014, 53, 856–860. [Google Scholar] [CrossRef]
- Li, L.; Hu, T.; Sun, H.; Zhang, J.; Wang, A. Pressure-Sensitive and Conductive Carbon Aerogels from Poplars Catkins for Selective Oil Absorption and Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 18001–18007. [Google Scholar] [CrossRef]
- Li, L.; Li, B.; Sun, H.; Zhang, J. Compressible and Conductive Carbon Aerogels from Wastepaper with Exceptional Performance for Oil/Water Separation. J. Mater. Chem. A 2017, 5, 14858–14864. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Li, L.; Wang, A. Ultralight, Compressible and Multifunctional Carbon Aerogels Based on Natural Tubular Cellulose. J. Mater. Chem. A 2016, 4, 2069–2074. [Google Scholar] [CrossRef]
- Kaang, B.K.; Han, N.; Jang, W.; Koo, H.Y.; Lee, Y.B.; Choi, W.S. Crossover Magnetic Amphiprotic Catalysts for Oil/Water Separation, the Purification of Aqueous and Non-Aqueous Pollutants, and Organic Synthesis. Chem. Eng. J. 2018, 331, 290–299. [Google Scholar] [CrossRef]
- Choi, W.S.; Yang, H.M.; Koo, H.Y.; Lee, H.J.; Lee, Y.B.; Bae, T.S.; Jeon, I.C. Smart microcapsules encapsulating reconfigurable carbon nanotube cores. Adv. Funct. Mater. 2010, 20, 820–825. [Google Scholar] [CrossRef]
- Zhong, L.S.; Hu, J.S.; Liang, H.P.; Cao, A.M.; Song, W.G.; Wan, L.J. Self-assembled 3D flowerlike iron oxide nanostructures and their application in water treatment. Adv. Mater. 2006, 18, 2426–2431. [Google Scholar] [CrossRef]
- Hu, J.S.; Zhong, L.S.; Song, W.G.; Wan, L.J. Synthesis of hierarchically structured metal oxides and their application in heavy metal ion removal. Adv. Mater. 2008, 20, 2977–2982. [Google Scholar] [CrossRef]
- Koo, H.Y.; Lee, H.J.; Go, H.A.; Lee, Y.B.; Bae, T.S.; Kim, J.K.; Choi, W.S. Graphene-based multifunctional iron oxide nanosheets with tunable properties. Chem. Eur. J. 2011, 17, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.; Ratcliffe, N.M.; Duffield, J.R.; Elder, G.R.; Patton, D. Functionalized paramagnetic nanoparticles for wastewater treatment. Chem. Commun. 2010, 46, 4583–4585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohse, S.; Murphy, C.J. Applications of colloidal inorganic nanoparticles: From medicine to energy. J. Am. Chem. Soc. 2012, 134, 15607–15620. [Google Scholar] [CrossRef] [PubMed]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef]
- Antochshuk, V.; Olkhovyk, O.; Jaroniec, M.; Park, I.S.; Ryoo, R. Benzoylthiourea-modified mesoporous silica for mercury(II) removal. Langmuir 2003, 19, 3031–3034. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schuth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Phetphaisit, C.W.; Yuanyang, S.; Chaiyasith, W.C. Polyacrylamido-2-methyl-1-propane sulfonic acid-grafted-natural rubber as bio-adsorbent for heavy metal removal from aqueous standard solution and industrial wastewater. J. Hazard. Mater. 2016, 301, 163–171. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.; Shen, M.; Tan, X.; Ding, Y.; Jiang, Z.; Wang, C. Preparation of phosphorylated polyacrylonitrile-based nanofiber mat and its application for heavy metal ion removal. Chem. Eng. J. 2015, 268, 290–299. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Q.; Liu, M.; Wang, K.; Wan, Q.; Deng, F.; Lu, L.; Zhang, X.; Wei, Y. Mussel inspired functionalization of carbon nanotubes for heavy metal ion removal. RSC Adv. 2015, 5, 68430–68438. [Google Scholar] [CrossRef]
- Li, X.; Zhou, H.; Wu, W.; Wei, S.; Xu, Y.; Kuang, Y. Studies of heavy metal ion adsorption on chitosan/sulfydryl-functionalized graphene oxide composites. J. Colloid Interface Sci. 2015, 448, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, M.; Siddiq, M.; Aktas, N.; Sahiner, N. Magnetic Co-Fe bimetallic nanoparticle containing modifiable microgels the removal of heavy metal ions, organic dyes and herbicides from aqueous media. RSC Adv. 2015, 5, 43873–43884. [Google Scholar] [CrossRef]
- Sanchez-Quiles, D.; Tovar-Sanchez, A. Sunscreens as a source of hydrogen peroxide production in coastal waters. Environ. Sci. Technol. 2014, 48, 9037–9042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozin, G.A.; Arsenault, A.C.; Cademaritiri, L. Nanochemistry, 2nd ed.; RSC Publishing: Cambridge, UK, 2009. [Google Scholar]
- Seger, B.; Kamat, P.V. Fuel Cell Geared in Reverse: Photocatalytic Hydrogen Production Using a TiO2/Nafion/Pt Membrane Assembly with No Applied Bias. J. Phys. Chem. C 2009, 113, 18946–18952. [Google Scholar] [CrossRef]
- Yoo, E.J.; Okata, T.; Akita, T.; Kohyama, M.; Nakamura, J.; Honma, I. Enhanced electrocatalytic activity of Pt subnanoclusters on graphene nanosheet surface. Nano Lett. 2009, 9, 2255–2259. [Google Scholar] [CrossRef] [PubMed]
- Ismaili, H.; Lagugne-Labarthet, F.; Workentin, M.S. Covalently assembled gold nanoparticle-carbon nanotube hybrids via a photoinitiated carbene addition reaction. Chem. Mater. 2011, 23, 1519–1525. [Google Scholar] [CrossRef]
- Cassagneau, T.; Fendler, J.H. Sandwich-like graphene nanocomposites armed with nanoneedles. J. Phys. Chem. B 1999, 103, 1789–1793. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Zhu, J. Grephene-Metal Particle Nanocomposites. J. Phys. Chem. C 2008, 112, 19841–19845. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, Y.; Liao, H.; Liu, J.; Aksay, I.A.; Yin, G.; Lin, Y. Graphene Decorated with PtAu Alloy Nanoparticles: Facile Synthesis and Promising Application for Formic Acid Oxidation. Chem. Mater. 2011, 23, 1079–1081. [Google Scholar] [CrossRef]
- Song, E.H.; Wen, Z.; Jiang, Q. CO Catalytic Oxidation on Copper-Embedded Graphene. J. Phys. Chem. C 2011, 115, 3678–3683. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, Y.; Liao, H.; Engelhard, M.H.; Yin, G.; Lin, Y. Polyelectrolyte-induced reduction of exfoliated graphite oxide: A facile route to synthesis of soluble graphene nanosheets. ACS Nano 2011, 5, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Su, F.; Han, Y.; Tian, Z.; Poh, C.K.; Liu, Z.; Lin, J.; Lee, J.Y.; Zhao, X.S. Pt nanoparticles supported on sandwiched Ru/carbon nanocomposite as a bimetallic catalyst for methanol electrooxidation. J. Phys. Chem. C 2008, 112, 15908–15914. [Google Scholar] [CrossRef]
- Steigerwalt, E.S.; Deluga, G.A.; Lukehart, C.M. Pt-Ru/Carbon fiber nanocomposites: Synthesis, characterization, and performance as anode catalysts of direct methanol fuel cells. A search for exceptional performance. J. Phys. Chem. B 2002, 106, 760–766. [Google Scholar] [CrossRef]
- Xiang, J.; Drzal, L.T. Electron and phonon transport in Au nanoparticle decorated graphene nanoplatelet nanostructured paper. ACS Appl. Mater. Interface 2011, 3, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; Nethravathi, C.; Deshpande, P.A.; Rajamathi, M.; Madras, G.; Ravishankar, N. Ultrafast microwave-assisted route to surfactant-free ultrafine Pt nanoparticles on graphene: Synergistic Co-reduction mechanism and high catalytic activity. Chem. Mater. 2011, 23, 2772–2780. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, S.; Hao, J.; Liu, H.; Wu, X.; Hu, J.; Walsh, M.P.; Wallington, T.J.; Zhang, K.M.; Stevanovic, S. On-road vehicle emissions and their control in China: A review and outlook. Sci. Total Environ. 2017, 574, 332–349. [Google Scholar] [CrossRef]
- Zhang, R.; Jing, J.; Tao, J.; Hsu, S.-C.; Wang, G.; Cao, J.; Lee, C.S.L.; Zhu, L.; Chen, Z.; Zhao, Y. Chemical characterization and source apportionment of PM2.5 in Beijing: Seasonal perspective. Atmos. Chem. Phys. 2013, 13, 7053–7074. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Woodward, A.; Vardoulakis, S.; Kovats, S.; Wilkinson, P.; Li, L.; Xu, L.; Li, J.; Yang, J.; Cao, L. Public Health and Mitigation Measures in China: A Review of the Current Evidence for Further Policy Response. Sci. Total Environ. 2017, 578, 148–157. [Google Scholar] [CrossRef]
- Seinfeld, J.H. Urban Air Pollution: State of the Science. Science 1989, 243, 745–752. [Google Scholar] [CrossRef]
- Maricq, M.M. Chemical characterization of particulate emissions from diesel engines: A review. J. Aerosol Sci. 2007, 38, 1079–1118. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G. Review of PM2.5 and PM10 Apportionment for Fossil Fuel Combustion and Other Sources by the Chemical Mass Balance Receptor Model. Energy Fuels 2002, 16, 222–260. [Google Scholar] [CrossRef]
- Wang, H.; Zhuang, Y.; Wang, Y.; Sun, Y.; Yuan, H.; Zhuang, G.; Hao, Z. Long-term monitoring and source apportionment of PM2.5/PM10 in Beijing, China. J. Environ. Sci. 2008, 20, 1323–1327. [Google Scholar] [CrossRef]
- Han, X.; Naeher, L.P. A review of traffic-related air pollution exposure assessment studies in the developing world. Environ. Int. 2006, 32, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; Otani, Y. Removal of nanoparticles from gas streams by fibrous filters: A review. Ind. Eng. Chem. Res. 2012, 52, 5–17. [Google Scholar] [CrossRef]
- Li, P.; Wang, C.; Zhang, Y.; Wei, F. Air filtration in the free molecular flow regime: A review of high-efficiency particulate air filters based on carbon nanotubes. Small 2014, 10, 4543–4561. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hsu, P.-C.; Lee, H.-W.; Ye, M.; Zheng, G.; Liu, N.; Li, W.; Cui, Y. Transparent air filter for high-efficiency PM 2.5 capture. Nat. Commun. 2015, 6, 6205. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Liu, C.; Hsu, P.-C.; Liu, K.; Zhang, R.; Liu, Y.; Cui, Y. Roll-to-roll transfer of electrospun nanofilter film for high-efficiency transparent air filter. Nano Lett. 2016, 16, 1270–1275. [Google Scholar] [CrossRef]
- Wang, C.-S. Electrostatic forces in fibrous filters—A review. Powder Technol. 2001, 118, 166–170. [Google Scholar] [CrossRef]
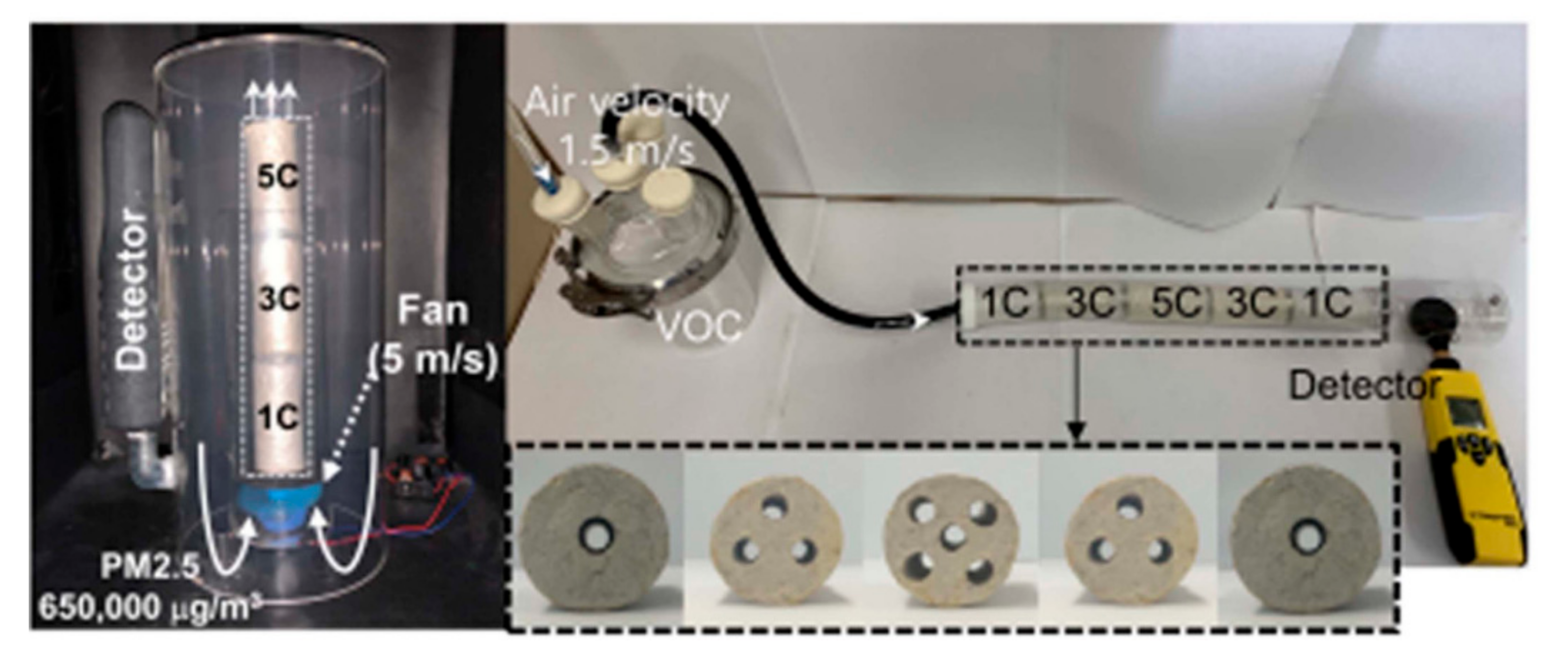
- Han, N.; Lee, Y.S.; Kaang, B.K.; Jang, W.; Koo, H.Y.; Choi, W.S. A lottery draw machine-inspired movable air filter with high removal efficiency and low pressure drop at a high flow rate. J. Mater. Chem. A 2019, 7, 6001–6011. [Google Scholar] [CrossRef]
- Chen, C.Y. Filtration of aerosols by fibrous media. Chem. Rev. 1955, 55, 595–623. [Google Scholar] [CrossRef]
- Leung, W.W.-F.; Hung, C.-H.; Yuen, P.-T. Effect of face velocity, nanofiber packing density and thickness on filtration performance of filters with nanofibers coated on a substrate. Sep. Purif. Technol. 2010, 71, 30–37. [Google Scholar] [CrossRef]
- Gong, G.; Zhou, C.; Wu, J.; Jin, X.; Jiang, L. Nanofibrous adhesion: The twin of gecko adhesion. ACS Nano 2015, 9, 3721–3727. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.M.; Park, H.-S.; Bae, G.-N.; Jung, J.H. Antimicrobial nanoparticlecoated electrostatic air filter with high filtration efficiency and low pressure drop. Sci. Total Environ. 2015, 533, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Cho, H.; Han, S.; Won, P.; Lee, H.; Hong, S.; Yeo, J.; Kwon, J.; Ko, S.H. High efficiency, transparent reusable, and active PM2.5 filters by hierarchical Ag nanowire percolation network. Nano Lett. 2017, 17, 4339–4346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalid, B.; Bai, X.; Wei, H.; Huang, Y.; Wu, H.; Cui, Y. Direct Blow-Spinning of Nanofibers on a Window Screen for Highly Efficient PM2.5 Removal. Nano Lett. 2017, 17, 1140–1148. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, C.; Hsu, P.-C.; Zhang, C.; Liu, N.; Zhang, J.; Lee, H.R.; Lu, Y.; Qiu, Y.; Chu, S.; et al. Nanofiber Air Filters with High-Temperature Stability for Efficient PM2.5 Removal from the Pollution Sources. Nano Lett. 2016, 16, 3642–3649. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Huang, X.; Zhang, T.; Wang, X.; Min, M.; Wang, L.; Huang, H.; Hsiao, B.S. Anionic surfactant-triggered steiner geometrical poly (vinylidene fluoride) nanofiber/nanonet air filter for efficient particulate matter removal. ACS Appl. Mater. Interfaces 2018, 10, 42891–42904. [Google Scholar] [CrossRef]
- Liu, H.; Huang, J.; Mao, J.; Chen, Z.; Chen, G.; Lai, Y. Transparent antibacterial nanofiber air filters with highly efficient moisture resistance for sustainable particulate matter capture. iScience 2019, 19, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Li, Y.; Hua, T.; Jiang, P.; Yin, X.; Yu, J.; Ding, B. Low-Resistance Dual-Purpose Air Filter Releasing Negative Ions and Effectively Capturing PM2.5. ACS Appl. Mater. Interfaces 2017, 9, 12054–12063. [Google Scholar] [CrossRef]
- Carpenter, W.A.; de Lannoy, F.C.; Wiesner, R.M. Cellulose nanomaterials in water treatment technologies. Environ. Sci. Technol. 2015, 49, 5277–5287. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Rodriguez, K.; Renneckar, S.; Vikesland, P.J. Environmental science and engineering applications of nanocellulose-based nanocomposites. Environ. Sci. Nano 2014, 1, 302–316. [Google Scholar] [CrossRef] [Green Version]
- Martınez-Sanz, M.; Mikkelsen, D.; Flanagan, B.; Gidley, M.J.; Gilbert, E.P. Multi-scale model for the hierarchical architecture of native cellulose hydrogels. Carbohydr. Polym. 2016, 147, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Li, J. Cellulose aerogels functionalized with polypyrrole and silver nanoparticles: In-situ synthesis, characterization and antibacterial activity. Carbohydr. Polym. 2016, 146, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Chouw, N.; Huang, L.; Kasal, B. Effect of alkali treatment on microstructure and mechanical properties of coir fibres, coir fibre reinforced-polymer composites and reinforced-cementitious composites. Constr. Build. Mater. 2016, 112, 168–182. [Google Scholar] [CrossRef]
- Devarayan, K.; Lei, D.; Kim, H.Y.; Kim, B.S. Flexible transparent electrode based on PANi nanowire/nylon nanofiber reinforced cellulose acetate thin film as supercapacitor. Chem. Eng. J. 2015, 273, 603–609. [Google Scholar] [CrossRef]
- Nielsen, H.L.; Engberg, J.; Ejlertsen, T.; Nielsen, H. Comparison of polycarbonate and cellulose acetate membrane filters for isolation of campylobacter concisus from stool samples. Diagn. Microbiol. Infect. Dis. 2013, 76, 549–550. [Google Scholar] [CrossRef]
- Kaang, B.K.; Lee, H.B.; Koo, H.Y.; Choi, W.S. Wastepaper-Based Cylindrical Hollow Air Filter Module for the Removal of Particulate Matter (PM10 and PM2.5) and HCHO. ACS Sustain. Chem. Eng. 2020, 8, 13984–13996. [Google Scholar] [CrossRef]
- Balgis, R.; Murata, H.; Goi, Y.; Ogi, T.; Okuyama, K.; Bao, L. Synthesis of dual-size cellulose–polyvinylpyrrolidone nanofiber composites via one-step electrospinning method for high-performance air filter. Langmuir 2017, 33, 6127–6134. [Google Scholar] [CrossRef]
- Nicosia, A.; Keppler, T.; Muller, F.A.; Vazquez, B.; Ravegnani, F.; Monticelli, P.; Belosi, F. Cellulose acetate nanofiber electrospun on nylon substrate as novel composite matrix for efficient, heat-resistant, air filters. Chem. Eng. Sci. 2016, 153, 284–294. [Google Scholar] [CrossRef]
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A review of the antibacterial activity of chitosan. Polim. Cienc. Tecnol. 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Z.-G.; Yan, X.; Wang, X.-X.; Zhao, H.; Guo, J.; Feng, J.-Y.; Long, Y.-Z. Chitosan nanostructures by in situ electrospinning for high-efficiency PM2.5 capture. Nanoscale 2017, 9, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.; Kit, K.; Li, J.; Davidson, P.M.; Zivanovic, S.; Meyer, H. Nanofibrous chitosan non-wovens for filtration applications. Polymer 2009, 50, 3661–3669. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, F.; Pei, H.; Li, J.; Cui, Z.; He, B. Antibacterial and environmentally friendly chitosan/polyvinyl alcohol blend membranes for air filtration. Carbohydr. Polym. 2018, 198, 241–248. [Google Scholar] [CrossRef]
- Bacsa, R.; Laurent, C.; Peigney, A.; Bacsa, W.; Vaugien, T.; Rousset, A. High specific surface area carbon nanotubes from catalytic chemical vapor deposition process. Chem. Phys. Lett. 2000, 323, 566–571. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-H.; Wang, S.; Wei, J.; Zhang, X.; Xu, C.; Luan, Z.; Wu, D.; Wei, B. Lead adsorption on carbon nanotubes. Chem. Phys. Lett. 2002, 357, 263–266. [Google Scholar] [CrossRef]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Maze, B.; Tafreshi, H.V.; Wang, Q.; Pourdeyhimi, B. A simulation of unsteady-state filtration via nanofiber media at reduced operating pressures. J. Aerosol Sci. 2007, 38, 550–571. [Google Scholar] [CrossRef]
- Zhang, R.; Wen, Q.; Qian, W.; Su, D.S.; Zhang, Q.; Wei, F. Superstrong ultralong carbon nanotubes for mechanical energy storage. Adv. Mater. 2011, 23, 3387–3391. [Google Scholar] [CrossRef]
- Yildiz, O.; Bradford, P.D. Aligned carbon nanotube sheet high efficiency particulate air filters. Carbon 2013, 64, 295–304. [Google Scholar] [CrossRef]
- Li, P.; Zong, Y.; Zhang, Y.; Yang, M.; Zhang, R.; Li, S.; Wei, F. In situ fabrication of depth-type hierarchical CNT/quartz fiber filters for high efficiency filtration of sub-micron aerosols and high water repellency. Nanoscale 2013, 5, 3367–3372. [Google Scholar] [CrossRef] [PubMed]
- Halonen, N.; Rautio, A.; Leino, A.-R.; Kyllonen, T.; Toth, G.; Lappalainen, J.; Kordás, K.; Huuhtanen, M.; Keiski, R.L.; Sápi, A. Three-dimensional carbon nanotube scaffolds as particulate filters and catalyst support membranes. ACS Nano 2010, 4, 2003–2008. [Google Scholar] [CrossRef] [PubMed]
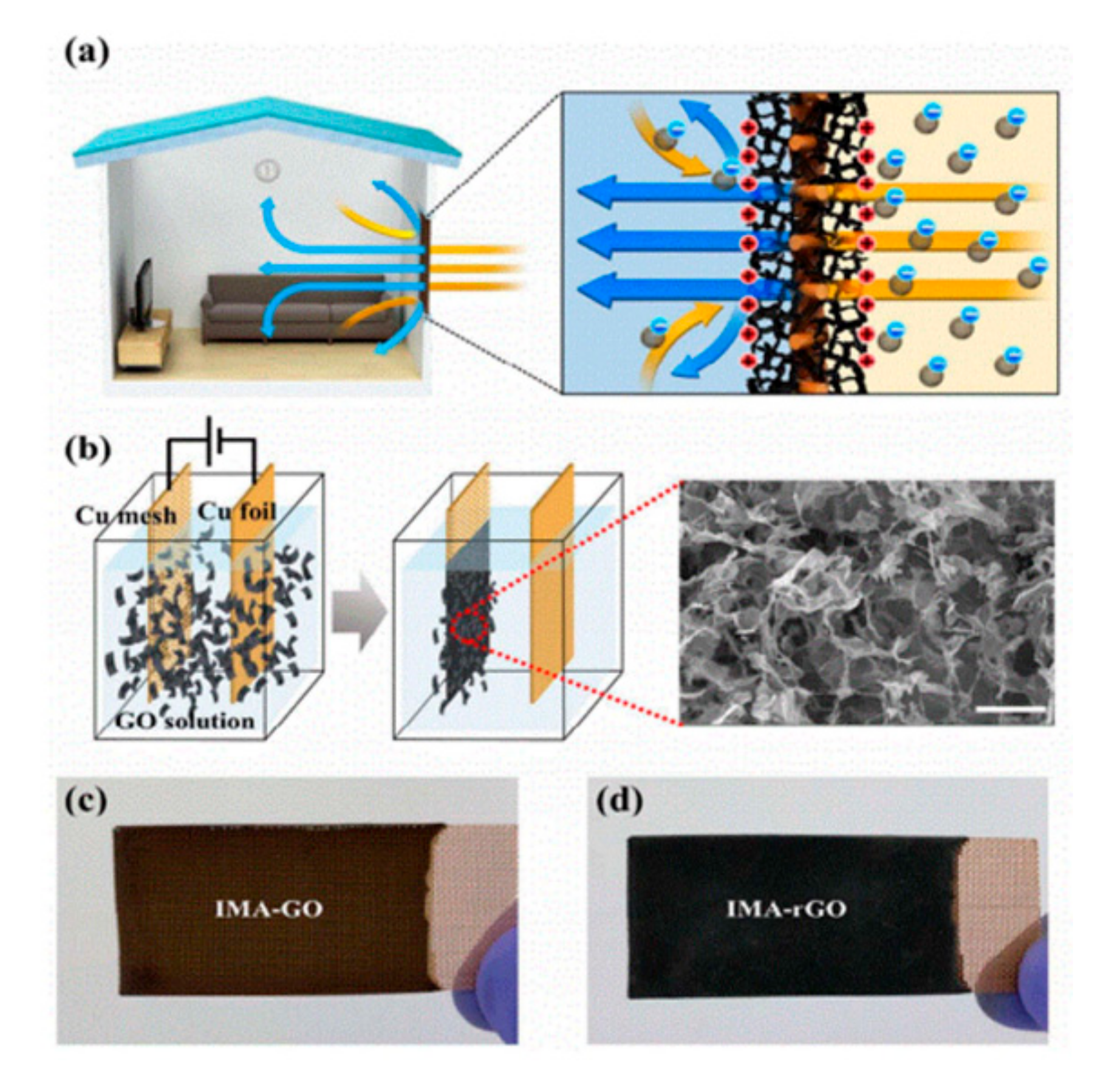
- Zhang, S.; Sun, J.; Hu, D.; Xiao, C.; Zhuo, Q.; Wang, J.; Qin, C.; Dai, L. Large-sized graphene oxide/modified tourmaline nanoparticle aerogel with stable honeycomb-like structure for high-efficiency PM 2.5 capture. J. Mater. Chem. A 2018, 6, 16139–16148. [Google Scholar] [CrossRef]
- Jung, W.; Lee, J.S.; Han, S.; Ko, S.H.; Kim, T.; Kim, Y.H. An efficient reduced graphene-oxide filter for PM 2.5 removal. J. Mater. Chem. A 2018, 6, 16975–16982. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel. Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Lett. 2003, 3, 1167–1171. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, W.; Xiao, K.; Berti, L.; Luo, J.; Tseng, H.P.; Fung, G.; Lam, K.S. Well-Defined, Reversible Boronate Crosslinked Nanocarriers for Targeted Drug Delivery in Response to Acidic pH Values and cis-Diols. Angew. Chem. Int. Ed. 2012, 51, 2864–2869. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Ding, B.; Wang, M.; Yin, Y. Self-assembly of phthalocyanine and polyacrylic acid composite multilayers on cellulose nanofibers. Carbohydr. Polym. 2010, 80, 839–844. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Xia, Y.; Jiao, X.; Chen, D. Electrospun flexible self-standing γ-alumina fibrous membranes and their potential as high-efficiency fine particulate filtration media. J. Mater. Chem. A 2014, 2, 15124–15131. [Google Scholar] [CrossRef]
- Mao, X.; Si, Y.; Chen, Y.; Yang, L.; Zhao, F.; Ding, B.; Yu, J. Silica nanofibrous membranes with robust flexibility and thermal stability for high-efficiency fine particulate filtration. RSC Adv. 2012, 2, 12216–12223. [Google Scholar] [CrossRef]
- Li, X.; Wang, N.; Fan, G.; Yu, J.; Gao, J.; Sun, G.; Ding, B. Electreted polyetherimide–silica fibrous membranes for enhanced filtration of fine particles. J. Colloid Interface Sci. 2015, 439, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, M.; Matsumoto, T.; Kobayashi, Y.; Jikihara, Y.; Nakayama, T.; Ohashi, H.; Honma, T.; Takei, T.; Haruta, M. Air purification by gold catalysts supported on PET nonwoven fabric. Appl. Catal. B Environ. 2013, 134, 130–135. [Google Scholar] [CrossRef]
- Jin, W.J.; Jeon, H.J.; Kim, J.H.; Youk, J.H. A study on the preparation of poly(vinylalcohol) nanofibers containing silver nanoparticles. Synth. Met. 2007, 157, 454–459. [Google Scholar] [CrossRef]
- Lala, L.N.; Ramaseshan, R.; Bojun, L.; Sundarrajan, S.S.; Barhate, R.; Ying-Jun, L.; Ramakrishna, S. Fabrication of nanofibers with antimicrobial functionality used as filters: Protection against bacterial contaminants. Biotechnol. Bioeng. 2007, 15, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Lan, Y.; Qu, J.; Hu, X.; Wang, A. Ag/AgBr/TiO2 visible light photocatalyst for destruction of azodyes and bacteria. J. Phys. Chem. B 2006, 110, 4066–4072. [Google Scholar] [CrossRef]
- Jin, M.; Zhang, X.; Nishimoto, S.; Liu, Z.; Tryk, D.A.; Emeline, A.V.; Murakami, T.; Fujishima, A. Light-stimulated composition conversion in TiO2-based nanofibers. J. Phys. Chem. C 2007, 111, 658–665. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Zorba, V.; Stratakis, E.; Barberoglou, M.; Spanakis, E.; Tzanetakis, P.; Anastasiadis, S.H.; Fotakis, C. Biomimetic artificial surfaces quantitatively reproduce the water repellency of a lotus leaf. Adv. Mater. 2008, 20, 4049–4054. [Google Scholar] [CrossRef]
- Yong, J.L.; Chen, F.; Fang, Y.; Huo, J.; Yang, Q.; Zhang, J.; Bian, H.; Hou, X. Bioinspired design of underwater superaerophobic and superaerophilic surfaces by femtosecond laser ablation for anti-or capturing bubbles. ACS Appl. Mater. Interfaces 2017, 9, 39863–39871. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, L. Definition of superhydrophobic states. Adv. Mater. 2007, 19, 3423–3424. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Li, M.; Yang, Q.; Fang, Y.; Huo, J.; Hou, X. Remarkably simple achievement of superhydrophobicity, superhydrophilicity, underwater superoleophobicity, underwater superoleophilicity, underwater superaerophobicity, and underwater superaerophilicity on femtosecond laser ablated PDMS surfaces. J. Mater. Chem. A 2017, 5, 25249–25257. [Google Scholar] [CrossRef] [Green Version]
- Larmour, I.A.; Bell, S.E.J.; Saunders, G.C. Remarkably simple fabrication of superhydrophobic surfaces using electroless galvanic deposition. Angew. Chem. Int. Ed. 2007, 46, 1710–1712. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, Y.; Wang, X.; Lin, T. Superhydrophobic cotton fabric fabricated by electrostatic assembly of silica nanoparticles and its remarkable buoyancy. Appl. Surf. Sci. 2010, 256, 6736–6742. [Google Scholar] [CrossRef]
- Babu, D.J.; Mail, M.; Barthlott, W.; Schneider, J.J. Superhydrophobic Vertically Aligned Carbon Nanotubes for Biomimetic Air Retention under Water (Salvinia Effect). Adv. Mater. Interfaces 2017, 4, 1700273. [Google Scholar] [CrossRef]
- Yong, J.L.; Chen, F.; Yang, Q.; Huo, J.; Hou, X. Superoleophobic surfaces. Chem. Soc. Rev. 2017, 46, 4168–4217. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Wang, S.; Wei, Z.; Song, Y.; Jiang, L. Bioinspired design of a superoleophobic and low adhesive water/solid interface. Adv. Mater. 2009, 21, 665–669. [Google Scholar] [CrossRef]
- Yong, J.L.; Chen, F.; Huo, J.; Fang, Y.; Yang, Q.; Bian, H.; Li, W.; Wei, Y.; Dai, Y.; Hou, X. Green, biodegradable, underwater superoleophobic wood sheet for efficient oil/water separation. ACS Omega 2018, 3, 1395–1402. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Wang, S.; Jiang, L. Nature-inspired superwettability systems. Nat. Rev. Mater. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Wen, G.; Guo, Z.; Liu, W. Biomimetic polymeric superhydrophobic surfaces and nanostructures: From fabrication to applications. Nanoscale 2017, 9, 3338–3366. [Google Scholar] [CrossRef]
- Genzer, J.; Efimenko, K. Recent developments in superhydrophobic surfaces and their relevance to marine fouling: A review. Biofouling 2006, 22, 339–360. [Google Scholar] [CrossRef]
- Wang, J.-N.; Zhang, Y.-L.; Liu, Y.; Zheng, W.; Lee, L.P.; Sun, H.-B. Recent developments in superhydrophobic graphene and graphene-related materials: From preparation to potential applications. Nanoscale 2015, 7, 7101–7114. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, Z. Superhydrophobic nanocoatings: From materials to fabrications and to applications. Nanoscale 2015, 7, 5922–5946. [Google Scholar]
- Wenzel, R.N. N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Yong, J.L.; Yang, Q.; Chen, F.; Zhang, D.; Du, G.; Bian, H.; Si, J.; Yun, F.; Hou, X. Superhydrophobic PDMS surfaces with three-dimensional (3D) pattern-dependent controllable adhesion. Appl. Surf. Sci. 2014, 288, 579–583. [Google Scholar] [CrossRef]
- Li, J.; Jing, Z.; Zha, F.; Yang, Y.; Wang, Q.; Lei, Z. Facile Spray-Coating Process for the Fabrication of Tunable Adhesive Superhydrophobic Surfaces with Heterogeneous Chemical Compositions Used for Selective Transportation of Microdroplets with Different Volumes. ACS Appl. Mater. Interfaces 2014, 6, 8868–8877. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Du, M.; Lai, H.; Zhang, N.; Sun, K. From petal effect to lotus effect: A facile solution immersion process for the fabrication of super-hydrophobic surfaces with controlled adhesion. Nanoscale 2013, 5, 2776–2783. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jiang, L. Switchable adhesion on liquid/solid interfaces. Adv. Funct. Mater. 2010, 20, 3753–3764. [Google Scholar] [CrossRef]
- Xia, F.; Jiang, L. Bio-inspired, smart, multiscale interfacial materials. Adv. Mater. 2008, 20, 2842–2858. [Google Scholar] [CrossRef]
- Tian, S.; Li, L.; Sun, W.; Xia, X.; Han, D.; Li, J.; Gu, C. Robust adhesion of flower-like few-layer graphene nanoclusters. Sci. Rep. 2012, 2, 511. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Zeng, X.; Li, H.; Lai, X.; Ye, C.; Xie, H. Study on the Wetting Behavior and Theoretical Models of Polydimethylsiloxane/silica Coating. Appl. Surf. Sci. 2013, 279, 458–463. [Google Scholar] [CrossRef]
- Erbil, H.Y.; Cansoy, C.E. Range of applicability of the Wenzel and Cassie−Baxter equations for superhydrophobic surfaces. Langmuir 2009, 25, 14135–14145. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Kwon, T.H. Effects of intrinsic hydrophobicity on wettability of polymer replicas of a superhydrophobic lotus leaf. J. Micromech. Microeng. 2007, 17, 687–692. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, W.B.; Shi, Z.; Wang, D.; Jin, J.; Jiang, L. Nanowire-haired inorganic membranes with superhydrophilicity and underwater ultralow adhesive superoleophobicity for high-efficiency oil/water separation. Adv. Mater. 2013, 25, 4192–4198. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, L.-P.; Xue, Z.; Feng, L.; Peng, J.; Wen, Y.; Wang, S.; Zhang, X. Dual-Scaled Porous Nitrocellulose Membranes with Underwater Superoleophobicity for Highly Efficient Oil/Water Separation. Adv. Mater. 2014, 26, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Duan, H.; Chen, G.Y.; Liu, X.; Yang, W.; Wang, D. Cleaning of Oil Fouling with Water Enabled by Zwitterionic Polyelectrolyte Coatings: Overcoming the Imperative Challenge of Oil-Water Separation Membranes. ACS Nano 2015, 9, 9188–9198. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhu, Y.; Liu, M.; Feng, L.; Wan, M.; Jiang, L. PANI nanowire film with underwater superoleophobicity and potential-modulated tunable adhesion for no loss oil droplet transport. Soft Matter 2012, 8, 9064–9068. [Google Scholar] [CrossRef]
- Liu, X.L.; Gao, J.; Xue, Z.X.; Chen, L.; Lin, L.; Jiang, L.; Wang, S.T. Bioinspired oil strider floating at the oil/water interface supported by huge superoleophobic force. ACS Nano 2012, 6, 5614–5620. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, M.; Wang, J.; Zhou, J.; Wang, L.; Song, Y.; Jiang, L. Functional Thin Films: Controllable Underwater Oil-Adhesion-Interface Films Assembled from Nonspherical Particle. Adv. Funct. Mater. 2011, 21, 4436–4441. [Google Scholar] [CrossRef]
- Wu, D.; Wu, S.Z.; Chen, Q.D.; Zhao, S.; Zhang, H.; Jiao, J.; Piersol, J.A.; Wang, J.N.; Sun, H.B.; Jiang, L. Facile creation of hierarchical PDMS microstructures with extreme underwater superoleophobicity for anti-oil application in microfluidic channels. Lab Chip 2011, 11, 3873–3879. [Google Scholar] [CrossRef]
- Lin, L.; Liu, M.J.; Chen, L.; Chen, P.P.; Ma, J.; Han, D.; Jiang, L. Bio-Inspired Hierarchical Macromolecule-Nanoclay Hydrogels for Robust Underwater Superoleophobicity. Adv. Mater. 2010, 22, 4826–4830. [Google Scholar] [CrossRef]
- Lim, Y.T.; Han, N.; Jang, W.; Jung, W.; Oh, M.; Han, S.W.; Koo, H.Y.; Choi, W.S. Surface Design of Separators for Oil/Water Separation with High Separation Capacity and Mechanical Stability. Langmuir 2017, 33, 8012–8022. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lee, H.B.; Koo, H.Y.; Choi, W.S. Remote-controlled magnetic sponge balls and threads for oil/water separation in a confined space and anaerobic reactions. ACS Appl. Mater. Interfaces 2019, 11, 40886–40897. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lim, Y.T.; Choi, W.S. One-step Synthesis of Environmentally Friendly Superhydrophilic and Superhydrophobic Sponges for Oil/water Separation. Materials 2019, 12, 1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and Superoleophilic PVDF Membranes for Effective Separation of Water-in-Oil Emulsions with High Flux. Adv. Mater. 2013, 25, 2071–2076. [Google Scholar] [CrossRef] [PubMed]
- Boakye-Ansah, S.; Lim, Y.T.; Lee, H.J.; Choi, W.S. Structure-Controllable Superhydrophobic Cu Meshes for Effective Separation of Oils with Different Viscosities and Aqueous Pollutant Purification. RSC Adv. 2016, 6, 17642–17650. [Google Scholar] [CrossRef]
- Zang, D.; Wu, C.; Zhu, R.; Zhang, W.; Yu, X.; Zhang, Y. Porous Copper Surfaces with Improved Superhydrophobicity under Oil and Their Application in Oil Separation and Capture from Water. Chem. Commun. 2013, 49, 8410–8412. [Google Scholar] [CrossRef] [Green Version]
- Xue, Z.; Cao, Y.; Liu, N.; Feng, L.; Jiang, L. Special wettable materials for oil/water separation. J. Mater. Chem. A 2014, 2, 2445–2460. [Google Scholar] [CrossRef]
- Wang, B.; Liang, W.X.; Guo, Z.G.; Liu, W.M. Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature. Chem. Soc. Rev. 2015, 44, 336–361. [Google Scholar] [CrossRef]
- Chu, Z.; Feng, Y.; Seeger, S. Oil/water separation with selective superantiwetting/superwetting surface materials. Angew. Chem. Int. Ed. 2015, 54, 2328–2338. [Google Scholar] [CrossRef]
- Yong, J.; Huo, J.; Chen, F.; Yang, Q.; Hou, X. Oil/water separation based on natural materials with super-wettability: Recent advances. Phys. Chem. Chem. Phys. 2018, 20, 25140–25163. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Z.; Mai, Z.; Ma, Y.; Liu, B.; Jiang, L.; Zhu, D. A super-hydrophobic and super-oleophilic coating mesh film for the separation of oil and water. Angew. Chem. Int. Ed. 2004, 43, 2012–2014. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, Y.; Jiang, L. Microscale and nanoscale hierarchical structured mesh films with superhydrophobic and superoleophilic properties induced by long-chain fatty acids. Nanotechnology 2007, 18, 015103. [Google Scholar] [CrossRef]
- Song, J.; Huang, S.; Lu, Y.; Bu, X.; Mates, J.E.; Ghosh, A.; Ganguly, R.; Carmalt, C.J.; Parkin, I.P.; Xu, W.; et al. Self-driven one-step oil removal from oil spill on water via selective-wettability steel mesh. ACS Appl. Mater. Interfaces 2014, 6, 19858–19865. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Li, S.H.; Ge, M.Z.; Wang, L.N.; Xing, T.L.; Chen, G.Q.; Liu, X.F.; Al-Deyab, S.S.; Zhang, K.Q.; Chen, T.; et al. Robust superhydrophobic TiO2@fabrics for UV shielding, self-cleaning and oil-water separation. J. Mater. Chem. A 2015, 3, 2825–2832. [Google Scholar] [CrossRef]
- Xue, C.-H.; Li, Y.-R.; Hou, J.-L.; Zheng, L.; Ma, J.-Z. Self-roughened superhydrophobic coatings for continuous oil-water separation. J. Mater. Chem. A 2015, 3, 10248–10253. [Google Scholar] [CrossRef]
- Liu, Q.; Patel, A.A.; Liu, L. Superhydrophilic and Underwater Superoleophobic Poly(sulfobetaine methacrylate)-Grafted Glass Fiber Filters for Oil-Water Separation. ACS Appl. Mater. Interfaces 2014, 6, 8996–9003. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, F.; Wang, D.; Pei, X.F.; Zhang, W.; Jin, J. A novel zwitterionic polyelectrolyte grafted PVDF membrane for thoroughly separating oil from water with ultrahigh efficiency. J. Mater. Chem. A 2013, 1, 5758–5765. [Google Scholar] [CrossRef]
- Yang, R.; Moni, P.; Gleason, K.K. Ultrathin Zwitterionic Coatings for Roughness- independent Underwater Superoleophobicity and Gravity-Driven Oil-Water Separation. Adv. Mater. Interfaces 2015, 2, 1400489. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Z. pH-Responsive Bidirectional Oil−Water Separation Material. Chem. Commun. 2013, 49, 9416–9418. [Google Scholar] [CrossRef]
- Dunderdale, G.J.; Urata, C.; Sato, T.; England, M.W.; Hozumi, A. Continuous, high-speed, and efficient oil/water separation using meshes with antagonistic wetting properties. ACS Appl. Mater. Interfaces 2015, 7, 18915–18919. [Google Scholar] [CrossRef]
- Liu, N.; Chen, Y.; Lu, F.; Cao, Y.; Xue, Z.; Li, K.; Feng, L.; Wei, Y. Straightforward Oxidation of a Copper Substrate Produces an Underwater Superoleophobic Mesh for Oil/Water Separation. ChemPhysChem 2013, 14, 3489–3494. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Yang, J.; Tang, Y.; Chen, L.; Liu, C.; Tang, H.; Li, C. Mechanical durability of superhydrophobic and oleophobic copper meshes. Appl. Surf. Sci. 2014, 316, 259–263. [Google Scholar] [CrossRef]
- Zhang, W.X.; Wen, X.G.; Yang, S.H.; Berta, Y.; Wang, Z.L. Single-Crystalline Scroll-Type Nanotube Arrays of Copper Hydroxide Synthesized at Room Temperature. Adv. Mater. 2003, 15, 822–825. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, N.; Fu, C.; Li, K.; Tao, L.; Feng, L.; Wei, Y. Thermo and pH Dual-Responsive Materials for Controllable Oil/Water Separation. ACS Appl. Mater. Interfaces 2014, 6, 2026–2030. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Lu, X.; Ren, G.; Zhu, Y.; Wan, M.; Jiang, L. Underwater self-cleaning PEDOT-PSS hydrogel mesh for effective separation of corrosive and Hot Oil/Water mixtures. Adv. Mater. Interfaces 2014, 1, 1400099. [Google Scholar] [CrossRef]
- Li, J.-J.; Zhou, Y.-N.; Luo, Z.-H. Smart Fiber Membrane for pH-Induced Oil/Water Separation. ACS Appl. Mater. Interfaces 2015, 7, 19643–19650. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.X.; Wang, S.T.; Lin, L.; Chen, L.; Liu, M.J.; Feng, L.; Jiang, L. A Novel Superhydrophilic and Underwater Superoleophobic Hydrogel-Coated Mesh for Oil/Water Separation. Adv. Mater. 2011, 23, 4270–4273. [Google Scholar] [CrossRef]
- Han, N.; Lim, Y.T.; Jang, W.; Koo, H.Y.; Choi, W.S. Polydopamine-Mediated All-in-One Device with Superhydrophilicity and Superhydrophobicity for One-Step Oil/Water Separation and Pollutant Purification. Polymer 2016, 107, 1–11. [Google Scholar] [CrossRef]
- Cortese, B.; Caschera, D.; Federici, F.; Ingo, G.M.; Gigli, G. Superhydrophobic Fabrics for Oil−Water Separation through a Diamond Like Carbon (DLC) Coating. J. Mater. Chem. A 2014, 2, 6781–6789. [Google Scholar] [CrossRef]
- Cortese, B.; Caschera, D.; Padeletti, G.; Ingo, G.M.; Gigli, G. A brief review of surface-functionalized cotton fabrics. Surf. Innov. 2013, 1, 140–156. [Google Scholar] [CrossRef]
- Cao, C.; Ge, M.; Huang, J.; Li, S.; Deng, S.; Zhang, S.; Chen, Z.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. Robust fluorine-free superhydrophobic PDMS–ormosil@ fabrics for highly effective self-cleaning and efficient oil–water separation. J. Mater. Chem. A 2016, 4, 12179–12187. [Google Scholar] [CrossRef]
- Zhang, J.; Seeger, S. Polyester materials with superwetting silicone nanofilaments for oil/water separation and selective oil absorption. Adv. Funct. Mater. 2011, 21, 4699–4704. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Xu, X.; Guo, F.; Zhu, X.; Men, X.; Ge, B. Robust and durable superhydrophobic cotton fabrics for oil/water separation. ACS Appl. Mater. Interfaces 2013, 5, 7208–7214. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, J.; Wang, G.; Liang, W.; Zhang, Y.; Shi, L.; Guo, Z.; Liu, W. Methodology for Robust Superhydrophobic Fabrics and Sponges from In Situ Growth of Transition Metal/Metal Oxide Nanocrystals with Thiol Modification and Their Applications in Oil/Water Separation. ACS Appl. Mater. Interfaces 2013, 5, 1827–1839. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, F.; Zhang, S. Durably superhydrophobic textile based on fly ash coating for oil/water separation and selective oil removal from water. Sep. Purif. Technol. 2016, 164, 138–145. [Google Scholar] [CrossRef]
- Xue, C.-H.; Jia, S.-T.; Chen, H.-Z.; Wang, M. Superhydrophobic cotton fabrics prepared by sol–gel coating of TiO2 and surface hydrophobization. Sci. Technol. Adv. Mater. 2008, 9, 035001. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Wang, C.; Wang, S.; Li, J. Fabrication of superhydrophobic cotton textiles for water–oil separation based on drop-coating route. Carbohydr. Polym. 2013, 97, 59–64. [Google Scholar] [CrossRef]
- Zhang, X.; Geng, T.; Guo, Y.; Zhang, Z.; Zhang, P. Facile fabrication of stable superhydrophobic SiO2/polystyrene coating and separation of liquids with different surface tension. Chem. Eng. J. 2013, 231, 414–419. [Google Scholar] [CrossRef]
- Li, K.; Zeng, X.; Li, H.; Lai, X.; Xie, H. Facile fabrication of superhydrophobic filtration fabric with honeycomb structures for the separation of water and oil. Mater. Lett. 2014, 120, 255–258. [Google Scholar] [CrossRef]
- Zhang, C.; Li, P.; Cao, B. Fabrication of superhydrophobic–superoleophilic fabrics by an etching and dip-coating two-step method for oil–water separation. Ind. Eng. Chem. Res. 2016, 55, 5030–5035. [Google Scholar] [CrossRef]
- Xue, C.-H.; Guo, X.-J.; Zhang, M.-M.; Ma, J.-Z.; Jia, S.-T. Fabrication of robust superhydrophobic surfaces by modification of chemically roughened fibers via thiol–ene click chemistry. J. Mater. Chem. A 2015, 3, 21797–21804. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun Nanofibers: Solving Global Issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Q.; Hua, D.; Xiong, R.; Zhao, J.; Rao, W.; Huang, S.; Zhan, X.; Chen, F.; Huang, C. Electrospun Fibers for Oil-Water Separation. RSC Adv. 2016, 6, 12868–12884. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Q.; Samal, S.K.; Wang, F.; Gao, B.; Pan, H.; Xu, H.; Yao, J.; Zhan, X.; De Smedt, S.C.; et al. Core-sheath structured electrospun nanofibrous membranes for oil–water separation. RSC Adv. 2016, 6, 41861–41870. [Google Scholar] [CrossRef]
- Alayande, S.O.; Dare, E.O.; Msagati, T.A.M.; Akinlabi, A.K.; Aiyedun, P.O. Superhydrophobic and superoleophillic surface of porous beadedelectrospun polystrene and polysytrene-zeolitefiber for crudeoil-water separation. Phys. Chem. Earth 2016, 92, 7–13. [Google Scholar] [CrossRef]
- Ning, L.Q.; Xu, N.K.; Wang, R.; Liu, Y. Fibrous Membranes Electrospun from the Suspension Polymerization Product of Styrene and Butyl Acrylate for Oil-Water Separation. RSC Adv. 2015, 5, 57101–57113. [Google Scholar] [CrossRef]
- Liu, C.-T.; Liu, Y.-L. pH-Induced Switches of the Oil- And Water-Selectivity of Crosslinked Polymeric Membranes for Gravity-Driven Oil-Water Separation. J. Mater. Chem. A 2016, 4, 13543–13548. [Google Scholar] [CrossRef]
- Gui, X.C.; Wei, J.Q.; Wang, K.L.; Cao, A.Y.; Zhu, H.W.; Jia, Y.; Shu, Q.K.; Wu, D.H. Carbon Nanotube Sponges. Adv. Mater. 2010, 22, 617–621. [Google Scholar] [CrossRef]
- Li, J.; Xu, C.C.; Zhang, Y.; Wang, R.F.; Zha, F.; She, H.D. Robust Superhydrophobic Attapulgite Coated Polyurethane sponge for Efficient Immiscible Oil/Water Mixture and Emulsion Separation. J. Mater. Chem. A 2016, 4, 15546–15553. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, Z.; Guo, J.; Shen, H.; Zhang, H.; Zhao, N.; Zhao, Y.P.; Chen, L.; Liang, S.M.; Jin, Y.; et al. Facile Fabrication of Robust Superhydrophobic Porous Materials and Their Application in Oil/ Water Separation. J. Mater. Chem. A 2015, 3, 23252–23260. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, Y.S.; Xiong, W.; Wang, M.; Fan, L.; Rabiee-Golgir, H.; Jiang, L.; Hou, W.; Huang, X.; Jiang, L.; et al. Highly Efficient and Recyclable Carbon Soot Sponge for Oil Cleanup. ACS Appl. Mater. Interfaces 2014, 6, 5924–5929. [Google Scholar] [CrossRef]
- Zhao, X.; Li, L.; Li, B.; Zhang, J.; Wang, A. Durable superhydrophobic/superoleophilic PDMS sponges and their applications in selective oil absorption and in plugging oil leakages. J. Mater. Chem. A 2014, 2, 18281–18287. [Google Scholar] [CrossRef]
- Du, R.; Feng, Q.; Ren, H.; Zhao, Q.; Gao, X.; Zhang, J. Hybrid-dimensional magnetic microstructure-based 3D substrates for remote controllable and ultrafast water remediation. J. Mater. Chem. A 2016, 4, 938–943. [Google Scholar]
- Lico, D.; Vuono, D.; Siciliano, C.; Nagy, J.B.; De Luca, P. Removal on unleaded gasoline from water by multi-walled carbon nanotubes. J. Environ. Manag. 2019, 237, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Pan, Q.; Liu, F. Facile Removal and Collection of Oils from Water Surfaces through Superhydrophobic and Superoleophilic Sponges. J. Phys. Chem. C 2011, 115, 17464–17470. [Google Scholar] [CrossRef]
- Zou, F.; Peng, L.; Fu, W.; Zhang, J.; Li, Z. Flexible superhydrophobic polysiloxane aerogels for oil-water separation via one-pot synthesis in supercritical CO2. RSC Adv. 2015, 5, 76346–76351. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, X.; Fang, J. Superhydrophobic and superoleophilic “sponge-like” aerogels for oil/water separation. J. Mater. Sci. 2015, 50, 5115–5124. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kaang, B.K.; Han, N.; Lee, H.J.; Choi, W.S. An Anti-Overturn Janus Sponge with Excellent Floating Stability for Simultaneous Pollutant Remediation and Oil/Water Separation. J. Mater. Chem. A 2018, 6, 16371–16381. [Google Scholar] [CrossRef]
- Kaang, B.K.; Lee, Y.S.; Han, N.; Choi, W.S. A Potential Amphiprotic Sponge with a Controlled Release Characteristic of Protons on Demand for Oil/Water Separation and Acid/Base Neutralization. Adv. Mater. Interfaces 2019, 6, 1900004. [Google Scholar] [CrossRef]

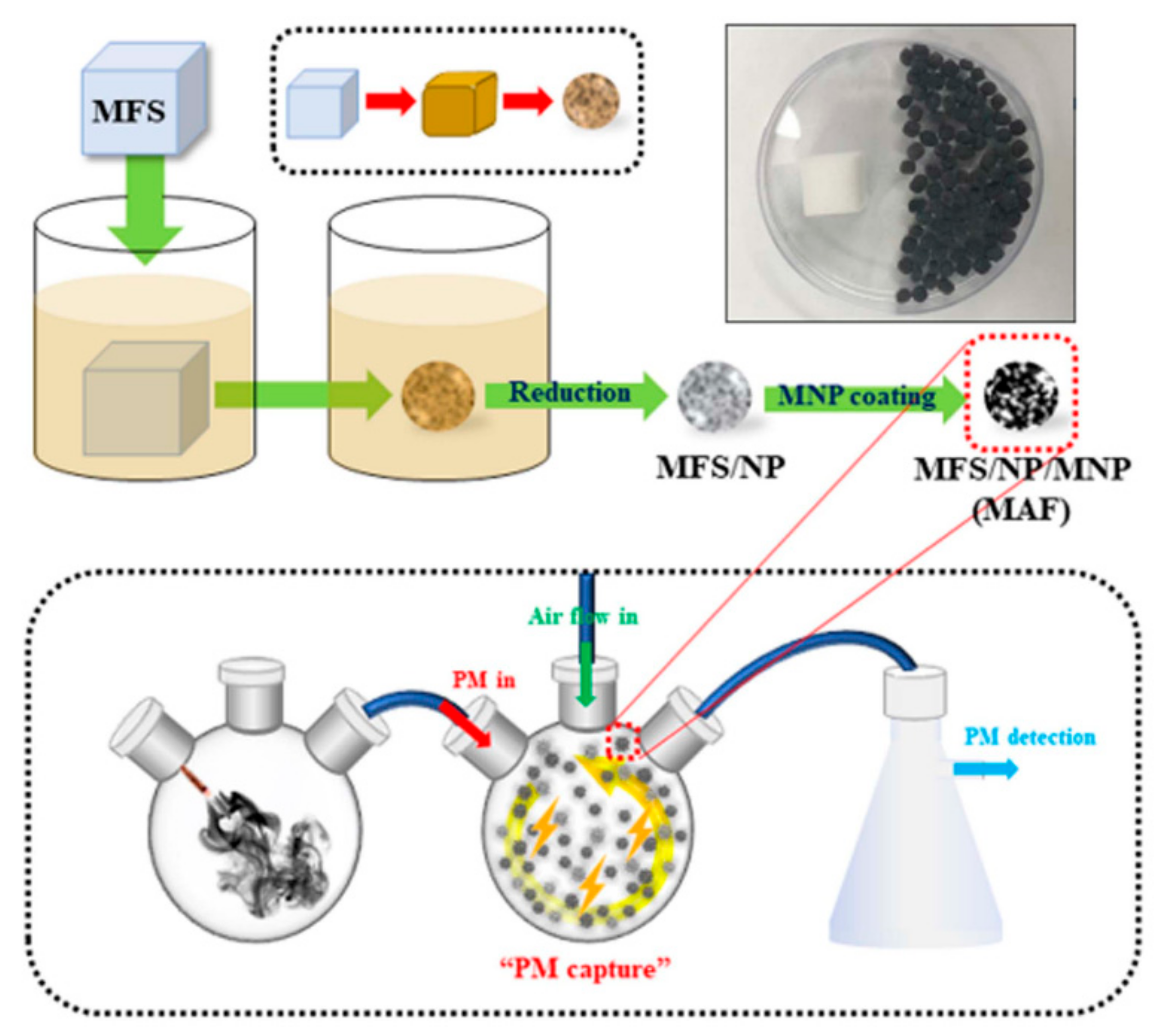

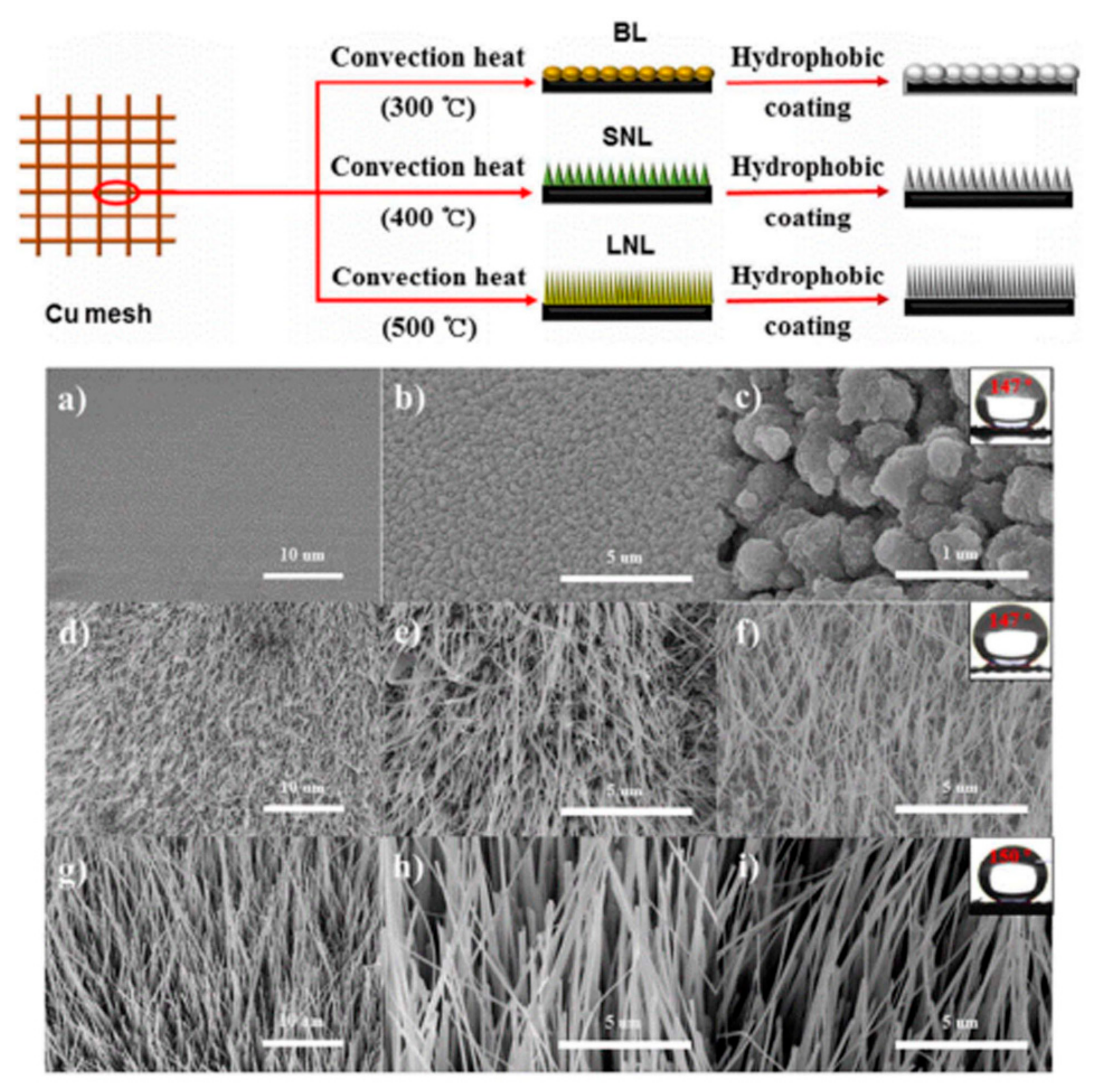
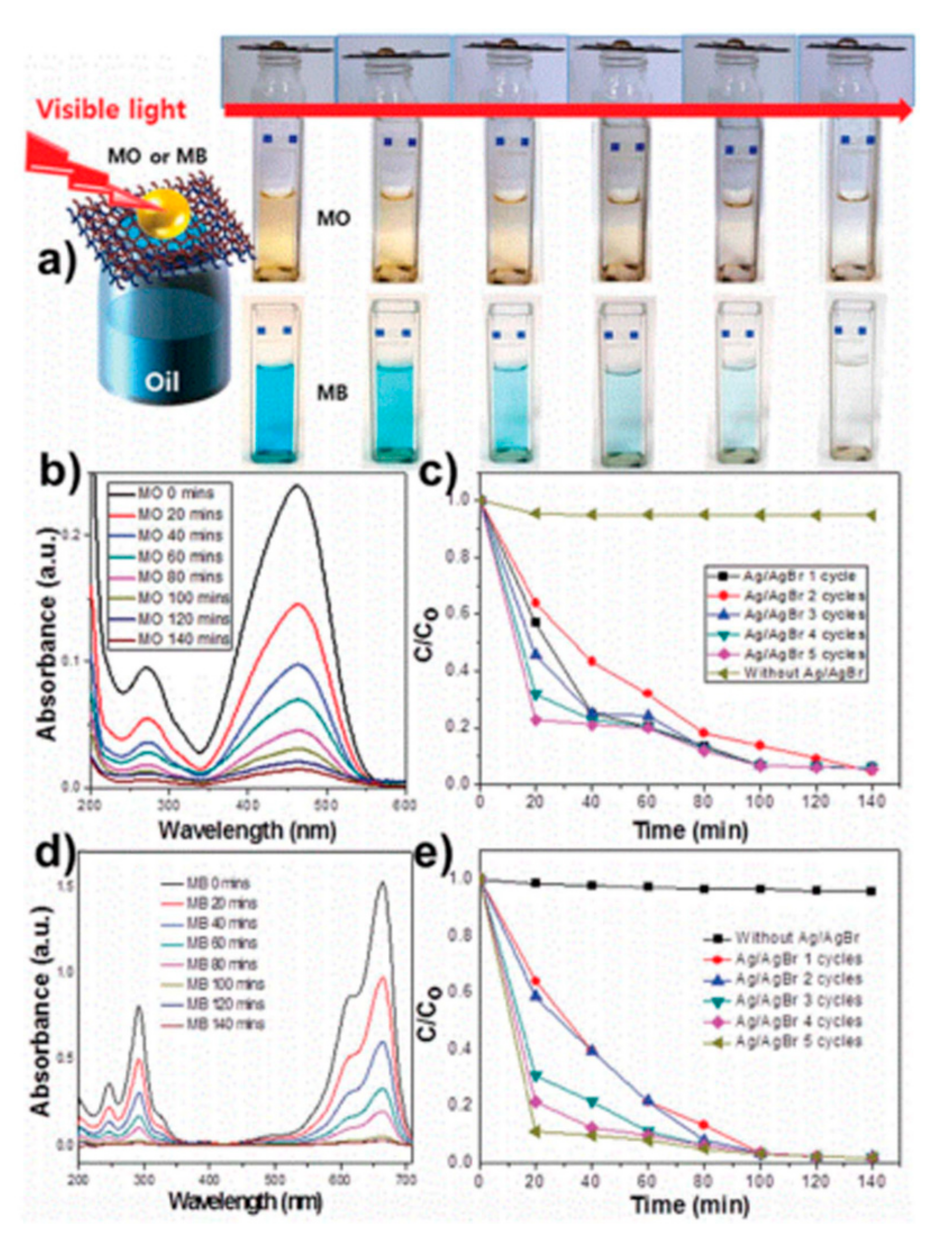
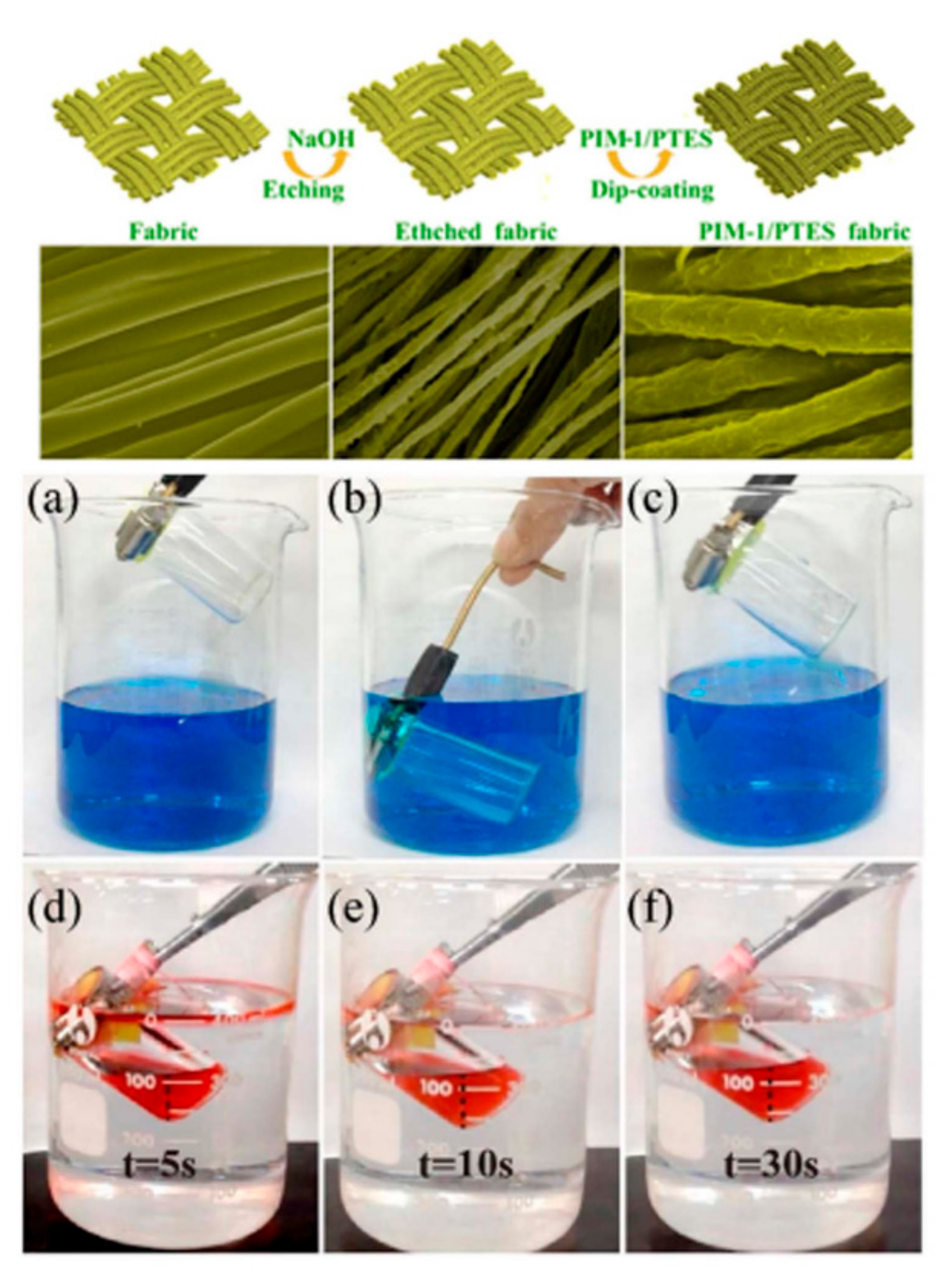
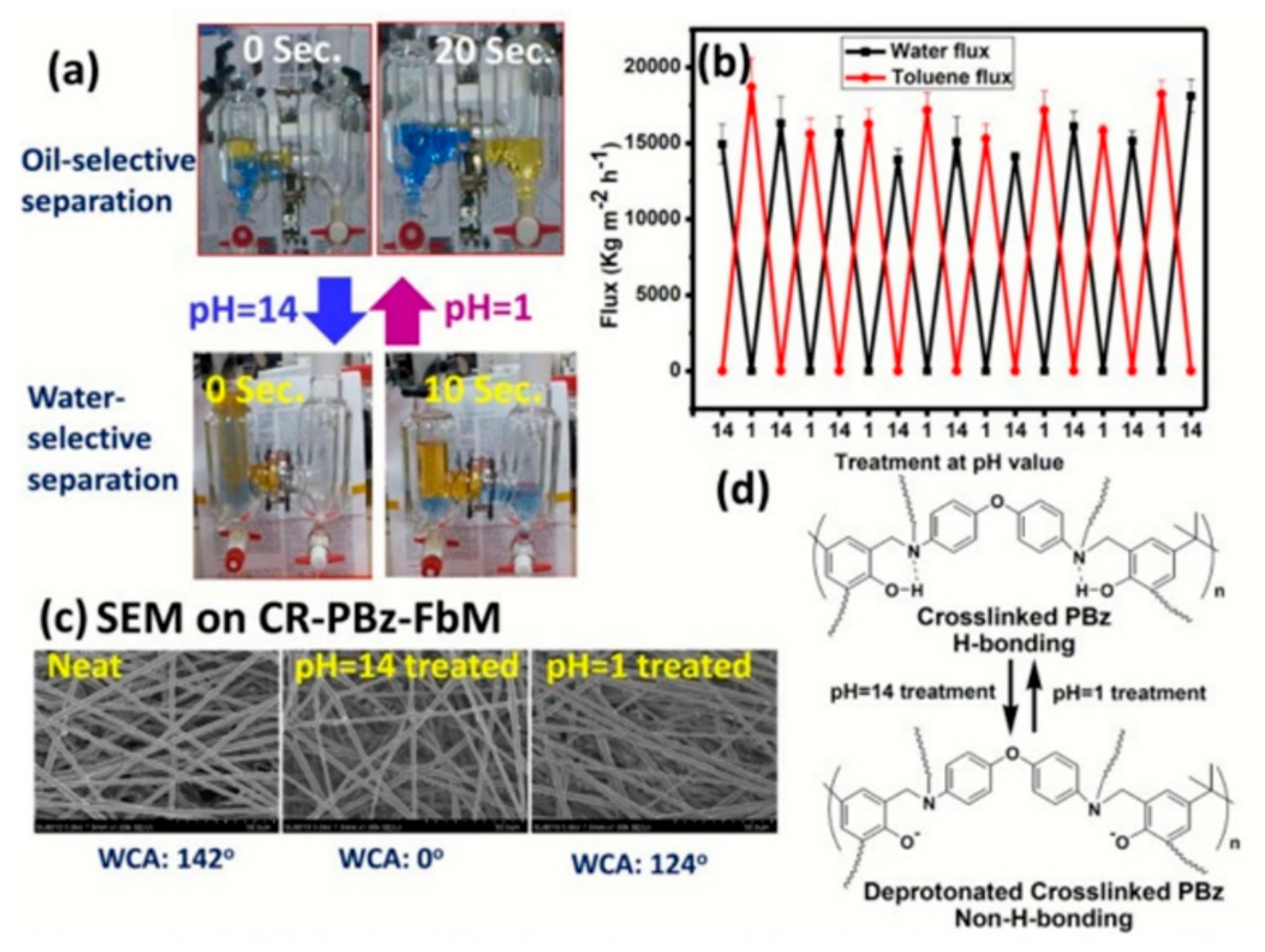
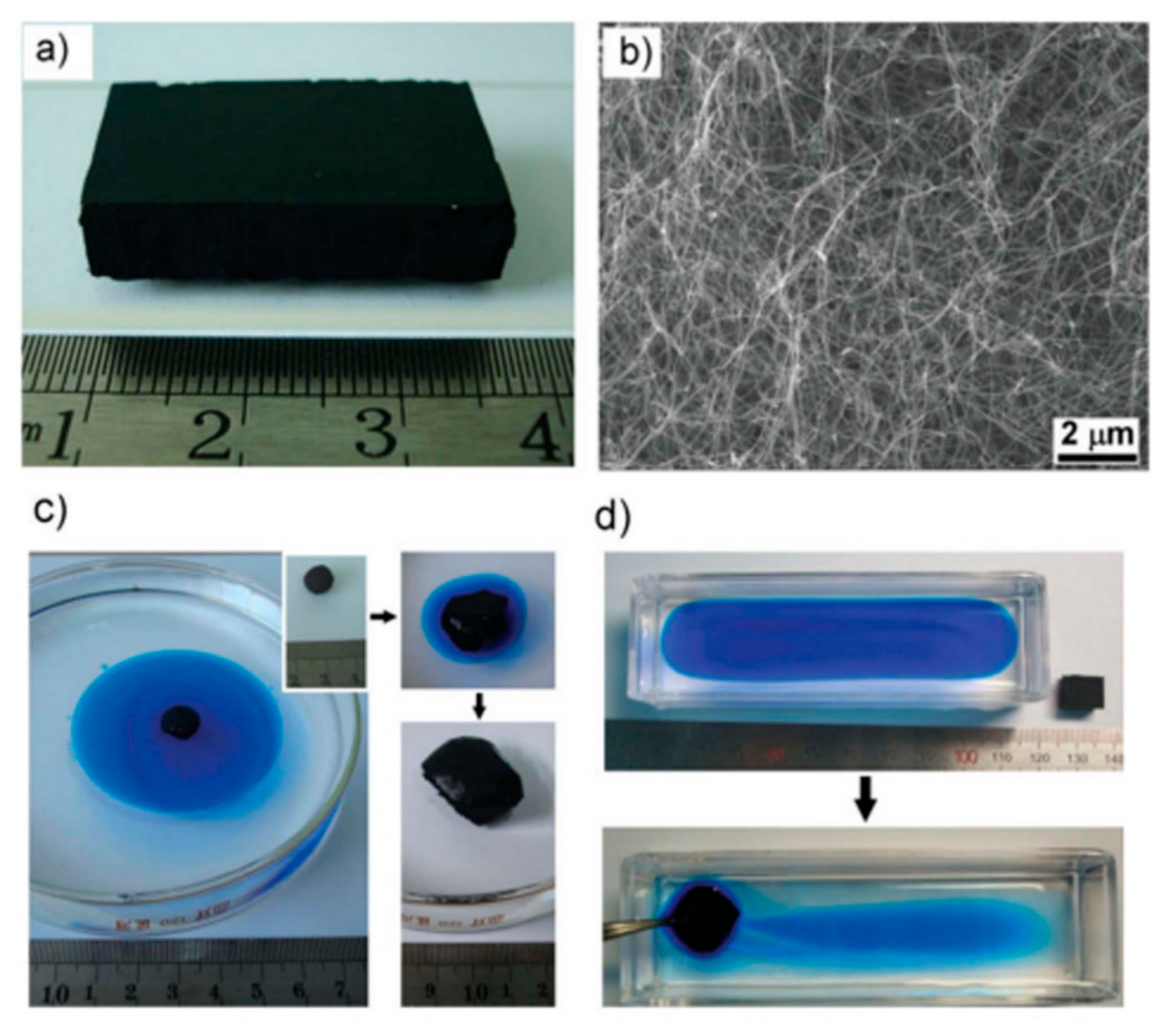
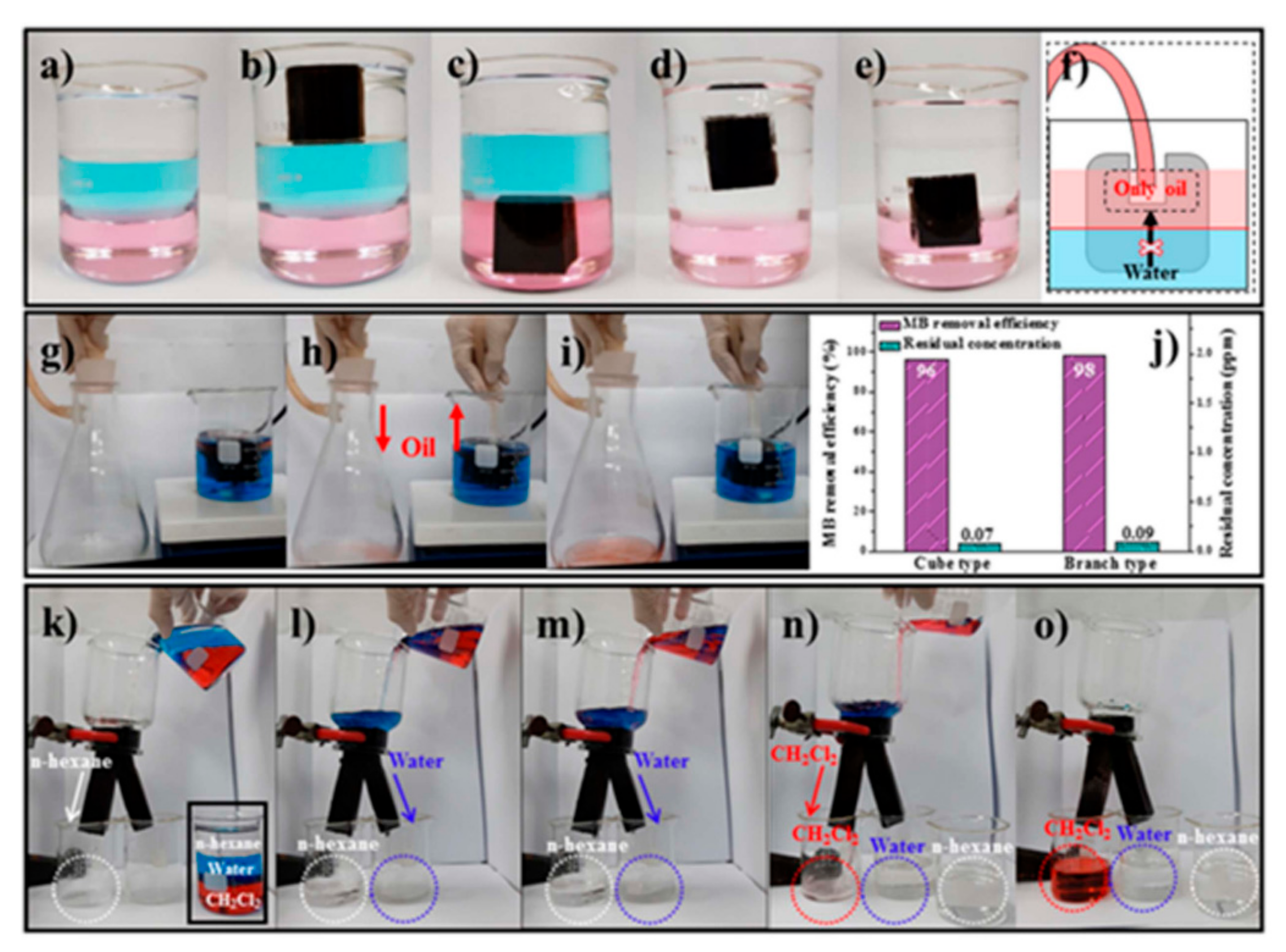
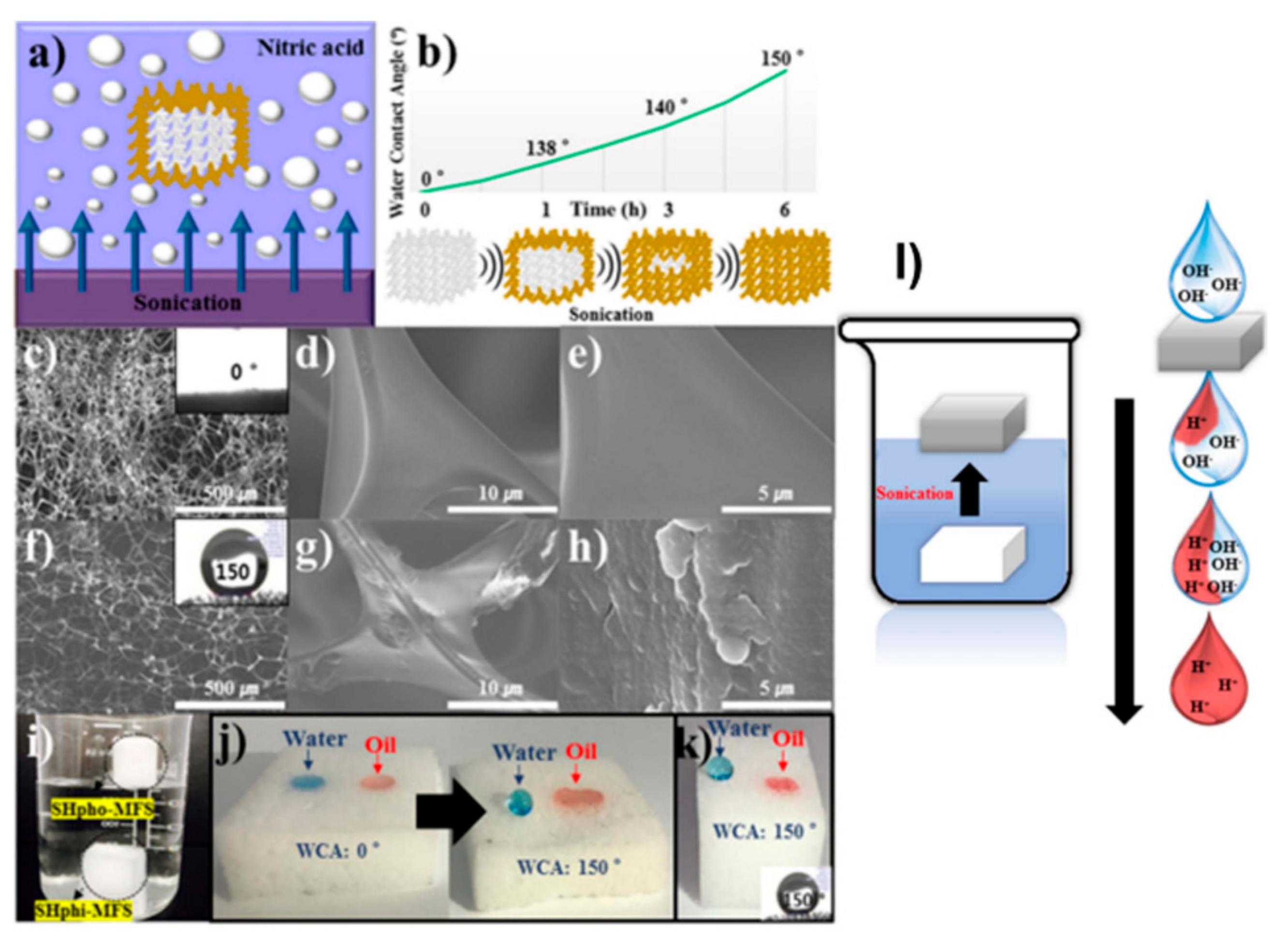
| Application Type | 2D | 3D |
|---|---|---|
| Air filter | Meshes Fiber nets Fabrics Papers | Sponges Sponge/polymer networks Sponge/paper networks |
| Oil/water separator | Meshes Membranes Fabrics | Sponges Forms Aerogels Rubber networks |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-J.; Choi, W.S. 2D and 3D Bulk Materials for Environmental Remediation: Air Filtration and Oil/Water Separation. Materials 2020, 13, 5714. https://doi.org/10.3390/ma13245714
Lee H-J, Choi WS. 2D and 3D Bulk Materials for Environmental Remediation: Air Filtration and Oil/Water Separation. Materials. 2020; 13(24):5714. https://doi.org/10.3390/ma13245714
Chicago/Turabian StyleLee, Ha-Jin, and Won San Choi. 2020. "2D and 3D Bulk Materials for Environmental Remediation: Air Filtration and Oil/Water Separation" Materials 13, no. 24: 5714. https://doi.org/10.3390/ma13245714





